Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China
Abstract
:1. Introduction
2. Materials and Methods
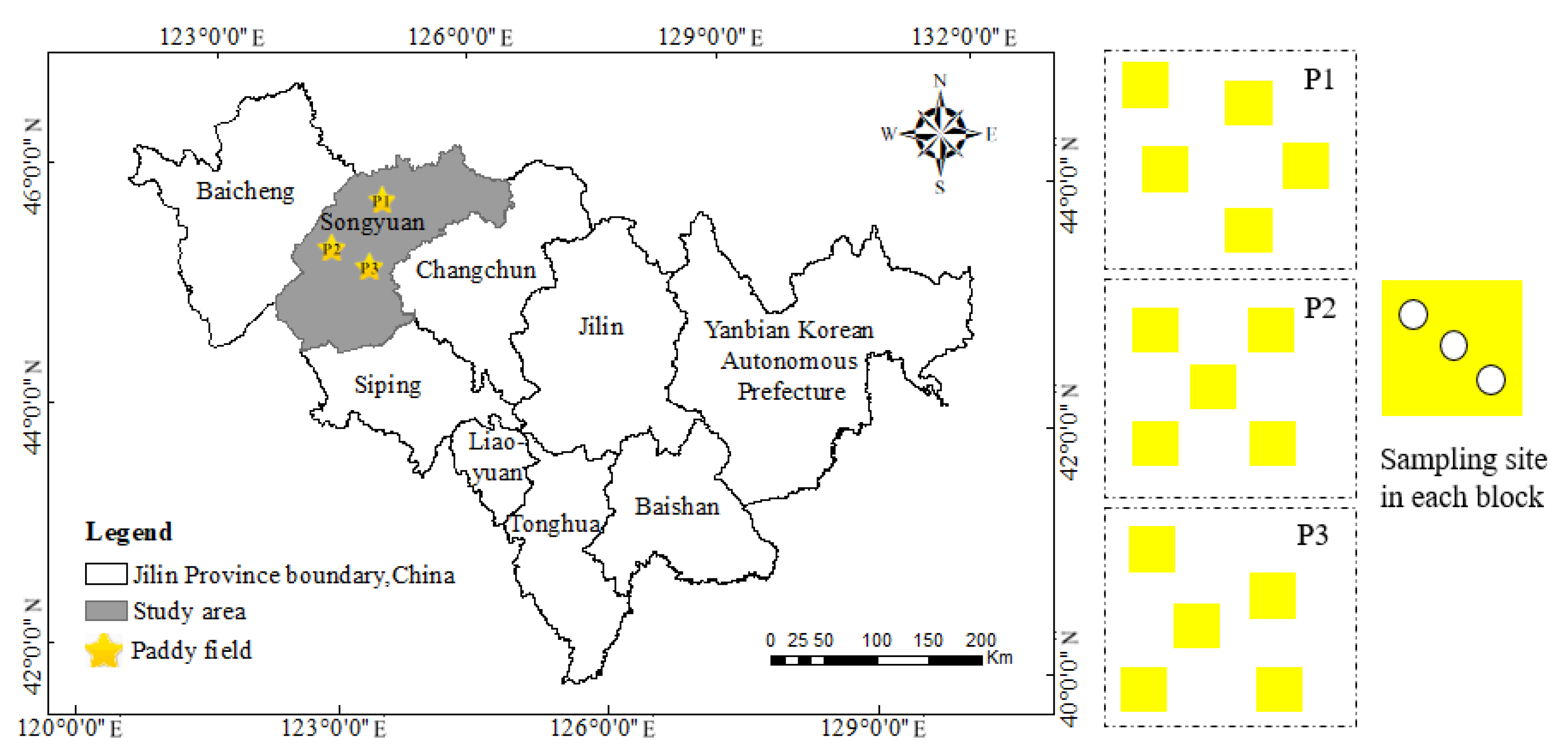
2.1. Study Area Description
2.2. Soil Sampling and Experimental Design
2.3. Soil Analysis
2.4. Soil Enzyme Activities
2.5. Biolog—ECO Plate Assay
2.6. Statistical Analysis
3. Results
3.1. Soil Physico-Chemical Properties
3.2. Soil Enzyme Activities
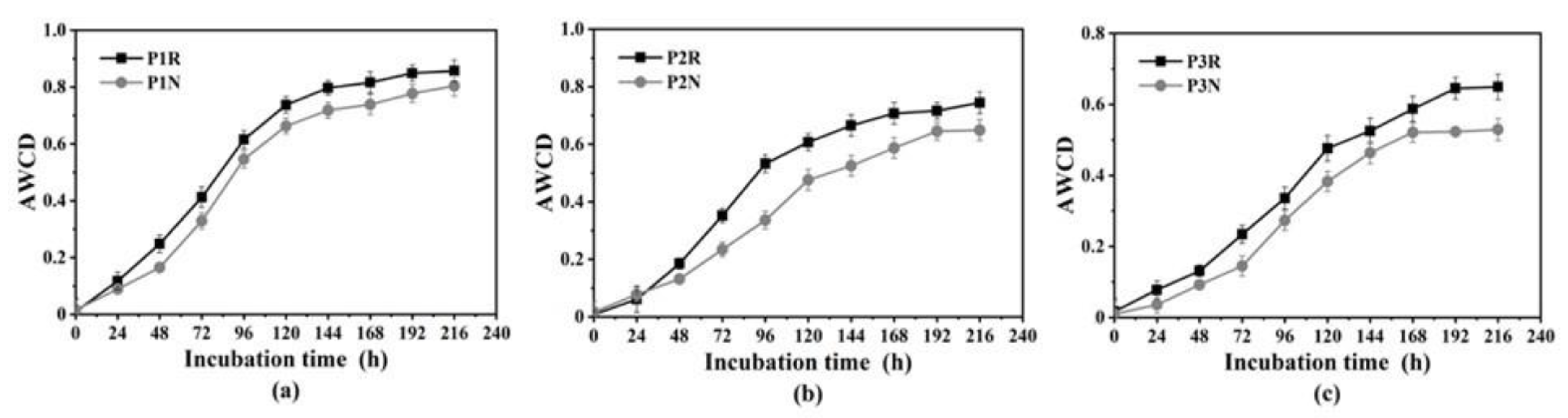
3.3. Community-Level Physiological Profile
3.4. Functional Diversity Index of Soil Microbial Community
3.5. Soil Microbial Utilization Intensity of Carbon Source
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Dou, S.; Wang, L.L.; Wu, J.S.; Wang, T.; Wang, C.Y.; Jiang, Z.D.; Ju, Z.S.; Wang, J.; Luo, M. Salinity Variability of Soda Meadow Alkaline Soil in Different Depths of Subsurface Pipe. Pol. J. Environ. Stud. 2018, 27, 2801–2809. [Google Scholar] [CrossRef]
- Huang, L.H.; Liang, Z.W.; Suarez, D.L.; Wang, Z.C.; Wang, M.M.; Yang, H.Y.; Liu, M. Impact of cultivation year, nitrogen fertilization rate and irrigation water quality on soil salinity and soil nitrogen in saline-sodic paddy fields in Northeast China. J. Agric. Sci. 2015, 154, 632–646. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Cui, B.; Yang, Z. Dynamics of the soil water and solute in the sodic saline soil in the Songnen Plain, China. Environ. Earth Sci. 2009, 59, 837–845. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, Z.; Biswas, A.; Yang, S.; Ding, J.; Wang, F. Updated information on soil salinity in a typical oasis agroecosystem and desert-oasis ecotone: Case study conducted along the Tarim River, China. Sci. Total Environ. 2020, 716, 135387. [Google Scholar] [CrossRef]
- Amezketa, E. An integrated methodology for assessing soil salinization, a pre-condition for land desertification. J. Arid. Environ. 2006, 67, 594–606. [Google Scholar] [CrossRef]
- Lin, N.F.; Tang, J.; Han, F.X. Eco-environmental problems and effective utilization of water resources in the Kashi Plain, western Terim Basin, China. Hydrogeol. J. 2001, 9, 202–207. [Google Scholar] [CrossRef]
- Liu, J.L.; Tang, L.; Gao, H.; Zhang, M.; Guo, C.H. Enhancement of alfalfa yield and quality by plant growth-promoting rhizobacteria under saline-alkali conditions. J. Sci. Food Agric. 2019, 99, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Takano, T.; Liu, S. Screening and Evaluation of Saline–Alkaline Tolerant Germplasm of Rice (Oryza sativa L.) in Soda Saline–Alkali Soil. Agronomy 2018, 8, 205. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 2007, 294, 263–276. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Singh, A. Soil salinization and waterlogging: A threat to environment and agricultural sustainability. Ecol. Indic. 2015, 57, 128–130. [Google Scholar] [CrossRef]
