Geochemical Classification of Global Mine Water Drainage
Abstract
:1. Introduction
2. Research Methodology
3. Results and Discussions
3.1. Geochemical Distribution of the Global AMD Dataset
3.2. Classification of the Global AMD Dataset Using the Hill (1968) Framework
3.3. Evaluation of Results and Framework Optimisation
4. Conclusions
- The addition of Class 0 to the framework for highly acidic and high concentration baring AMD. The research results found that 11% of the referenced mine water sites exceeded Class I specifications. Class 0 is proposed as an addition to aid policy makers identify these sites as uniquely contaminated mine waters and aid remediators to identify suitable remediation techniques.
- Revisions to Class I to enable the classification of all the geochemical variations of non-neutralised and unoxidised mine water sources. The research results showed that 38.7% of non-neutralised and unoxidised referenced mine waters did not meet the specification of the original Class I of the Hill (1968) framework. The proposed revisions comprised of changes to the lower limits of Fe2+, acidity and SO4 concentrations to enable the classification of all mine water sources.
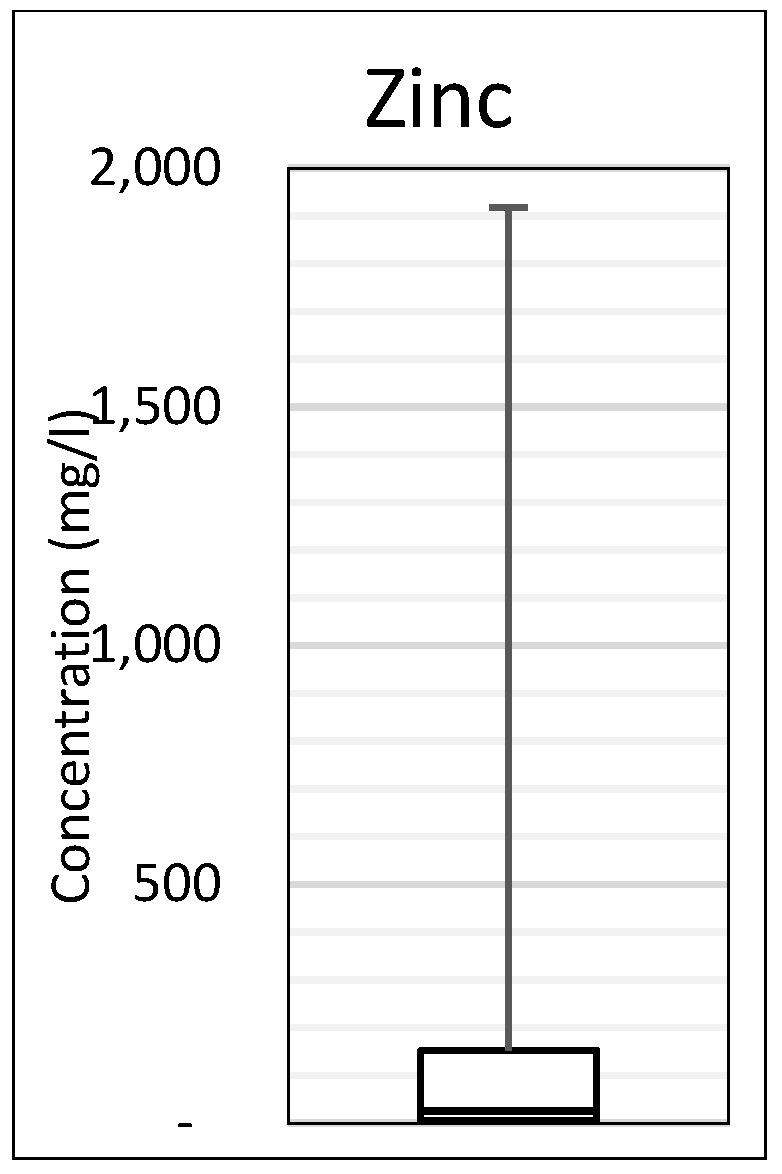
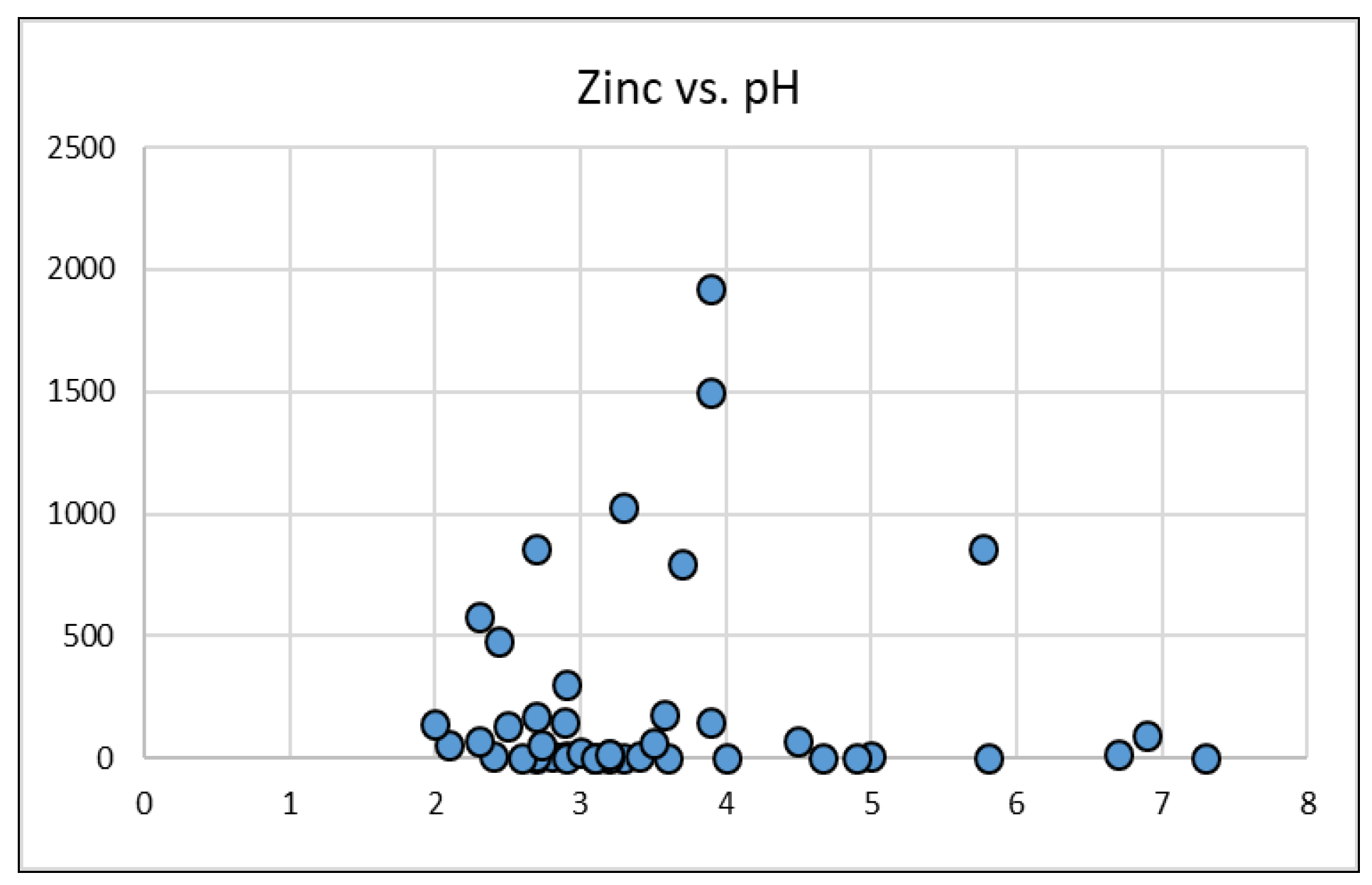
- The addition of an indicator species for cytotoxic cation AMD contaminants Zn, Ni, Pb, As and Cd. Zinc was selected as the indicator species for these contaminants with a categorisation of low, median and high proposed for classification. The proposed addition of Zn will enable regulators and mine water remediators to greater understand the environmental impacts of the AMD source and the mine water remediation requirements.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Braungardt, C.B.; Achterberg, E.P.; Elbaz-Poulichet, F.; Morley, N.H. Metal geochemistry in a mine-polluted estuarine system in Spain. Appl. Geochem. 2003, 18, 1757–1771. [Google Scholar] [CrossRef]
- Byrne, P.; Wood, P.J.; Reid, I. The Impairment of River Systems by Metal Mine Contamination: A Review Including Remediation Options. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2017–2077. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.; Nkambule, T.T.; Mamba, B. Synthesis and application of hematite nanoparticles for acid mine drainage treatment. J. Environ. Chem. Eng. 2018, 6, 1865–1874. [Google Scholar] [CrossRef]
- McCarthy, T.S. The impact of acid mine drainage in South Africa. South Afr. J. Sci. 2011, 107, 1–7. [Google Scholar] [CrossRef]
