Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees
Abstract
1. Introduction
2. Materials and Methods
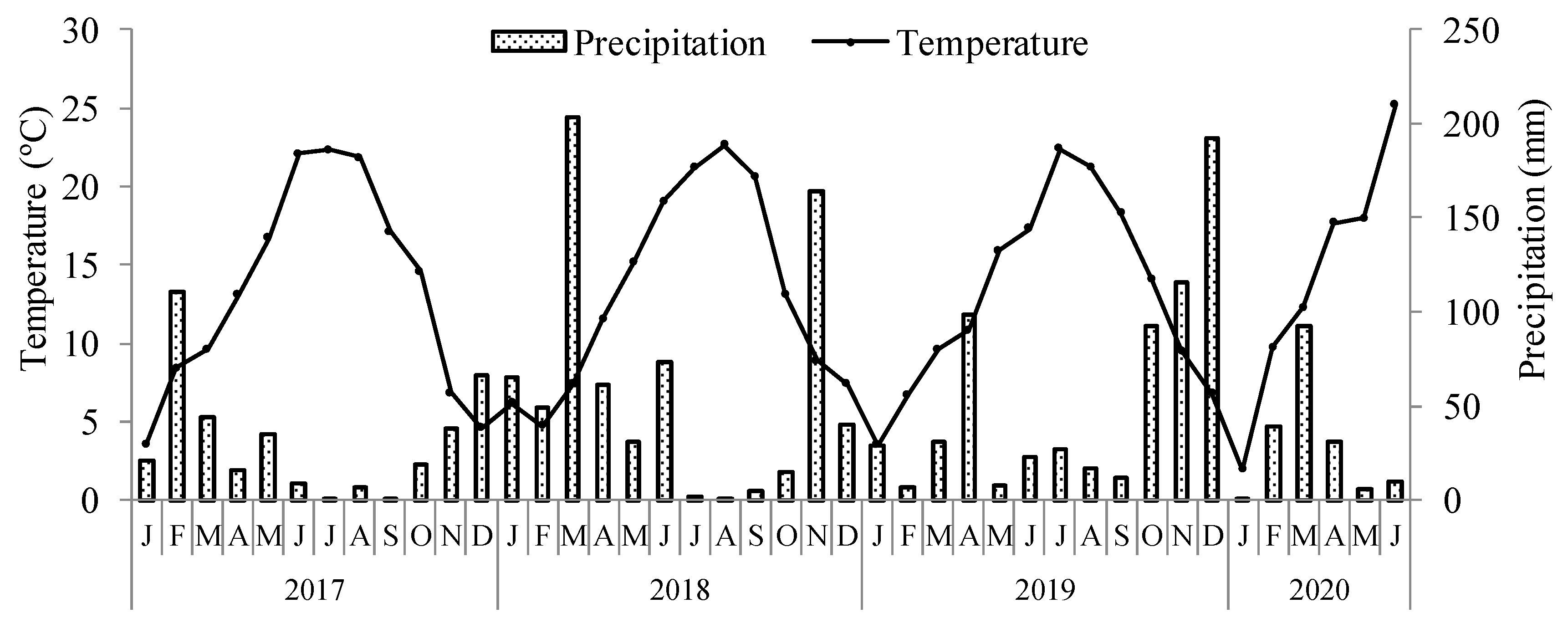
2.1. Study Site
2.2. Experimental Design and Management
2.3. Field Determinations
2.4. Laboratory Analysis
2.5. Data Analysis
3. Results
3.1. Plant Growth
3.2. Nutrient Concentration in Plant Tissues
3.3. Leaf Gas Exchange
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodrigues, M.A.; Ladeira, L.C.; Arrobas, M. Azotobacter-enriched organic manures to increase nitrogen fixation and crop productivity. Eur. J. Agron. 2018, 93, 88–94. [Google Scholar] [CrossRef]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs): An introduction. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 3–29. [Google Scholar]
- Guaya, D.; Mendoza, A.; Valderrama, C.; Farran, A.; Sauras-Yera, T.; Cortina, J.L. Use of nutrient-enriched zeolite (NEZ) from urban wastewaters in amended soils: Evaluation of plant availability of mineral elements. Sci. Total. Environ. 2020, 727, 138646. [Google Scholar] [CrossRef]
- Bernardi, A.C.D.C.; Bezerram, M.; Monte, D.M.; Renato, P.; Paiva, P.; Werneck, C.G. Dry matter production and nutrient accumulation after successive crops of lettuce, tomato, rice, and andropogon grass in a substrate with zeolite. Rev. Bras. Ciência 2010, 34, 435–442. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef]
- Litaor, M.I.; Katz, L.; Shenker, M. The influence of compost and zeolite co-addition on the nutrients status and plant growth in intensively cultivated Mediterranean soils. Soil Use Manag. 2017, 33, 72–80. [Google Scholar] [CrossRef]
- Villarreal-Núñez, J.E.; Barahona-Amores, L.A.; Castillo-Ortiz, O.A. Efecto de zeolita sobre la eficiencia de fertilizantes nitrogenados en el cultivo de arroz. Agron. Mesoam. 2015, 26, 315. [Google Scholar] [CrossRef]
- Palanivell, P.; Ahmed, O.H.; Majid, N.M. Minimizing ammonia volatilization from urea, improving lowland rice (cv. MR219) seed germination, plant growth variables, nutrient uptake, and nutrient recovery using clinoptilolite zeolite. Arch. Agron. Soil Sci. 2016, 62, 708–724. [Google Scholar] [CrossRef]
- Ryakhovskaya, N.I.; Gainatulina, V.V. Potato and oat yield in short-cycle crop rotation with zeolite application. Russ. Agric. Sci. 2009, 35, 153–155. [Google Scholar] [CrossRef]
- Assimakopoulou, A.; Dimitroulia, D.; Kosmidis, S.; Doula, M.K. Growth, yield and nutrient status of pepper plants grown on a soil substrate with olive mill waste sludge and natural zeolite addition. J. Plant Nutr. 2020, 43, 629–640. [Google Scholar] [CrossRef]
- Cooper, J.E.; Scherer, H.W. Nitrogen fixation. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 389–408. [Google Scholar]
- López-Ráez, J.A.; Pozo, M.J. Chemical signalling in the arbuscular mycorrhizal symbiosis: Biotechnological applications. In Symbiotic Endophytes; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 215–232. [Google Scholar]
- Lanfranco, L.; Bonfante, P.; Genre, A. The mutualistic interaction between plants and arbuscular mycorrhizal fungi. Microbiol. Spectr. 2016, 4, 1–20. [Google Scholar] [CrossRef]