- Silvia, D.; Anton, H.; Rüdiger, H. Ecological Biochemistry: Environmental and Interspecies Interactions; Wiley-Blackwell: Weinheim, Germany, 2014. [Google Scholar]
- Watt, M.; Kirkegaard, J.A.; Passioura, J.B. Rhizosphere biology and crop productivity—A review. Soil Res. 2006, 44, 299. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interations with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Duan, C.; Fang, L.; Yang, C.; Chen, W.; Cui, Y.; Li, S. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol. Environ. Saf. 2018, 156, 106–115. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.; Yang, Y.; Xing, G.; Deng, C.; Shen, Y.; Luo, L.; Li, B.; Yuan, H. A proposal of “core enzyme” bioindicator in long-term Pb-Zn ore pollution areas based on topsoil property analysis. Environ. Pollut. 2016, 213, 760–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Zeglin, L.H. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Roldán, A.; Salinas-García, J.R.; Alguacil, M.M.; Caravaca, F. Changes in soil enzyme activity, fertility, aggregation and C sequestration mediated by conservation tillage practices and water regime in a maize field. Appl. Soil Ecol. 2005, 30, 11–20. [Google Scholar] [CrossRef]
- Tiwari, M.B.; Tiwari, B.K.; Mishra, R.R. Enzyme activity and carbon dioxide evolution from upland and wetland rice soils under three agricultural practices in hilly regions. Biol. Fertil. Soils 1989, 7, 359–364. [Google Scholar] [CrossRef]
- Zhang, X.; Miao, M.; Wu, Z.; Zhang, Z.; Gao, R.; Shi, L. Effects of Helianthus annuus varieties on rhizosphere soil enzyme activities and microbial community functional diversity of saline-alkali land in Xinjiang. Acta Ecol. Sin. 2017, 37, 1659–1666. [Google Scholar]
- Wan, Z.; Song, C. Advance on Response of Soil Enzyme Activity to Ecological Environment. Chin. J. Soil Sci. 2009, 40, 951–956. [Google Scholar]
- Freeman, C.; Liska, G.; Ostle, N.J.; Lock, M.A.; Reynolds, B.; Hudson, J. Microbial activity and enzymic decomposition processes following peatland water table drawdown. Plant Soil 1996, 180, 121–127. [Google Scholar] [CrossRef]
