Seaweed Production Potential in the Brazilian Northeast: A Study on the Eastern Coast of the State of Rio Grande do Norte, RN, Brazil
Abstract
:1. Introduction
2. Study Object Characterization
Study Area
3. Methods
3.1. Crop Area Selection Criteria
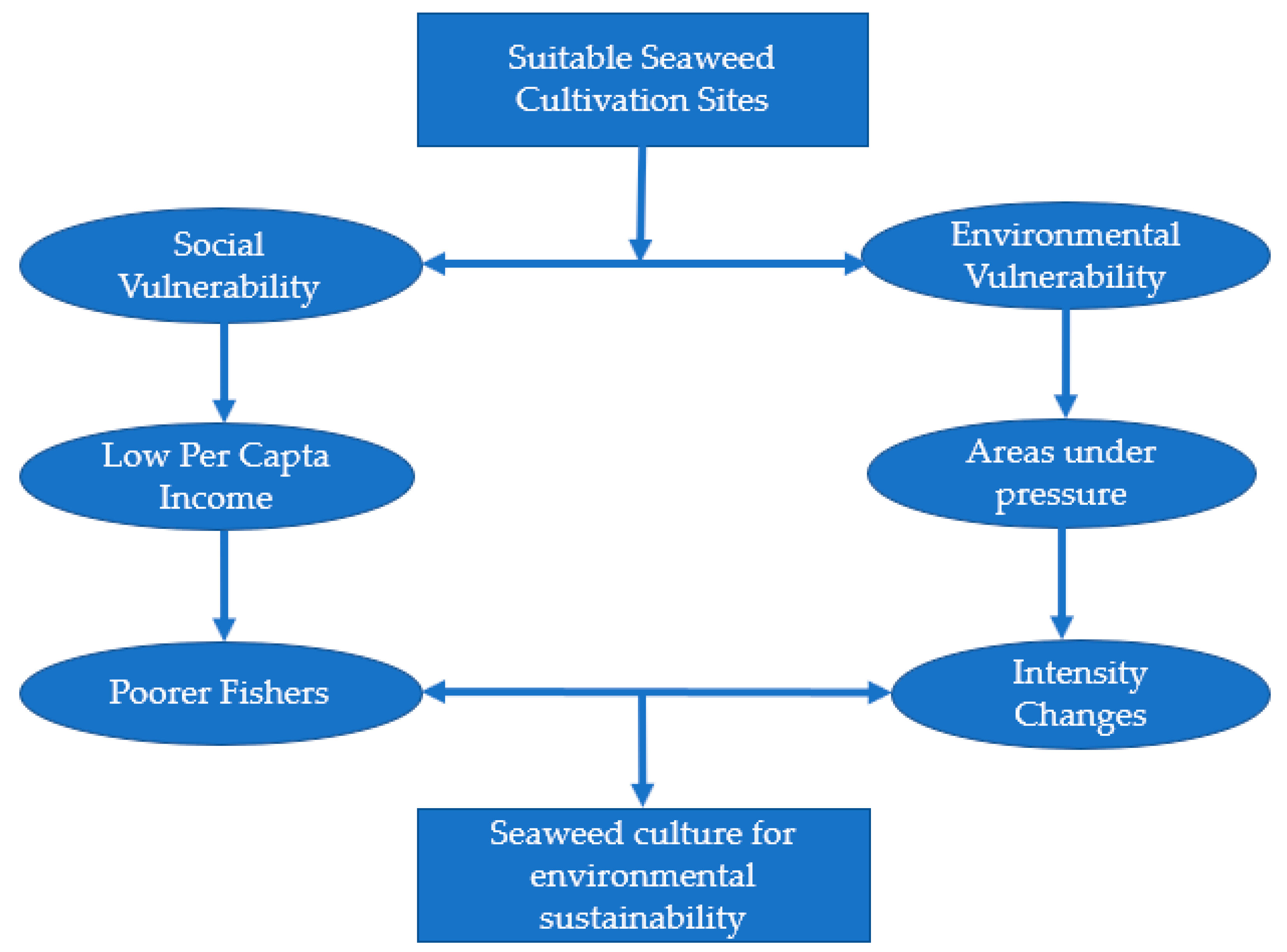
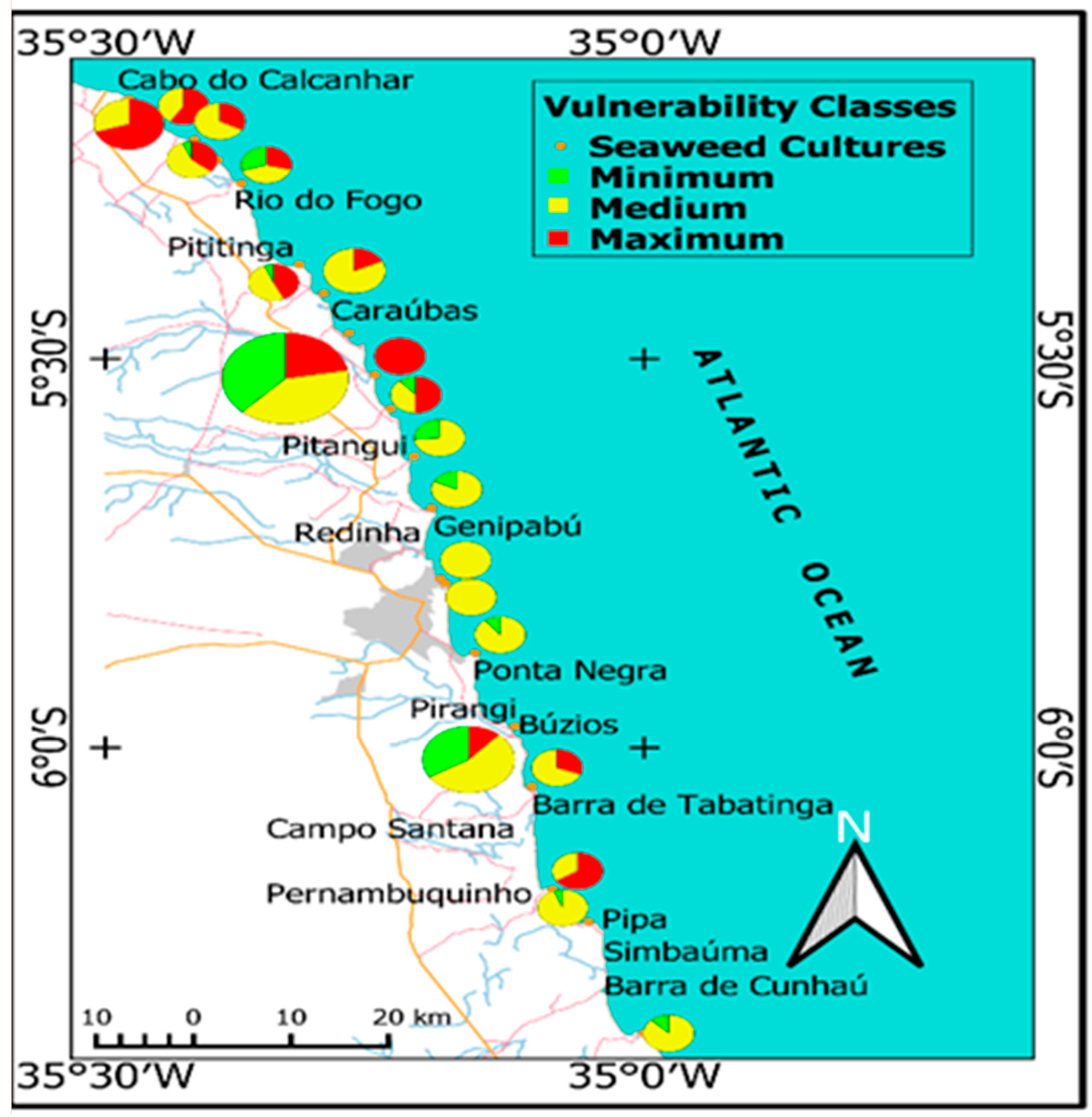
3.1.1. Social and Environmental Vulnerability Indicators
3.1.2. Social and Environmental Sustainability Indicators
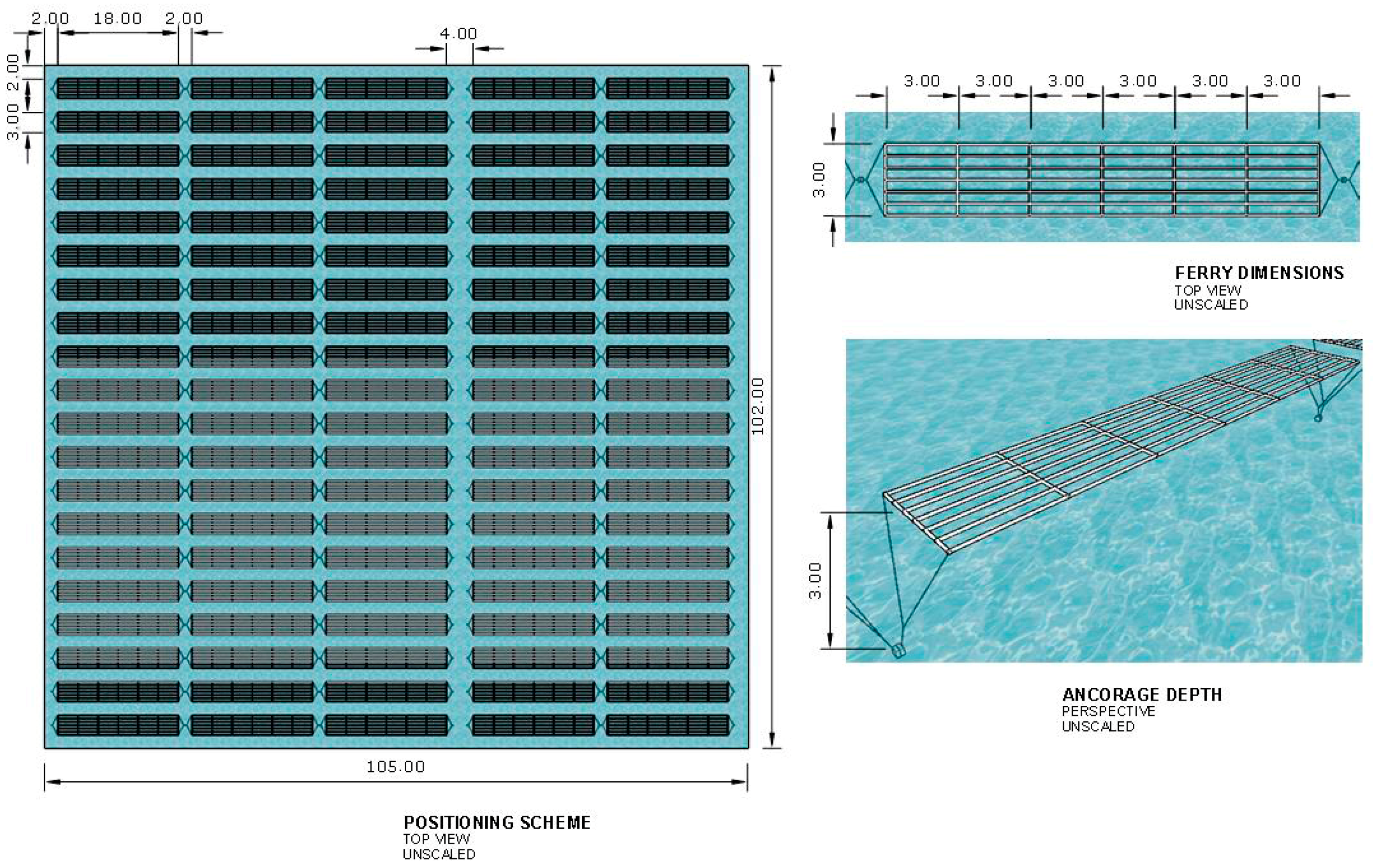
3.2. Potential Seaweed Biomass Production Estimation
3.3. Bioproduct Productive Potential Estimates
3.3.1. Bioethanol