- Younger, P.L.; Banwart, S.A.; Hedin, R.S. Mine Water: Hydrology, Pollution, Remediation Dordrecht; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid Mine Drainage Remediation Options: A Review. Sci. Total. Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Gihring, T.M.; Banfield, J.F. Seasonal Variations in Microbial Populations and Environmental Conditions in an Extreme Acid Mine Drainage Environment. Appl. Environ. Microbiol. 1999, 65, 3627–3632. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento, A.M.; Nieto, J.M.; Olías, M.; Cánovas, C.R. Hydrochemical characteristics and seasonal influence on the pollution by acid mine drainage in the Odiel river Basin (SW Spain). Appl. Geochem. 2009, 24, 697–714. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Gray, N.F. Environmental Impact and Remediation of Acid Mine Drainage: A Management Problem. Environ. Earth Sci. 1997, 30, 62–71. [Google Scholar] [CrossRef]
- de Klerk, A.R.; Oberholster, P.J.; van Wyk, J.H.; Truter, J.C.; Schaefer, L.M.; Botha, A.M. The Effect of Rehabilitation Measures on Ecological Infrustructure in Response to Acid Mine Drainage from Coal Mining. Ecol. Eng. 2016, 95, 463–474. [Google Scholar] [CrossRef]
- Shabalala, A.N.; Ekolu, S.O.; Diop, S.; Solomon, F. Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage—Column study. J. Hazard. Mater. 2017, 323, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Rose, P. Long-term sustainability in the management of acid mine drainage wastewaters—Development of the Rhodes BioSURE Process. Water South Afr. 2013, 39, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Udayabhanu, G.; Prasad, B. Studies on environmental impact of acid mine drainage generation and its treatment: An appraisal. Indian J. Environ. Prot. 2010, 30, 953–967. [Google Scholar]
- Mafanya, L. Flow Model for the Treatment of Acid Mine Drainage Using Pervious Concrete. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2020. [Google Scholar]
- Kefeni, K.K.; Msagati, T.A.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef]
- Mafanya, L.; Kallon, D.V.V.; Simelane, S.P. Chemical Analysis of AMD Properties Based on Factorial Method. In Proceedings of the Open Innovations Conference, OI 2019, Cape Town, South Africa, 2–4 October 2019. [Google Scholar]
- Mafanya, L.; Kallon, D.V.V.; Simelane, S.P. Flow Properties Upon Treatment of Acid Mine Drainage Using Pervious Concrete Slabs. In Proceedings of the SAIIE NeXXXt, Port Elizabeth, South Africa, 30 September–2 October 2019; pp. 399–403. [Google Scholar]
- Soni, A.K.; Mishra, B.; Singh, S. Pit lakes as an end user of mining: A review. J. Min. Environ. 2014, 5, 99–111. [Google Scholar]
- Department of Mineral Resources. Progress on the Management and Rehabilitation of Derelict and Ownerless Mines: Presentation to the Parliamentary Select Committee on Finance; DMR: Pretoria, South Africa, 2010.