- You, Y.M.; Wang, J.; Huang, X.M.; Tang, Z.X.; Liu, S.R.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Guo, X. Effects of biochar on the diversity and community structure of soil fungi in intercropping system. Appl. Ecol. Environ. Res. 2019, 17, 8817–8834. [Google Scholar] [CrossRef]
- Jeanbille, M.; Buée, M.; Bach, C.; Cébron, A.; Frey-Klett, P.; Turpault, M.P.; Uroz, S. Soil Parameters Drive the Structure, Diversity and Metabolic Potentials of the Bacterial Communities Across Temperate Beech Forest Soil Sequences. Microb. Ecol. 2016, 71, 482–493. [Google Scholar] [CrossRef]
- Asemoloye, M.D.; Jonathan, S.G.; Ahmad, R. Synergistic plant-microbes interactions in the rhizosphere: A potential headway for the remediation of hydrocarbon polluted soils. Int. J. Phytoremediat. 2019, 21, 71–83. [Google Scholar] [CrossRef]
- Jiang, L.L.; Han, G.M.; Lan, Y.; Liu, S.N.; Gao, J.P.; Yang, X.; Meng, J.; Chen, W.F. Corn cob biochar increases soil culturable bacterial abundance without enhancing their capacities in utilizing carbon sources in Biolog Eco-plates. J. Integr. Agric. 2017, 16, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.M.; Tang, J.; Zhang, L.S.; Ma, H.Y.; Zhao, J. Arsenic distribution and geological factors in the western Jilin province, China. J. Geochem. Explor. 2012, 112, 347–356. [Google Scholar] [CrossRef]
- Tang, J.; Liang, S.; Li, Z.Y.; Zhang, H.; Lou, Y.; Wang, J.J. Effect of freeze-thaw cycles on carbon stocks of saline-alkali paddy soil. Arch. Agron. Soil Sci. 2016, 62, 1640–1653. [Google Scholar] [CrossRef]
- Tang, J.; Liang, S.; Li, Z.Y.; Zhang, H.; Wang, S.N.; Zhang, N. Emission Laws and Influence Factors of Greenhouse Gases in Saline-Alkali Paddy Fields. Sustainability 2016, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Zheng, B.Z. Technical Guide for Soil Analysis; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Zhao, Q.; Tang, J.; Li, Z.Y.; Yang, W.; Duan, Y.C. The Influence of Soil Physico-Chemical Properties and Enzyme Activities on Soil Quality of Saline-Alkali Agroecosystems in Western Jilin Province, China. Sustainability 2018, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.G. Principles and Methods of Soil Microbiology Research; Education Press: Beijing, China, 2010. [Google Scholar]
- Rutgers, M.; Wouterse, M.; Drost, S.M.; Breure, A.M.; Mulder, C.; Stone, D.; Creamer, R.E.; Winding, A.; Bloem, J. Monitoring soil bacteria with community-level physiological profiles using Biolog™ ECO-plates in the Netherlands and Europe. Appl. Soil Ecol. 2016, 97, 23–35. [Google Scholar] [CrossRef]