- Miransari, M. Arbuscular mycorrhizal fungi and uptake of nutrients. In Symbiotic Endophytes; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 253–270. [Google Scholar]
- Hu, J.; Lin, X.; Wang, J.; Dai, J.; Cui, X.; Chen, R.; Zhang, J. Arbuscular mycorrhizal fungus enhances crop yield and P-uptake of maize (Zea mays L.): A field case study on a sandy loam soil as affected by long-term P-deficiency fertilization. Soil Biol. Biochem. 2009, 41, 2460–2465. [Google Scholar] [CrossRef]
- Mechri, B.; Attia, F.; Tekaya, M.; Cheheb, H.; Hammami, M. Colonization of olive trees (Olea europaea L.) with the arbuscular mycorrhizal fungus Glomus sp. modified the glycolipids biosynthesis and resulted in accumulation of unsaturated fatty acids. J. Plant Physiol. 2014, 171, 1217–1220. [Google Scholar] [CrossRef]
- Ortas, I.; Bykova, A. The Effect of Mycorrhiza Inoculation and Phosphorus Application on Phosphorus Efficiency of Wheat Plants. Commun. Soil Sci. Plant Anal. 2018, 49, 1199–1207. [Google Scholar] [CrossRef]
- Koller, R.; Rodriguez, A.; Robin, C.; Scheu, S.; Bonkowski, M. Protozoa enhance foraging efficiency of arbuscular mycorrhizal fungi for mineral nitrogen from organic matter in soil to the benefit of host plants. New Phytol. 2013, 199, 203–211. [Google Scholar] [CrossRef]
- Garcia, K.; Zimmermann, S.D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 2014, 5, 337. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Mbarki, N.; Cheheb, H.; Hammami, M.; Attia, F. Arbuscular mycorrhizal fungus Rhizophagus irregularis influences key physiological parameters of olive trees (Olea europaea L.) and mineral nutrient profile. Photosynthetica 2017, 55, 308–316. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R. Arbuscular mycorrhizal fungi and the tolerance of plants to drought and salinity. In Symbiotic Endophytes; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 271–288. [Google Scholar]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Bati, C.B.; Santilli, E.; Lombardo, L. Effect of arbuscular mycorrhizal fungi on growth and on micronutrient and macronutrient uptake and allocation in olive plantlets growing under high total Mn levels. Mycorrhiza 2015, 25, 97–108. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Yue, X.; He, Y.; Xia, Y.-S.; Wang, Y. Arbuscular mycorrhizal fungi enhance antioxidant defence in the leaves and the retention of heavy metals in the roots of maize. Environ. Sci. Pollut. Res. 2018, 25, 24338–24347. [Google Scholar] [CrossRef]
- Berdeni, D.; Cotton, T.E.A.; Daniell, T.J.; Bidartondo, M.I.; Cameron, D.D.; Evans, K.L. The Effects of Arbuscular Mycorrhizal Fungal Colonisation on Nutrient Status, Growth, Productivity, and Canker Resistance of Apple (Malus pumila). Front. Microbiol. 2018, 9, 1461. [Google Scholar] [CrossRef]
- Godbold, D.L.; Hoosbeek, M.R.; Lukac, M.; Cotrufo, M.F.; Janssens, I.A.; Ceulemans, R.; Polle, A.; Velthorst, E.J.; Scarascia-Mugnozza, G.; De Angelis, P.; et al. Mycorrhizal Hyphal Turnover as a Dominant Process for Carbon Input into Soil Organic Matter. Plant Soil 2006, 281, 15–24. [Google Scholar] [CrossRef]