- Van Zyl, H.; Bond-Smith, M.; Minter, T.; Botha, M.; Leiman, A. Financial Provisions for Rehabilitation and Closure in South African Mining; Discussion document of challenges and recommended improvements; WWF–South Africa: Cape Town, South Africa, 2012. [Google Scholar]
- Dean, A.P.; Lynch, S.; Rowland, P.; Toft, B.D.; Pittman, J.K.; White, K.N. Natural Wetlands Are Efficient at Providing Long-Term Metal Remediation of Freshwater Systems Polluted by Acid Mine Drainage. Environ. Sci. Technol. 2013, 47, 12029–12036. [Google Scholar] [CrossRef]
- DeGraff, J.V. Addressing the toxic legacy of abandoned mines on public land in the western United States. Rev. Eng. Geol. 2007, 17, 1–8. [Google Scholar]
- Unger, C.; Lechner, A.M.; Glenn, V.; Edraki, M.; Mulligan, D.R. Mapping and prioritising rehabilitation of abandoned mines in Australia. In Proceedings of the Life-of-Mine Conference, Brisbane, Australia, 10–12 July 2012; pp. 259–265. [Google Scholar]
- Johnston, D.; Potter, H.; Jones, C.; Rolley, S.; Watson, I.; Pritchard, J. Abandoned Mines and the Water Environment. In Environment Agency Science Project SC030136-41; Environment Agency: Bristol, UK, 2008. [Google Scholar]
- Caraballo, M.A.; Macías, F.; Rötting, T.S.; Nieto, J.M.; Ayora, C. Long term remediation of highly polluted acid mine drainage: A sustainable approach to restore the environmental quality of the Odiel river basin. Environ. Pollut. 2011, 159, 3613–3619. [Google Scholar] [CrossRef]
- Clyde, E.J.; Champagne, P.; Jamieson, H.E.; Gorman, C.; Sourial, J. The use of a passive treatment system for the mitigation of acid mine drainage at the Williams Brothers Mine (California): Pilot-scale study. J. Clean. Prod. 2016, 130, 116–125. [Google Scholar] [CrossRef]
- Dinu, L.; Stefanescu, M.; Balaiu, M.; Cosma, I.; Criste, C.; Badescu, V. Acid mine water treatment using the high density sludge technology. J. Environ. Prot. Ecol. 2014, 15, 1700–1717. [Google Scholar]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Papirio, S.; Villa-Gomez, D.K.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Acid Mine Drainage Treatment in Fluidized-Bed Bioreactors by Sulfate-Reducing Bacteria: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2545–2580. [Google Scholar] [CrossRef]
- Skousen, J. Overview of Acid Mine Drainage Treatment with Chemicals. Acid Mine Drain. Rock Drain. Acid Sulfate Soils 2014, 26, 325–337. [Google Scholar] [CrossRef]
- Taylor, J.; Pape, S.; Murphy, N. A summary of passive and active treatment technologies for acid and metalliferous drainage (AMD). In Proceedings of the 5th Australian Workshop on Acid Mine Drainage, Frementle, Australia, 29–31 August 2005. [Google Scholar]
- Naidu, G.; Ryu, S.; Thiruvenkatachari, R.; Choi, Y.; Jeong, S.; Vigneswaran, S. A critical review on remediation, reuse, and resource recovery from acid mine drainage. Environ. Pollut. 2019, 247, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Opitz, J.; Timms, W. Mine water discharge quality—A review of classification frameworks. In Proceedings of the International Mine Water Association, IMWA, Leipzig, Germany, 11–15 July 2016; pp. 17–26. [Google Scholar]
- McCourt, J.L. Environmental legislation and water management issues during mine closure in South Africa. In Proceedings of the International Mine Water Association 1999, Sevilla, Spain, 29 September–4 October 2012. [Google Scholar]
- Stark, L. Breaking New Ground—Mining, Minerals, and Sustainable Development: The Report of the MMSD Project; Earthscan: London, UK; Sterling, TX, USA, 2002. [Google Scholar]
- Obreque-Contreras, J.; Pérez-Flores, D.; Gutiérrez, P.; Chávez-Crooker, P. Acid Mine Drainage in Chile: An Opportunity to Apply Bioremediation Technology. J. Waste Water Treat. Anal. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Thomashausen, S.; Maennling, N.; Mebratu-Tsegaye, T. A comparative overview of legal frameworks governing water use and waste water discharge in the mining sector. Resour. Policy 2018, 55, 143–151. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Jones, B.W.; Millar, G.J. Alternative neutralisation materials for acid mine drainage treatment. J. Water Process. Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, A.P.; Younger, P.L. Broadening the scope of mine water environmental impact assessment. Environ. Impact Assess. Rev. 2000, 20, 85–96. [Google Scholar] [CrossRef]
- INAP. Global Acid Mine Drainage Guide (GARD Guide). 2009. Available online: http://www.gardguide.com (accessed on 10 July 2020).
- AUSTRALIA MC. Water Accounting Framework for the Minerals Industry. 2014. Available online: https://minerals.org.au/sites/default/files/WAF_UserGuide_v1.3_(Jan_2014).pdf (accessed on 12 July 2020).
- Timms, W.; Holley, C. Mine site water-reporting practices, groundwater take and governance frameworks in the Hunter Valley coalfield, Australia. Water Int. 2016, 41, 351–370. [Google Scholar] [CrossRef]
- Gray, N.F. The Use of an Objective Index for the Assessment of the Contamination of Surface Water and Groundwater by Acid Mine Drainage. Water Environ. J. 1996, 10, 332–340. [Google Scholar] [CrossRef]