- Klimek, B.; Niklinska, M.; Jazwa, M.; Tarasek, A.; Tekielak, I.; Musielok, L. Covariation of soil bacteria functional diversity and vegetation diversity along an altitudinal climatic gradient in the Western Carpathians. Pedobiologia 2015, 58, 105–112. [Google Scholar] [CrossRef]
- Zhang, H.; Li, G.; Song, X.; Yang, D.; Li, Y.; Qiao, J.; Zhang, J.; Zhao, S. Changes in soil microbial functional diversity under different vegetation restoration patterns for Hulunbeier Sandy Land. Acta. Ecol. Sin 2013, 33, 38–44. [Google Scholar] [CrossRef]
- Wu, X.; Xu, J.; Dong, F.; Liu, X.; Zheng, Y. Responses of soil microbial community to different concentration of fomesafen. J. Hazard. Mater. 2014, 273, 155–164. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Z.K.; Li, B.; Sun, X.; Wang, J.; Wang, J.H.; Zhu, L.S. Evaluating toxicity of 1-octyl-3-methylimidazolium hexafluorophosphate to microorganisms in soil. Chemosphere 2018, 210, 762–768. [Google Scholar] [CrossRef]
- Dobranic, J.K.; Zak, J.C. A microtiter plate procedure for evaluating fungal functional diversity. Mycologia 1999, 91, 756–765. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Xu, W.; Hu, Y.; Hu, Y.; Zhang, Y. The microbiome and functions of black soils are altered by dibutyl phthalate contamination. Appl. Soil Ecol. 2016, 99, 51–61. [Google Scholar] [CrossRef]
- Li, N.; Shao, T.Y.; Zhu, T.S.; Long, X.H.; Gao, X.M.; Liu, Z.P.; Shao, H.B.; Rengel, Z. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci. Rep. 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Loinaz, G.; Onaindia, M.; Amezaga, I.; Mijangos, I.; Garbisu, C. Relationship between vegetation diversity and soil functional diversity in native mixed-oak forests. Soil Biol. Biochem. 2008, 40, 49–60. [Google Scholar] [CrossRef]
- Leonard, S.G.; Miles, R.L.; Tueller, P.T. Vegetation-Soil Relationships on Arid and Semiarid Rangelands; Springer Netherlands: Dordrecht, The Netherlands, 1988. [Google Scholar]
- Yin, R.; Deng, H.; Wang, H.-l.; Zhang, B. Vegetation type affects soil enzyme activities and microbial functional diversity following re-vegetation of a severely eroded red soil in sub-tropical China. CATENA 2014, 115, 96–103. [Google Scholar] [CrossRef]
- Bacmaga, M.; Wyszkowska, J.; Kucharski, J. Bioaugmentation of Soil Contaminated with Azoxystrobin. Water Air Soil Poll. 2017, 228, 9. [Google Scholar] [CrossRef] [Green Version]