3.3.2. Biofertilizer
3.3.3. Agar
4. Results and Discussion
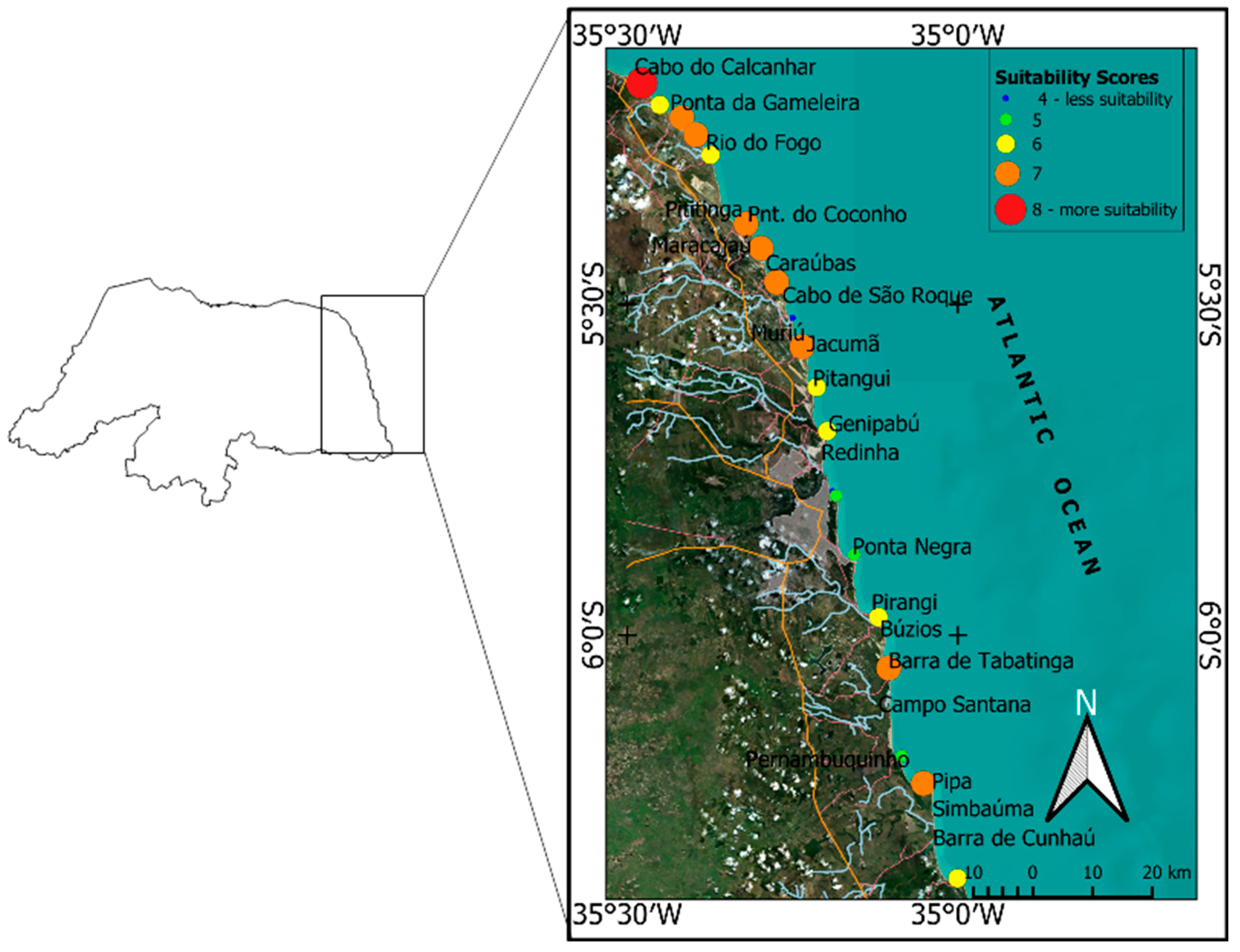
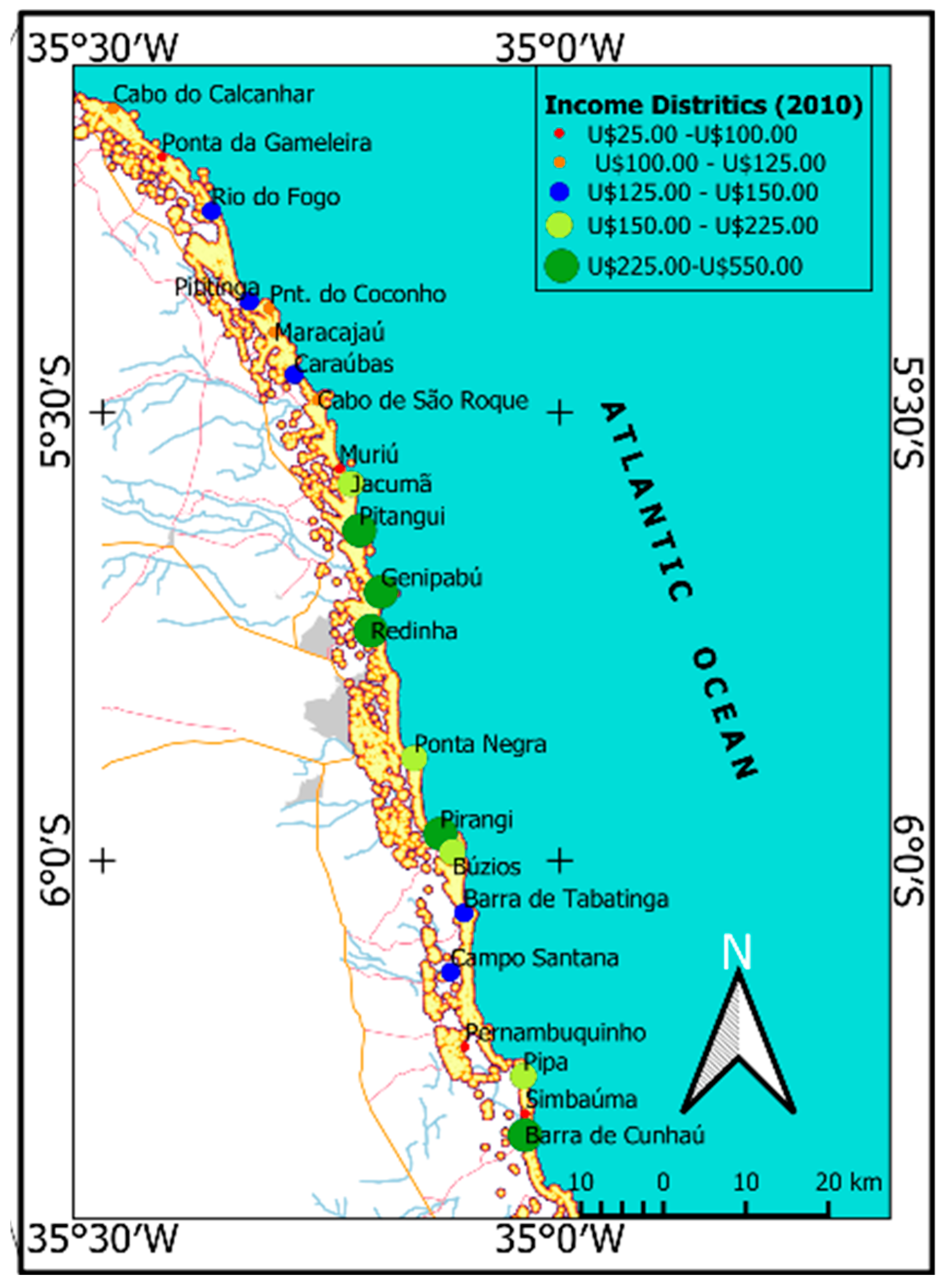
4.1. Social and Environmental Sustainability of Potential Cultivation Areas
4.2. Potential Seaweed Biomass Estimated
- (i)
- Scenario I considers the use of the areas classified as of maximum vulnerability (very attractive), comprising the most attractive physical and socio-environmental indicators for seaweed cultivation. These areas total 601.92 ha, where 89% are concentrated in the territorial strip located to the north of the city of