- Kuma, J.S.Y.; Younger, P.L.; Buah, W.K. Numerical Indices of the Severity of Acidic Mine Drainage: Broadening the Applicability of the Gray Acid Mine Drainage Index. Mine Water Environ. 2010, 30, 67–74. [Google Scholar] [CrossRef]
- Hill, R.D. Mine Drainage Treatment: State of the Art and Research Needs; U.S. Department of the Interior, Ed.; Mine Drainage Control Activities, Federal Water Pollution Control Administration: Cincinnati, OH, USA, 1968.
- Xiong, W.; Chen, X.; Zhu, C.; Zhang, J.; Lan, T.; Liu, L.; Mo, B.; Chen, X. Arabidopsis ribosomal proteins RPL23aA and RPL23aB are functionally equivalent. In Comprehensive AMD Index to Evaluate Environmental Impacts of Mining in Malaysian Metallic Ex-Mines; Research Square: Durham, NC, USA, 2020. [Google Scholar]
- Antunes, I.; Valente, T.; Gomes, P.; Costa, M.R.; Fonseca, R.; Moreno, F. Spatial Distribution of Acid Mine Drainage Indexes in Different Water Environments. In Proceedings of the International Mine Water Association, Perm, Russia, 15–19 July 2019; pp. 334–338. [Google Scholar]
- Johnson, D.B. Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut. 2003, 3, 47–66. [Google Scholar] [CrossRef]
- Ayres, D.M.; Davis, A.P.; Gietka, P.M. Removing Heavy Metals from Wastewater; Engineering Research Centre; University of Maryland: College Park, MD, USA, 1994; p. 90. [Google Scholar]
- Edraki, M.; Golding, S.; Baublys, K.; Lawrence, M. Hydrochemistry, mineralogy and sulfur isotope geochemistry of acid mine drainage at the Mt. Morgan mine environment, Queensland, Australia. Appl. Geochem. 2005, 20, 789–805. [Google Scholar] [CrossRef]
- Harris, D.L.; Lottermoser, B.G.; Duchesne, J. Ephemeral acid mine drainage at the Montalbion silver mine, north Queensland. Aust. J. Earth Sci. 2003, 50, 797–809. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Edraki, M.; Hardie, K.; Kadletz, O.; Hall, T. Identification of acid rock drainage sources through mesotextural classification at abandoned mines of Croydon, Australia: Implications for the rehabilitation of waste rock repositories. J. Geochem. Explor. 2014, 137, 11–28. [Google Scholar] [CrossRef]
- Galhardi, J.A.; Bonotto, D.M. Hydrogeochemical features of surface water and groundwater contaminated with acid mine drainage (AMD) in coal mining areas: A case study in southern Brazil. Environ. Sci. Pollut. Res. 2016, 23, 18911–18927. [Google Scholar] [CrossRef] [Green Version]
- De Andrade, R.P.; Figueiredo, B.R.; De Mello, J.W.V.; Dos Santos, J.; Zandonadi, L.U. Control of geochemical mobility of arsenic by liming in materials subjected to acid mine drainage. J. Soils Sediments 2008, 8, 123–129. [Google Scholar] [CrossRef]
- Fernandes, H.M.; Franklin, M.R. Assessment of acid rock drainage pollutants release in the uranium mining site of Poços de Caldas—Brazil. J. Environ. Radioact. 2001, 54, 5–25. [Google Scholar] [CrossRef]
- Lichtner, P.C.; Waber, N. Redox front geochemistry and weathering: Theory with application to the Osamu Utsumi uranium mine, Poços de Caldas, Brazil. J. Geochem. Explor. 1992, 45, 521–564. [Google Scholar] [CrossRef]
- Campaner, V.P.; Luiz-Silva, W. Physico-chemical Processes in Acid Mine Drainage in Coal Mining South Brazil [Processos Físico-químicos Em Drenagem Ácida De Mina Em Mineração De Carvão No Sul Do Brasil]. Química Nova. 2009, 32, 146–152. [Google Scholar] [CrossRef]
- Mackasey, W.O. Abandoned Mines in Canada. In A Review for Mining Watch Canada; WOM Geological Associations Inc.: Sudbury, ON, Canada, 2000. [Google Scholar]
- Genty, T.; Bussiere, B.; Paradie, M.; Neculita, C.M. Passive biochemical treatment of ferriferous mine drainage: Lorraine mine site, Northern Quebec, Canada. In Proceedings of the IMWA, Freiberg, Germany, 11–15 July 2016. [Google Scholar]
- Lyew, D.; Sheppard, J.D. Effects of physical parameters of a gravel bed on the activity of sulphate-reducing bacteria in the presence of acid mine drainage. J. Chem. Technol. Biotechnol. 1997, 70, 223–230. [Google Scholar] [CrossRef]
- Sracek, O.; Choquette, M.; Gélinas, P.; Lefebvre, R.; Nicholson, R. Geochemical characterization of acid mine drainage from a waste rock pile, Mine Doyon, Québec, Canada. J. Contam. Hydrol. 2004, 69, 45–71. [Google Scholar] [CrossRef]
- Wu, P.; Tang, C.; Liu, C.; Zhu, L.; Pei, T.; Feng, L. Geochemical distribution and removal of As, Fe, Mn and Al in surface water system affected by acid mine drainage coalfield in Southwestern China. Environ. Geol. 2009, 57, 1457–1467. [Google Scholar] [CrossRef]
- Zhao, F.; Cong, Z.; Sun, H.; Ren, D. The geochemistry of rare earth elements (REE) in acid mine drainage from the Sitai coal mine, Shanxi Province, North China. Int. J. Coal Geol. 2007, 70, 184–192. [Google Scholar] [CrossRef]
- Hao, C.; Wang, L.; Gao, Y.; Zhang, L.; Dong, H. Microbial diversity in acid mine drainage of Xiang Mountain sulfide mine, Anhui Province, China. Extremophiles 2010, 14, 465–474. [Google Scholar] [CrossRef]
- Chen, L.-X.; Hu, M.; Huang, L.-N.; Hua, Z.-S.; Kuang, J.-L.; Li, S.-J.; Shu, W.-S. Comparative metagenomic and metatranscriptomic analyses of microbial communities in acid mine drainage. ISME J. 2015, 9, 1579–1592. [Google Scholar] [CrossRef]