- Högberg, M.N.; Skyllberg, U.; Högberg, P.; Knicker, H. Does ectomycorrhiza have a universal key role in the formation of soil organic matter in boreal forests? Soil Biol. Biochem. 2020, 140, 107635. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Piroli, L.B.; Forcelini, D.; Raimundo, S.; Domingues, L.S.; Cassol, L.C.; Correia, C.M.; Arrobas, M. Use of commercial mycorrhizal fungi in stress-free growing conditions of potted olive cuttings. Sci. Hortic. 2021, 275, 109712. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Ferreira, H.F.; Correia, C.M. Changes in growth, gas exchange, xylem hydrualic properties and water use efficiency of three olive cultivars under contrasting water availability regimes. Environ. Exp. Bot. 2007, 60, 183–192. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Luzio, A.; Silva, E.; Gonçalves, A.; Meijón, M.; Escandón, M.; Arrobas, M.; Rodrigues, M.A.; Moutinho-Pereira, J.; et al. Kaolin and salicylic acid alleviate summer stress in rainfed olive orchards by modulation of distinct physiological and biochemical response. Sci. Hortic. 2019, 246, 201–211. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Silva, E.; Gonçalves, A.; Matos, C.; Rodrigues, M.A.; Moutinho-Pereira, J.; Barros, A.; Correia, C. Kaolin and salicylic acid foliar application modulate yield, quality and phytochemical composition of olive pulp and oil from rainfed trees. Sci. Hortic. 2018, 237, 176–183. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Claro, A.M.; Rodrigues, M.A. Soil management in rainfed olive orchards may result in conflicting effects on olive production and soil fertility. Span. J. Agric. Res. 2013, 11, 472. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Dimande, P.; Pereira, E.; Ferreira, I.Q.; Freitas, S.; Correia, C.M.; Moutinho-Pereira, J.; Arrobas, M. Early-maturing annual legumes: An option for cover cropping in rainfed olive orchards. Nutr. Cycl. Agroecosyst. 2015, 103, 153–166. [Google Scholar] [CrossRef]
- Arrobas, M.; Santos, D.; Ribeiro, A.; Pereira, E.; Rodrigues, M.A. Soil and foliar nitrogen and boron fertilization of almond trees grown under rainfed conditions. Eur. J. Agron. 2019, 106, 39–48. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.A.; Arrobas, M. Soil and foliar applied boron in olive: Tree crop growth and yield, and boron remobilization within plant tissues. Span. J. Agric. Res. 2019, 17, e0901. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Technical Paper 9; ISRIC, FAO: Wageningen, The Netherlands, 2002. [Google Scholar]
- Balbino, L.R. La Méthode Egner-Riehm et la Détermination du Phosfore et du Potassium «assimilável» des sols du Portugal. II Col. Medit Cont. Fert. Plantas Cultivadas; Facultad de Ciencias: Sevilla, Spain, 1968; pp. 55–65. [Google Scholar]
- Jones, J.B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Temminghoff, E.E.J.M.; Houba, V.G. Plant Analysis Orocedures, 2nd ed.; Klwuwer Academic Publishers: AA Dordrecht, The Netherlands, 2004. [Google Scholar]