- Pistocchi, C.; Ragaglini, G.; Colla, V.; Branca, T.A.; Tozzini, C.; Romaniello, L. Exchangeable Sodium Percentage decrease in saline sodic soil after Basic Oxygen Furnace Slag application in a lysimeter trial. J. Environ. Manag. 2017, 203, 896–906. [Google Scholar] [CrossRef]
- Kunito, T.; Akagi, Y.; Park, H.D.; Toda, H. Influences of nitrogen and phosphorus addition on polyphenol oxidase activity in a forested Andisol. Eur. J. For. Res. 2009, 128, 361–366. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef] [Green Version]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.P.; Zhu, L.S.; Wang, J.H.; Wang, J.; Xie, H.; Lv, D.D. Enzymatic activities and microbial biomass in black soil as affected by azoxystrobin. Environ. Earth Sci. 2015, 74, 1353–1361. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A.; Piotrowska, A.; Palumbo, G.; Colombo, C. Soil enzyme activities as affected by anthropogenic alterations: Intensive agricultural practices and organic pollution. Sci. Total Environ. 2005, 341, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, Z.; Song, Z. Effects of di-n-butyl phthalate on rhizosphere and non-rhizosphere soil microbial communities at different growing stages of wheat. Ecotoxicol. Environ. Saf. 2019, 174, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.M.; Liao, M.; Yang, J.; Chai, J.J.; Fang, S.; Wang, R.H. Influence of root-exudates concentration on pyrene degradation and soil microbial characteristics in pyrene contaminated soil. Chemosphere 2012, 88, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Zavarzina, A.G.; Leontievsky, A.A.; Golovleva, L.A.; Trofimov, S.Y. Biotransformation of soil humic acids by blue laccase of Panus tigrinus 8/18: An in vitro study. Soil Biol. Biochem. 2004, 36, 359–369. [Google Scholar] [CrossRef]
- Girelli, A.M.; Mattei, E.; Messina, A.; Tarola, A.M. Inhibition of Polyphenol Oxidases Activity by Various Dipeptides. J. Agric. Food Chem. 2004, 52, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Jianchao, H.; Yanyou, W.; Bin, L.; Chundu, W. Properties of PolyphenolOx idase in Soil and its Significance. Chin. J. Soil Sci. 2006, 37, 470–474. [Google Scholar]
- Pérez-Montaño, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sun, L.; Ling, N.; Zhu, C.; Chi, F.Q.; Li, W.Q.; Hao, X.Y.; Zhang, W.; Bian, J.Y.; Chen, L.; et al. Exploring Soil Factors Determining Composition and Structure of the Bacterial Communities in Saline-Alkali Soils of Songnen Plain. Front. Microbiol. 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Hmid, A.; Al Chami, Z.; Sillen, W.; De Vocht, A.; Vangronsveld, J. Olive mill waste biochar: A promising soil amendment for metal immobilization in contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 1444–1456. [Google Scholar] [CrossRef]