- Pino, L.; Vargas, C.; Schwarz, A.; Bórquez, R. Influence of operating conditions on the removal of metals and sulfate from copper acid mine drainage by nanofiltration. Chem. Eng. J. 2018, 345, 114–125. [Google Scholar] [CrossRef]
- Dold, B. Evolution of Acid Mine Drainage Formation in Sulphidic Mine Tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Dold, B.S.; Fontboté, L. A mineralogical and geochemical study of element mobility in sulfide mine tailings of Fe oxide Cu–Au deposits from the Punta del Cobre belt, northern Chile. Chem. Geol. 2002, 189, 135–163. [Google Scholar] [CrossRef]
- Abarca, M.; Guerra, P.; Arce, G.; Montecinos, M.; Escauriaza, C.; Coquery, M.; Pastén, P. Response of suspended sediment particle size distributions to changes in water chemistry at an Andean mountain stream confluence receiving arsenic rich acid drainage. Hydrol. Process. 2016, 31, 296–307. [Google Scholar] [CrossRef]
- Montecinos, M.; Coquery, M.; Alsina, M.A.; Bretier, M.; Gaillard, J.-F.; Dabrin, A.; Pastén, P. Partitioning of copper at the confluences of Andean rivers. Chemosphere 2020, 259, 127318. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.; Baumann, N.; Krawczyk-Bärsch, E.; Brockmann, S.; Zimmermann, U.; Jenk, U.; Weiß, S. Identification of the uranium speciation in an underground acid mine drainage environment. Geochim. Cosmochim. Acta 2011, 75, 2200–2212. [Google Scholar] [CrossRef]
- Geller, W.; Klapper, H.; Salomons, W. (Eds.) Acidic Mining Lakes: Acid Mine Drainage, Limnology and Reclamation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Haferburg, G.; Reinicke, M.; Merten, D.; Büchel, G.; Kothe, E. Microbes adapted to acid mine drainage as source for strains active in retention of aluminum or uranium. J. Geochem. Explor. 2007, 92, 196–204. [Google Scholar] [CrossRef]
- Grawunder, A.; Lonschinski, M.; Merten, D.; Büchel, G. istribution and bonding of residual contamination in glacial sediments at the former uranium mining leaching heap of Gessen/Thuringia, Germany. Geochemistry 2009, 69, 5–19. [Google Scholar] [CrossRef]
- Ulrich, K.-U.; Bethge, C.; Guderitz, I.; Heinrich, B.; Neumann, V.; Nitsche, C.; Benthaus, F.-C. In-Lake Neutralization: Quantification and Prognoses of the Acid Load into a Conditioned Pit Lake (Lake Bockwitz, Central Germany). Mine Water Environ. 2012, 31, 320–338. [Google Scholar] [CrossRef]
- Kuma, J.S.; Younger, P.L. Water quality trends in the Tarkwa gold-mining district, Ghana. Bull. Int. Assoc. Eng. Geol. 2004, 63, 119–132. [Google Scholar] [CrossRef]
- Kortatsi, B.K.; Tay, C.K.; Anornu, G.; Hayford, E.; Dartey, G.A. Hydrogeochemical evaluation of groundwater in the lower Offin basin, Ghana. Environ. Earth Sci. 2007, 53, 1651–1662. [Google Scholar] [CrossRef]
- Tay, C.K.; Hayford, E.K.; Hodgson, I.O.A. Application of multivariate statistical technique for hydrogeochemical assessment of groundwater within the Lower Pra Basin, Ghana. Appl. Water Sci. 2017, 7, 1131–1150. [Google Scholar] [CrossRef] [Green Version]
- Akabzaa, T.M.; Jamieson, H.E.; Jorgenson, N.; Nyame, K. The Combined Impact of Mine Drainage in the Ankobra River Basin, SW Ghana. Mine Water Environ. 2009, 28, 50–64. [Google Scholar] [CrossRef]
- Herrera, P.; Uchiyama, H.; Igarashi, T.; Asakura, K.; Ochi, Y.; Iyatomi, N.; Nagae, S. Treatment of acid mine drainage through a ferrite formation process in central Hokkaido, Japan: Evaluation of dissolved silica and aluminium interference in ferrite formation. Miner. Eng. 2007, 20, 1255–1260. [Google Scholar] [CrossRef]
- Fukushi, K.; Sasaki, M.; Sato, T.; Yanase, N.; Amano, H.; Ikeda, H. A natural attenuation of arsenic in drainage from an abandoned arsenic mine dump. Appl. Geochem. 2003, 18, 1267–1278. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tomiyama, S.; Metugi, H.; Ii, H.; Ueda, A. Flow and geochemical modeling of drainage from Tomitaka mine, Miyazaki, Japan. J. Environ. Sci. 2015, 36, 130–143. [Google Scholar] [CrossRef]
- Kano, K. Prevention of hazard in mining waste heap. In Proceedings of the Third Asia-Pacific Regional Workshop on Hazardous Waste Management in Mining Industry, APCHW, Beijing, China, 19–21 April 2000. [Google Scholar]
- Romero, F.M.; Núñez, L.; Gutiérrez, M.E.; Armienta, M.A.; Ceniceros-Gómez, A. Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco mining area, Mexico. Arch. Environ. Contam. Toxicol. 2011, 60, 191–203. [Google Scholar] [CrossRef]
- Jallath, J.E.S.; Romero, F.M.; Argüelles, R.I.; Macedo, A.C.; Arenas, J.G. Acid drainage neutralization and trace metals removal by a two-step system with carbonated rocks, Estado de Mexico, Mexico. Environ. Earth Sci. 2018, 77, 86. [Google Scholar] [CrossRef]
- Rivera-Uria, M.Y.; Romero, F.M.; Sedov, S.; Ramos, D.; Solleiro-Rebolledo, E.; Díaz-Ortega, J. Effects of the interaction between an acid solution and pedogenic carbonates: The case of the Buenavista del Cobre Mine, Mexico. Rev. Mex. Cienc. Geológicas 2019, 36, 308–320. [Google Scholar] [CrossRef]