Natal/RN, between the municipalities of Ceará-Mirim/RN and Touros/RN. This range already records cultivation activities of the assessed seaweed, and therefore, due to its set of attractions, is considered a priority for the development of seaweed algaculture in the region. According to the conditions presented in item 3.2, an estimated 52,728 t of fresh biomass may be produced per year, which results in 5272 t/year of commercially available dry biomass if 100% of the available areas are used. This estimate reaches 26,364 t when 50% of the areas are used and is reduced to 10,545 t when cultivation is restricted to 20% of the same areas;
- (ii)
- Scenario II considers the use of the sum of areas classified as presenting high (very attractive) and medium vulnerability (attractive), comprising 1825.47 ha distributed along the entire coast of RN. According to this scenario, under the conditions presented in item 3.2, an annual supply of 159,911 fresh biomass is estimated, resulting in 16,000 t/year of dry biomass to be sold, in the case of the use of 100% of the classified area. When 50% of the areas are used, 79,955tons are produced, and when cultivation is reduced to 20% of the same area, 31,981 tons are produced;
- (iii)
- Scenario III, less likely, estimates the use of all areas with presenting the potential for seaweed cultivation, around 2227.59 ha comprising areas of maximum, medium and minimum vulnerability – unattractive –. According to this scenario, under the conditions presented in item 3.2, the annual supply of fresh biomass would reach 195,136. t, which corresponds to 19,000 t of annual dry biomass, when using 100% of the classified areas. When using 50% of the same area, 97,568. t are produced, decreasing to 38,997. t when the cultivation area is of only 20%.