- Rapoport, H.L. Botânica e Morfología. In El Cultivo del Olivo, 7th ed.; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2017; pp. 35–64. [Google Scholar]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of Arbuscular Mycorrhizal Fungi on Photosynthesis, Water Status, and Gas Exchange of Plants Under Salt Stress—A Meta-Analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Bryson, G.; Mills, H.; Sasseville, D.; Jones, J.B., Jr.; Barker, A. Plant Analysis Handbook III: A Guide to Sampling, Preparation, Analysis and Interpretation for Agronomic and Horticultural Crops; Micro-Macro Publishing: Athens, GA, USA, 2014. [Google Scholar]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.M.; Rodrigues, M.A. The effect of nitrogen applications on the growth of young olive trees and nitrogen use efficiency. Turk. J. Agric. For. 2020, 44, 278–289. [Google Scholar] [CrossRef]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, an Introduction to Nutrient Management, 8th ed.; Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Jarrell, W.M.; Beverly, R.B. The Dilution Effect in Plant Nutrition Studies. Adv. Agron. 1981, 34, 197–224. [Google Scholar] [CrossRef]
- Arrobas, M.; Afonso, S.; Rodrigues, M.A. Diagnosing the nutritional condition of chestnut groves by soil and leaf analyses. Sci. Hortic. 2018, 228, 113–121. [Google Scholar] [CrossRef]
- Ågren, G.I.A.; Hyvönen, R.; Baskaran, P. Ectomycorrhiza, Friend or Foe? Ecosystems 2019, 22, 1561–1572. [Google Scholar] [CrossRef]
- Marschner, P.; Solaiman, Z.; Rengel, Z. Growth, phosphorus uptake, and rhizosphere microbial-community composition of a phosphorus-efficient wheat cultivar in soils differing in pH. J. Plant Nutr. Soil Sci. 2005, 168, 343–351. [Google Scholar] [CrossRef]
- Chaney, K. Phosphate Fertilizers (Cap. 6). In Ullmann’s Agrochemicals 1; Wiley-VCH, Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Rodrigues, M.A.; Pavão, F.; Lopes, J.I.; Gomes, V.; Arrobas, M.; Moutinho-Pereira, J.; Ruivo, S.; Cabanas, J.E.; Correia, C.M. Olive Yields and Tree Nutritional Status during a Four-Year Period without Nitrogen and Boron Fertilization. Commun. Soil Sci. Plant Anal. 2011, 42, 803–814. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.; Rodrigues, M.A. Olive response to potassium applications under different water regimes and cultivars. Nutr. Cycl. Agroecosyst. 2018, 112, 387–401. [Google Scholar] [CrossRef]
- Dag, A.; Yermiyahu, U.; Ben-Gal, A.; Zipori, I.; Kapulnik, Y. Nursery and post-transplant field response of olive trees to arbuscular mycorrhizal fungi in an arid region. Crop. Pasture Sci. 2009, 60, 427–433. [Google Scholar] [CrossRef]
- Arrobas, M.; Moutinho-Pereira, J. Fertilização do olival. In Manual da Safra e Contra Safra do Olival; Rodrigues, M.A., Correia, C., Eds.; Instituto Politécnico de Bragança: Bragança, Portugal, 2009; pp. 21–39. ISBN 978-972-745-103-6. [Google Scholar]