- Esteller, M.; Domínguez-Mariani, E.; Garrido, S.E.; Avilés, M. Groundwater pollution by arsenic and other toxic elements in an abandoned silver mine, Mexico. Environ. Earth Sci. 2015, 74, 2893–2906. [Google Scholar] [CrossRef]
- Lghoul, M.; Maqsoud, A.; Hakkou, R.; Kchikach, A. Hydrogeochemical behavior around the abandoned Kettara mine site, Morocco. J. Geochem. Explor. 2014, 144, 456–467. [Google Scholar] [CrossRef]
- Nadeif, A.; Taha, Y.; Bouzahzah, H.; Hakkou, R.; Benzaazoua, M. Desulfurization of the Old Tailings at the Au-Ag-Cu Tiouit Mine (Anti-Atlas Morocco). Minerals 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Goumih, A.; El Adnani, M.; Hakkou, R.; Benzaazoua, M. Geochemical Behavior of Mine Tailings and Waste Rock at the Abandoned Cu–Mo–W Azegour Mine (Occidental High Atlas, Morocco). Mine Water Environ. 2013, 32, 121–132. [Google Scholar] [CrossRef]
- Iavazzo, P.; Ducci, D.; Adamo, P.; Trifuoggi, M.; Migliozzi, A.; Boni, M. Impact of Past Mining Activity on the Quality of Water and Soil in the High Moulouya Valley (Morocco). Water Air Soil Pollut. 2011, 223, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Davies, H.; Weber, P.; Lindsay, P.; Craw, D.; Pope, J. Characterisation of acid mine drainage in a high rainfall mountain environment, New Zealand. Sci. Total. Environ. 2011, 409, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.; Harding, J. Acid Mine Drainage Index (AMDI): A benthic invertebrate biotic index for assessing coal mining impacts in New Zealand streams. N. Z. J. Mar. Freshw. Res. 2012, 46, 335–352. [Google Scholar] [CrossRef] [Green Version]
- Pope, J.; Trumm, D. Controls on Zn Concentrations in Acidic and Neutral Mine Drainage from New Zealand’s Bituminous Coal and Epithermal Mineral Deposits. Mine Water Environ. 2015, 34, 455–463. [Google Scholar] [CrossRef]
- Giles, E.; Jenkins, I.; Williams, S.; Kirk, A.; Fellows, D.; Press, R. Tui Mine Remediation Detailed Design Report. Underground Mine, Access Road, Waste Rock Stack Remediation Works; URS Ltd.: Waikato, New Zealand, 2010. [Google Scholar]
- Morrell, W.J. An Assessment of the Revegetation Potential of Base-Metal Tailings from the Tui Mine, Te Aroha, New Zealand: A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Doctor of Philosophy in Soil Science at Massey University. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 1997. [Google Scholar]
- Rybnikova, L.; Rybnikov, P. Water quality of the abandoned sulfide mines of the Middle Urals (Russia). In Proceedings of the 13th International Mine Water Association Congress—Mine Water & Circular Economy, Lappeenranta, Finland, 25–30 June 2017. [Google Scholar]
- Myagkaya, I.; Lazareva, E.; Zaikovskii, V.; Zhmodik, S. Interaction of natural organic matter with acid mine drainage: Authigenic mineralization (case study of Ursk sulfide tailings, Kemerovo region, Russia). J. Geochem. Explor. 2020, 211, 106456. [Google Scholar] [CrossRef]
- Khayrulina, E.; Khmurchik, V.; Maksimovich, N. The Kizel Coal Basin (the Western Urals, Russia): Environmental problems and Solutions. In Mining Meets Water-Conflicts and Solutions. In Proceedings of the IMWA2016 Annual Conference, Leipzig, Germany, 11–15 July 2016; pp. 761–767. [Google Scholar]
- Myagkaya, I.; Lazareva, E.V.; Gustaytis, M.; Zhmodik, S. Gold and silver in a system of sulfide tailings. Part 1: Migration in water flow. J. Geochem. Explor. 2016, 160, 16–30. [Google Scholar] [CrossRef]
- Tutu, H.; McCarthy, T.; Cukrowska, E. The chemical characteristics of acid mine drainage with particular reference to sources, distribution and remediation: The Witwatersrand Basin, South Africa as a case study. Appl. Geochem. 2008, 23, 3666–3684. [Google Scholar] [CrossRef]
- Aurecon. Feasibility Study for a Long-Term Solution to Address the Acid Mine Drainage Associated with the East, Central and West Rand Underground Mining Basins: Treatment Technology Options, 1st ed.; Study Report No. 5.4 P RSA 000/00/16512/4; Council for Geoscience: Pretoria, South Africa, 2013. [Google Scholar]
- Expert Team of the Inter Ministerial Committee. Mine Water Management in the Witwatersrand Gold Fields with Special Emphasis on Acid Mine Drainage-Report to the Inter-Ministerial Committee on Acid Mine Drainage; Council of Geoscience: Pretoria, South Africa, 2010. [Google Scholar]
- Dold, B.S.; Wade, C.; Fontboté, L. Water management for acid mine drainage control at the polymetallic Zn–Pb–(Ag–Bi–Cu) deposit Cerro de Pasco, Peru. J. Geochem. Explor. 2009, 100, 133–141. [Google Scholar] [CrossRef]
- Kuyucak, N.; Chavez, J.; Castillo, J.R.; Ruiz, J. Technical Feasibility Studies and Uses of Treated Acid Mine Drainage at Kingsmill Tunnel, Peru. In Proceedings of the Sixth International Conference on Acid Rock Drainage, Cairns, Queensland, Australia, 12–18 July 2003. [Google Scholar]
- Sevink, J.; Verstraten, J.M.; Kooijman, A.M.; Loayza-Muro, R.A.; Hoitinga, L.; Palomino, E.J.; Jansen, B. Rare Moss-Built Microterraces in a High-Altitude, Acid Mine Drainage-Polluted Stream (Cordillera Negra, Peru). Water Air Soil Pollut. 2015, 226, 201. [Google Scholar] [CrossRef] [Green Version]