- Klimek, B.; Chodak, M.; Jazwa, M.; Niklinska, M. Functional diversity of soil microbial communities in boreal and temperate Scots pine forests. Eur. J. For. Res. 2016, 135, 731–742. [Google Scholar] [CrossRef] [Green Version]
- van Groenigen, K.J.; Forristal, D.; Jones, M.; Smyth, N.; Schwartz, E.; Hungate, B.; Dijkstra, P. Using metabolic tracer techniques to assess the impact of tillage and straw management on microbial carbon use efficiency in soil. Soil Biol. Biochem. 2013, 66, 139–145. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Review: Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 2010, 165, 66–70. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and Bacterial Diversity: To What Extent Does the Concentration of Salt Affect the Bacterial Community in a Saline Soil? PLoS ONE 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lv, G.H.; Li, Y. Soil microbial functional diversity of rhizosphere and non-rhizosphere of three dominant herbaceous plants in the Dushanzi District. Acta Ecol. Sin. 2018, 38, 3110–3117. [Google Scholar]
- Li, C.G.; Li, X.M.; Wang, J.G. Effect of soybean continuous cropping on bulk and rhizosphere soil microbial community function. Acta Ecol. Sin. 2006, 26, 1144–1150. [Google Scholar]



| Sampling Site | pH | ESP (%) | EC (ds cm−1) | Soil Classification | Clay (%) | Silt (%) | Sand (%) | Fertilization Treatment |
|---|---|---|---|---|---|---|---|---|
| P1 | 8.23 | 5.85 | 0.35 | Loam | 14.07 | 40.18 | 45.75 | N, P and K |
| P2 | 9.37 | 8.09 | 0.31 | Silty loam | 10.75 | 67.33 | 21.92 | N, P and K |
| P3 | 9.94 | 13.20 | 0.45 | Sandy loam | 4.58 | 31.30 | 64.12 | N, P and K |
| Sampling Site | P1R | P1N | P2R | P2N | P3R | P3N |
|---|---|---|---|---|---|---|
| pH | 8.07 ± 0.16 Aa | 8.37 ± 0.05 Ab | 8.91 ± 0.12 Ba | 9.19 ± 0.14 Bb | 9.33 ± 0.16 Ca | 9.57 ± 0.15 Cb |
| ESP (%) | 6.35 ± 0.06 Aa | 6.63 ± 0.08 Bb | 7.45 ± 0.11 Ba | 8.07 ± 0.13 Bb | 12.21 ± 0.23 Ca | 14.18 ± 0.31 Cb |
| EC (dS m−1) | 0.39 ± 0.07 Aa | 0.28 ± 0.05 Ab | 0.27 ± 0.06 Aa | 0.36 ± 0.07 Bb | 0.53 ± 0.07 Ba | 0.41 ± 0.01 Bb |
| SWC (%) | 0.54 ± 0.06 Aa | 0.38 ± 0.03 Ab | 0.50 ± 0.06 Aa | 0.41 ± 0.09 Ab | 0.51 ± 0.21 Aa | 0.32 ± 0.19 Ab |