- Liu, A.; Hamel, C.; Elmi, A.; Costa, C.; Ma, B.; Smith, D.L. Concentrations of K, Ca and Mg in maize colonized by arbuscular mycorrhizal fungi under field conditions. Can. J. Soil Sci. 2002, 82, 271–278. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wan, S.; Li, X. The Significance of Calcium in Photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef]
- Valentine, A.J.; Mortimer, P.E.; Kleinert, A.; Kang, Y.; Benedito, V.A. Carbon metabolism and costs of arbuscular mycorrhizal associations to host roots. In Symbiotic Endophytes; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 233–252. [Google Scholar]






| Soil Properties | Soil Properties (cont.) | ||
|---|---|---|---|
| 1 Organic carbon (g kg−1) | 4.5 ± 0.9 | 5 Extract. Zn (mg kg−1) | 0.5 ± 0.12 |
| 2 pH (H2O) | 4.5 ± 0.12 | 5 Extract. Cu (mg kg−1) | 0.9 ± 0.47 |
| 2 pH (KCl) | 3.6 ± 0.06 | 6 Exchang. Ca (cmolc kg−1) | 4.8 ± 0.96 |
| 3 Extract. P (mg P2O5 kg−1) | 32.0 ± 4.3 | 6 Exchang. Mg (cmolc kg−1) | 1.1 ± 0.28 |
| 3 Extract. K (mg K2O kg−1) | 81.5 ± 15.2 | 6 Exchang. K (cmolc kg−1) | 0.3 ± 0.04 |
| 4 Extract. B (mg kg−1) | 0.5 ± 0.06 | 6 Exchang. Na (cmolc kg−1) | 0.2 ± 0.01 |
| 5 Extract. Fe (mg kg−1) | 36.2 ± 3.28 | 7 Exchang. acidity (cmolc kg−1) | 0.1 ± 0.08 |
| 5 Extract. Mn (mg kg−1) | 76.1 ± 15.50 | CEC (cmolc kg−1) | 6.4 ± 1.24 |
| General Formula: (Ca, K2, Na2, Mg)4Al8Si40O96.24H2O | |||
|---|---|---|---|
| Mineral composition (%) | Chemical composition (%) | ||
| Clinoptilolite | 84 | SiO2 | 65.0–71.3 |
| Cristobalite | 8 | Al2O3 | 11.5–13.1 |
| Clay mica | 4 | CaO | 2.7–5.2 |
| Plagioclase | 3–4 | K2O | 2.2–3.4 |
| Rutile | 0.1–0.3 | FeO3 | 0.7–1.9 |
| Quartz | Traces | MgO | 0.6–1.2 |
| Na2O | 0.2–0.3 | ||
| Ion exchange (mol kg−1) | Physical and mechanical properties | ||
| Ca2+ | 0.64–0.08 | Volume density | 1600–1800 kg m−3 |
| K+ | 0.22–0.45 | Porosity | 24–32% |
| Mg2+ | 0.06–0.19 | Diameter of pores | 0.4 nm |
| Na+ | 0.01–0.19 | Specific surface | 30–60 m2 g−1 |
| CEC | 1.2–1.59 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, J.I.; Arrobas, M.; Brito, C.; Gonçalves, A.; Silva, E.; Martins, S.; Raimundo, S.; Rodrigues, M.Â.; Correia, C.M. Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees. Sustainability 2020, 12, 10630. https://doi.org/10.3390/su122410630
Lopes JI, Arrobas M, Brito C, Gonçalves A, Silva E, Martins S, Raimundo S, Rodrigues MÂ, Correia CM. Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees. Sustainability. 2020; 12(24):10630. https://doi.org/10.3390/su122410630
Chicago/Turabian StyleLopes, João I., Margarida Arrobas, Cátia Brito, Alexandre Gonçalves, Ermelinda Silva, Sandra Martins, Soraia Raimundo, Manuel Ângelo Rodrigues, and Carlos M. Correia. 2020. "Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees" Sustainability 12, no. 24: 10630. https://doi.org/10.3390/su122410630
APA StyleLopes, J. I., Arrobas, M., Brito, C., Gonçalves, A., Silva, E., Martins, S., Raimundo, S., Rodrigues, M. Â., & Correia, C. M. (2020). Mycorrhizal Fungi were More Effective than Zeolites in Increasing the Growth of Non-Irrigated Young Olive Trees. Sustainability, 12(24), 10630. https://doi.org/10.3390/su122410630