- Wade, C.; Dold, B.S.; Fontboté, L. Geochemistry and Mineralogy of the Quiulacocha Tailings Impoundment from the Polymetallic Zn-Pb-(Ag-Bi-Cu) Deposit Cerro de Pasco, Peru. J. Am. Soc. Min. Reclam. 2006, 2006, 2198–2206. [Google Scholar] [CrossRef]
- Peterson, H.E. Unsaturated hydrology, evaporation, and geochemistry of neutral and acid rock drainage in highly heterogeneous mine waste rock at the Antamina mine, Peru. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2014. [Google Scholar]
- Chon, H.-T.; Hwang, J.-H. Geochemical Characteristics of the Acid Mine Drainage in the Water System in the Vicinity of the Dogye Coal Mine in Korea. Environ. Geochem. Health 2000, 22, 155–172. [Google Scholar] [CrossRef]
- Kim, D.H. Study on mine reclamation regimes for redeveloping closed mines of Korea. Econ. Environ. Geol. 2009, 42, 619–626. [Google Scholar]
- Kim, J.J.; Kim, S.J.; Kim, Y.Y. Mineralogy of evaporation residues and geochemistry of acid mine drainage in the Donghae mine area. Econ. Environ. Geol. 2003, 36, 103–109. [Google Scholar]
- Park, C.Y.; Jeong, Y.J. Seasonal variation of heavy metal content in acid mine drainage from Kwangyang mine. J. Korean Soc. Min. Energy Resour. Eng. 1999, 36, 91–102. [Google Scholar]
- España, J.S. Acid mine drainage in the Iberian Pyrite Belt: An overview with special emphasis on generation mechanisms, aqueous composition and associated mineral phases. Macla Rev. Soc. Española Mineral. 2008, 10, 34–43. [Google Scholar]
- Olías, M.; Nieto, J.; Sarmiento, A.; Cerón, J.; Cánovas, C. Seasonal water quality variations in a river affected by acid mine drainage: The Odiel River (South West Spain). Sci. Total. Environ. 2004, 333, 267–281. [Google Scholar] [CrossRef]
- Nieto, J.M.; Sarmiento, A.M.; Olías, M.; Canovas, C.R.; Riba, I.; Kalman, J.; DelValls, T.A. Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environ. Int. 2007, 33, 445–455. [Google Scholar] [CrossRef]
- Romero, A.; González, I.; Galán, E. Stream water geochemistry from mine wastes in Peña de Hierro, Riotinto area, SW Spain: A case of extreme acid mine drainage. Environ. Earth Sci. 2011, 62, 645–656. [Google Scholar] [CrossRef]
- Burnside, N.M.; Banks, D.; Boyce, A.J. Sustainability of thermal energy production at the flooded mine workings of the former Caphouse Colliery, Yorkshire, United Kingdom. Int. J. Coal Geol. 2016, 164, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Banks, D.; Athresh, A.; Al-Habaibeh, A.; Burnside, N. Water from abondoned mines as a heat source: Practical experiences of open- and closed-loop strategies, United Kingdom. Sustain. Water Resour. Manag. 2019, 50, 5–29. [Google Scholar]
- Kay, C.M.; Rowe, O.F.; Rocchetti, L.; Coupland, K.; Hallberg, K.B.; Johnson, D.B. Evolution of microbial “streamer” growths in an acidic, metal-contaminated stream draining an abandoned underground copper mine. Life 2013, 3, 189–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rose, L.M. Physical and Chemical Controls on Natural and Anthropogenic Remediation of Two Streams Impacted by Acid Mine Drainage in the Raccoon Creek Watershed, Ohio. Ph.D. Thesis, Ohio University, Athens, OH, USA, 2011. [Google Scholar]
- Hammarstrom, J.M.; Sibrell, P.L.; Belkin, H.E. Characterization of limestone reacted with acid-mine drainage in a pulsed limestone bed treatment system at the Friendship Hill National Historical Site, Pennsylvania, USA. Appl. Geochem. 2003, 18, 1705–1721. [Google Scholar] [CrossRef]
- Zwain, H.M.; Vakili, M.; Dahlan, I. Waste Material Adsorbents for Zinc Removal from Wastewater: A Comprehensive Review. Int. J. Chem. Eng. 2014, 2014, 1–13. [Google Scholar] [CrossRef]


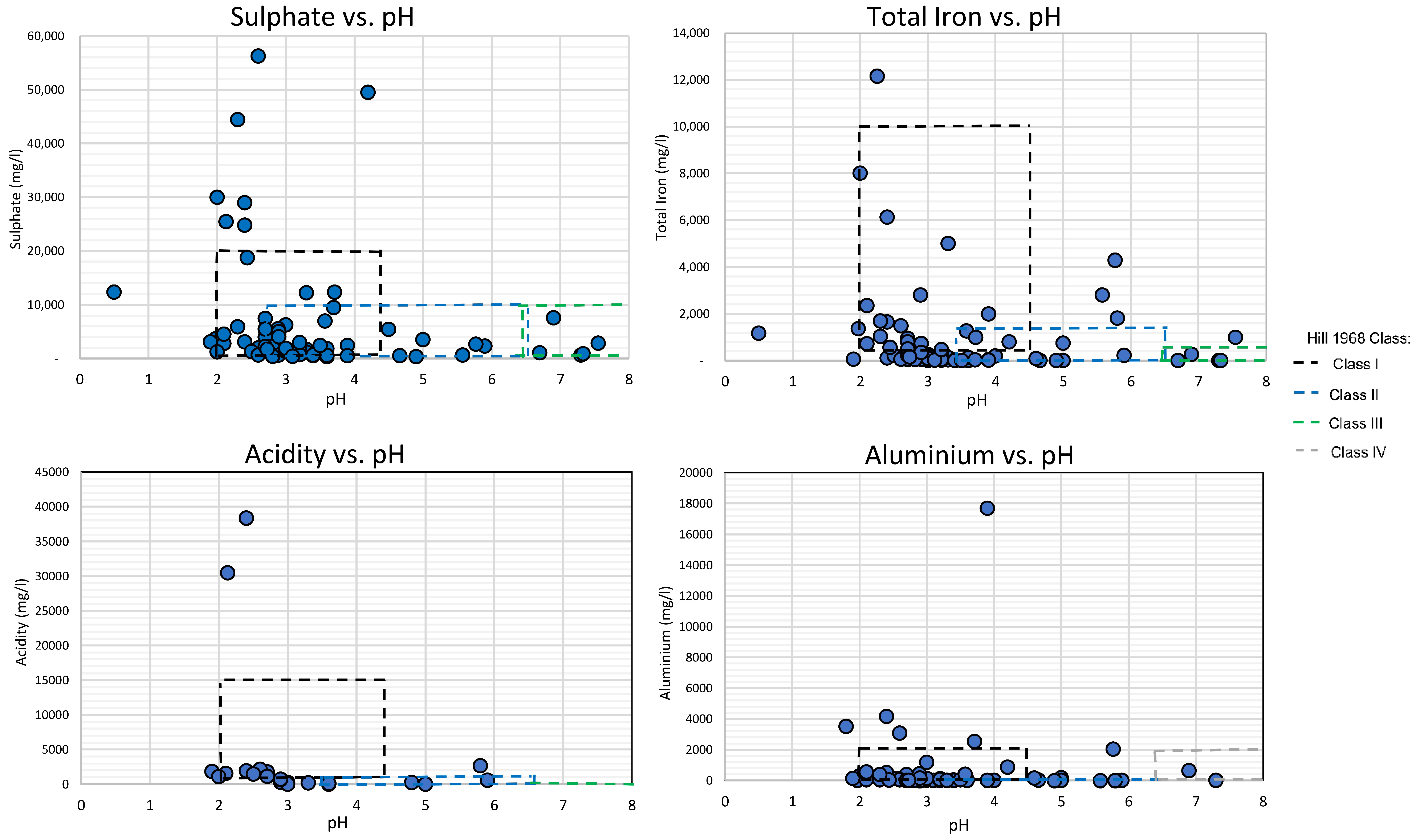
| Class | Class Description | Thresholds | ||
|---|---|---|---|---|
| Class I | Acid mine drainage | pH = 2.0–4.5 Acidity = 1–15 g/L | Fe2+ = 500–10,000 mg/L Fe3+ = 0 mg/L | SO4 = 1–20 g/L Al = 0–2000 mg/L |
| Class II | Partially oxidised and/or neutralised | pH = 3.5–6.6 Acidity = 0–1 g/L | Fe2+ = 0–500 mg/L Fe3+ = 0–1.000 mg/L | SO4 = 500–10,000 mg/L Al = 0–20 mg/L |
| Class III | Neutral and not oxidised | pH = 6.5–8.5 Acidity = 0 mg/L | Fe2+ = 0–500 mg/L Fe3+ = 0 mg/L | SO4 = 500–10,000 mg/L Al = 0–2000 mg/L |
| Class IV | Oxidised and neutralised/alkaline | pH = 6.5–8.5 Acidity = 0 mg/L | Fe2+ = 0 mg/L Fe3+ = 0 mg/L | SO4 = 500–10,000 mg/L Al = 0 mg/L |
| No | Country | Minerals | Sites | References |
|---|---|---|---|---|
| 1 | Australia | Au | Mount Ida Goldfield | [18,53,54,55] |
| Sn | Jumna mine | |||
| Ag | Montalbion mine | |||
| Au, Cu | Mount Morgan mine, Arnold’s Gully | |||
| 2 | Brazil | Coal | Coal mining area southern Brazil, Pedras stream | [56,57,58,59,60] |
| Au | Iron Quadrangle, Velhas river basin | |||
| U | Osamu Utsumi uranium mine, Pocos de Caldas | |||
| Coal | Coal mine in Figueira municipality, State of Paraná | |||
| 3 | Canada | Zn, Cu, Pb, Ag | Mattabi Mine | [61,62,63,64,65,66] |
| Fe | Lorraine mine site | |||
| Zn, Au, Ag | Les Mines Gallen | |||
| Au | Doyon mine, Québec | |||
| 4 | China | Coal | Xingren mine | [65,66,67,68] |
| Rare earth metals | Sitai mine | |||
| Cu | Tongling mine | |||
| Pyrite | Xiang Mountain sulphide mine | |||