| SOC (g kg−1) | 12.35 ± 0.11 Aa | 10.31 ± 0.17 Ab | 11.27 ± 0.07 Ba | 8.36 ± 0.16 Bb | 9.58 ± 0.23 Ca | 7.49 ± 0.27 Cb |
| AMY | INV | CAT | PPO | H | E | D | |
|---|---|---|---|---|---|---|---|
| pH | −0.53 | −0.92 * | −0.431 | 0.263 | −0.744 | −0.458 | −0.887 * |
| ESP | −0.45 | −0.71 | −0.59 | 0.51 | −0.62 | −0.34 | −0.76 |
| EC | −0.08 | −0.26 | 0.01 | 0.11 | −0.14 | 0.15 | −0.34 |
| SWC | 0.88 * | 0.62 | 0.93 * | −0.84 * | 0.87 * | 0.93 ** | 0.71 |
| SOC | 0.89 * | 0.90 * | 0.71 | −0.63 | 0.96 ** | 0.83 * | 0.97 ** |
| Field | Shannon-Wiener Index (H) | Evenness Index (E) | Simpson Index (D) | |||
|---|---|---|---|---|---|---|
| R | N | R | N | R | N | |
| P1 | 2.71 ± 0.38Aa | 1.28 ± 0.11Ab | 0.88 ± 0.02Aa | 0.29 ± 0.02Ab | 0.80 ± 0.08Aa | 0.55 ± 0.05Ab |
| P2 | 2.08 ± 0.56Ba | 0.91 ± 0.12Bb | 0.76 ± 0.01Ba | 0.28 ± 0.02Ab | 0.63 ± 0.15Ba | 0.42 ± 0.36Bb |
| P3 | 1.45 ± 0.04Ca | 0.61 ± 0.27Ab | 0.64 ± 0.02Ca | 0.26 ± 0.03Ab | 0.44 ± 0.02Ca | 0.35 ± 0.28Ca |
| Horizon | 1 a–5 a–9 a | 2 a–6 a–10 a | 3 a–7 a–11 a | 4 a–8 a–12 a |
|---|---|---|---|---|
| A b | Water | CH:β-methyl-D-glucoside | CH:D-galactonic acid γ-lactone | AA:L-arginine |
| B b | CA:Pyruvatic acid methyl ester | CH:D-xylose | CA:D-galacturonic acid | AA:L-asparagine |
| C b | PM:Tween 40 | CH:I-erythritol | PA:2-hydroxybenzoic acid | AA:L-phenylalanine |
| D b | PM:Tween80 | CH:D-mannitol | PA:4-hydroxybenzoic acid | AA:L-serine |
| E b | PM:α-cyclodextrin | CH:N-acetyl-D-glucosamine | CA:R-hydroxybutyric acid | AA:L-threonine |
| F b | PM:Glycogen | CA:D-glucosaminic acid | CA:D-galacturonic acid | AA:Glycyl-L-glutamic acid |
| G b | CH:D-cellobiose | CH:Glucose-1-phosphate | CA:a-ketobutyric acid | AM:Phenyl ethylamine |
| H b | CH:α-D-lactose | CH:D,L-α-glycerol phosphate | CA:D-malic acid | AM:Putrescine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. https://doi.org/10.3390/su122310095
Qu Y, Tang J, Li Z, Zhou Z, Wang J, Wang S, Cao Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability. 2020; 12(23):10095. https://doi.org/10.3390/su122310095
Chicago/Turabian StyleQu, Yunke, Jie Tang, Zhaoyang Li, Zihao Zhou, Jingjing Wang, Sining Wang, and Yidan Cao. 2020. "Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China" Sustainability 12, no. 23: 10095. https://doi.org/10.3390/su122310095
APA StyleQu, Y., Tang, J., Li, Z., Zhou, Z., Wang, J., Wang, S., & Cao, Y. (2020). Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability, 12(23), 10095. https://doi.org/10.3390/su122310095