| 5 | Chile | Cu | Active copper mine | [69,70,71,72,73] |
| Cu | Chuquicamata porphyry copper mine | |||
| Cu, Au | Punta del Cobre belt | |||
| Cu | Andean mountain mines—Azufre River | |||
| 6 | Germany | U | Konigstein mine | [74,75,76,77,78] |
| Coal | Lusatian Lignite District | |||
| U | Gessenhalde near Ronneburg, Thuringia, | |||
| Lignite | Mine pit, Lake Bockwitz, south of Leipzig | |||
| 7 | Ghana | Ag | Tarkwa gold-mining district | [79,80,81,82] |
| Ag | Lower Offin basin | |||
| Ag | Lower Pra Basin | |||
| Ag | Iduapriem Gold Mine | |||
| 8 | Japan | Au | Tomitaka | [83,84,85,86] |
| Coal | Hokutan Horonai coal mine | |||
| As | Honshu | |||
| As | Nishinomaki | |||
| 9 | Mexico | Ag, Zn, Pb | Taxco Mining Area | [87,88,89,90] |
| Zn, Pb, Cu, Ag, Au | Estado de Mexico | |||
| Cu | Buenavista del Cobre Mine | |||
| Ag | Huautla mine | |||
| 10 | Morocco | Au, Ag, Cu | Tiouit mine | [91,92,93,94] |
| Cu, Mo, W | Azegour mine | |||
| Pb | Zeïda mine | |||
| Pyrrhotite ore | Kettara mine site | |||
| 11 | New Zealand | Coal | Mangatini stream | [95,96,97,98,99] |
| Coal | Stockton coal mine—Mangatini stream catchment | |||
| Coal | Stockton Denniston Plateau | |||
| Cu, Pb, Zn | Tui Mine | |||
| 12 | Russia | Coal | Levikha mine | [100,101,102,103] |
| Coal | Berikul tailing | |||
| Cu, Zn | Ursk tailings, Kemerovo region | |||
| Coal | The Kizel Coal Basin | |||
| 13 | South Africa | Au | Western basin | [104,105,106] |
| Au | Witwatersrand basin | |||
| Au | Central Basin | |||
| Coal | Witbank | |||
| 14 | Peru | Zn, Pb, Ag, Bi, Cu | Polymetallic Cerro de Pasco deposit | [107,108,109,110,111] |
| Ag, Cu, Pb, Zn | Kingsmill Tunnel, Central Andes | |||
| Ag, Au, Cu | Rio Santiago Stream, Cordillera Negra | |||
| Cu, Zn | Antamina mine | |||
| 15 | South Korea | Cu | Ilgwang | [112,113,114,115] |
| Coal | Donghae mine area | |||
| Coal | Dogye coal mine | |||
| Au, Ag | Kwangyang | |||
| 16 | Spain | Ag, Au, Cu, Fe, Pb, Tn | Iberian Pyrite Belt (from 25 mines) | [116,117,118,119] |
| Ag, Au, Cu, Fe, Pb, Tn | Odiel River basin | |||
| Ag, Au, Cu, Fe, Pb, Tn | Tinto river | |||
| Cu, Fe, Zn | Peña de Hierro, Riotinto area | |||
| 17 | United Kingdom | Cu | Parys Mountain copper mine | [24,51,120,121,122] |
| Coal | Yorkshire colliery | |||
| Coal | Derbyshire colliery | |||
| Sn, Cu | Wheal Jane | |||
| 18 | United States | Coal | South Carolina | [25,123,124,125] |
| Coal | Solomon Creek, Pennsylvania | |||
| Cu | Racoon Creek, Ohio | |||
| Cu | Friendship Hill |
| Distribution | pH | Acidity | Aluminium | Sulphate | Total Iron |
|---|---|---|---|---|---|
| 25th percentile (Q1) | 0–2.6 | 0–215 mg/L | 0–11 mg/L | 0–1217 mg/L | 0–40 mg/L |
| 50th percentile (Q2) | 2.6–3.1 | 215–712 mg/L | 11–56 mg/L | 1217–2444 mg/L | 40–209 mg/L |
| 75th percentile (Q3) | 3.1–4.0 | 712–1788 mg/L | 56–343 mg/L | 2444–6081 mg/L | 209–988 mg/L |
| Distribution | Zinc |
|---|---|
| 25th percentile (Q1) | 0–5 mg/L |
| 50th percentile (Q2) | 5–25 mg/L |
| 75th percentile (Q3) | 25–152 mg/L |
| Classification | AMD Geochemistry Distribution | |||
|---|---|---|---|---|
| Acidity vs. pH | Total Fe vs. pH | Aluminium vs. pH | Sulphate vs. pH | |
| Class I | 53.3% | 46% | 76.2% | 69.5% |
| Class II | 20% | 12% | 11.1% | 11.1% |
| Class III | 10% | 4% | 2.7% | 4.2% |
| Class IV | - | - | 1.6% | - |
| Outliers | 16.7% | 38% | 22.2% | 15.2% |
| Class | Class Description | Thresholds | ||
|---|---|---|---|---|
| Class 0 ** | Highly concentrated and acidic mine drainage ** | pH = 0.5–3 ** Acidity = 5–45 g/L ** | Total Fe = 1000–12,000 mg/L ** | SO4 = 10–60 g/L ** Al = 1000–18,000 mg/L ** |
| Class I | Acid mine drainage | pH = 2.0–4.5 Acidity = 0–15 g/L ** | Fe2+ = 0–10,000 mg/L ** Fe3+ = 0 mg/L | SO4 = 0–20 g/L ** Al = 0–2000 mg/L |
| Class II | Partially oxidised and/or neutralised | pH = 3.5–6.6 Acidity = 0–1 g/L | Fe2+ = 0–500 mg/L Fe3+ = 0–1.000 mg/L | SO4 = 500–10,000 mg/L Al = 0–20 mg/L |
| Class III | Neutral and not oxidised | pH = 6.5–8.5 Acidity = 0 mg/L | Fe2+ = 0–500 mg/L Fe3+ = 0 mg/L | SO4 = 500–10,000 mg/L Al = 0–2000 mg/L |
| Class IV | Oxidised and neutralised/alkaline | pH = 6.5–8.5 Acidity = 0 mg/L | Fe2+ = 0 mg/L Fe3+ = 0 mg/L | SO4 = 500–10,000 mg/L Al = 0 mg/L |
| Category ** | L = Zinc ≤ 1 mg/L** | M = Zinc ≤ 25 mg/L ** | H = Zinc > 25 mg/L ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thisani, S.K.; Kallon, D.V.V.; Byrne, P. Geochemical Classification of Global Mine Water Drainage. Sustainability 2020, 12, 10244. https://doi.org/10.3390/su122410244
Thisani SK, Kallon DVV, Byrne P. Geochemical Classification of Global Mine Water Drainage. Sustainability. 2020; 12(24):10244. https://doi.org/10.3390/su122410244
Chicago/Turabian StyleThisani, Sandisiwe Khanyisa, Daramy Vondi Von Kallon, and Patrick Byrne. 2020. "Geochemical Classification of Global Mine Water Drainage" Sustainability 12, no. 24: 10244. https://doi.org/10.3390/su122410244
APA StyleThisani, S. K., Kallon, D. V. V., & Byrne, P. (2020). Geochemical Classification of Global Mine Water Drainage. Sustainability, 12(24), 10244. https://doi.org/10.3390/su122410244