4.3. Bioproduct Potential Estimates
4.3.1. Bioethanol
- (i)
- Scenario I—In this scenario, an estimated 569,464 liters per year of bioethanol could be produced if 100% of the available areas were cultivated, reaching 284,732 liters using 50% of the areas and 113,892 liters when cultivation decreases to 20% of the areas defined in this scenario.
- (ii)
- Scenario II—This scenario offers 1,727.04 liters of bioethanol if 100% of the available areas were to be used. This estimate reaches 863,515 liters when 50% of the areas are used and is reduced to 345,40 liters when cultivation is restricted to 20% of the same areas.
- (iii)
- Scenario III—The annual supply of bioethanol for this scenario the reaches 2,107.48 liters when using 100% of the classified areas, decreasing to 1,053,73 liters when using 50% and reducing to 421,18 liters when the cultivation area reaches only 20%.
4.3.2. Biofertilizer
- (i)
- Scenario I—This scenario estimated 26,364 liters of biofertilizer per year if 100% of the available areas are cultivated, decreasing to 13,182 liters when 50% of the areas are used and to 5,272 liters is assumed when the cultivation is only in 20% of the defined areas in this scenario.
- (ii)
- Scenario II—This scenario estimates the production of 79,955 liters of biofertilizer if 100% of the areas are used, decreasing to 39,977 liters when 50% of the areas are used and to 15,990 liters when the cultivation is restricted to 20% of the same areas.
- (iii)
- Scenario III—The estimated annual supply of biofertilizer for this scenario totals 97,568 liters, in 100% of the classified areas. If 50% of this same area is used, 48,784 liters are obtained, reduced to 19,498 liters when the cultivation area reaches only 20%.
4.3.3. Agar
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rioux, L.-E.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 141–192. [Google Scholar]
- Sudhakar, K.; Mamat, R.; Samykano, M.; Azmi, W.; Ishak, W.; Yusaf, T. An overview of marine macroalgae as bioresource. Renew. Sustain. Energy Rev. 2018, 91, 165–179. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Fernand, F.; Israel, A.; Skjermo, J.; Wichard, T.; Timmermans, K.R.; Golberg, A. Offshore macroalgae biomass for bioenergy production: Environmental aspects, technological achievements and challenges. Renew. Sustain. Energy Rev. 2017, 75, 35–45. [Google Scholar] [CrossRef]
- Sondak, C.F.A.; Ang, P.O.; Beardall, J.; Bellgrove, A.; Boo, S.M.; Gerung, G.S.; Hepburn, C.D.; Hong, D.D.; Hu, Z.; Kawai, H.; et al. Carbon dioxide mitigation potential of seaweed aquaculture beds (SABs). J. Appl. Phycol. 2017, 29, 2363–2373. [Google Scholar] [CrossRef]
- Lago, C.; Herrera, I.; Caldés, N.; Lechón, Y. Nexus Bioenergy–Bioeconomy. In The Role of Bioenergy in the Bioeconomy; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 3–24. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 92-5-104958-0. [Google Scholar]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vasquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I.; et al. Seaweeds: An opportunity for wealth and sustainable livelihood for coastal communities. Environ. Boil. Fishes 2014, 26, 1939–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezerra, A.D.F. Cultivo de Algas Marinhas Como Desenvolvimento de Comunidades Costeiras; UFRN: Natal, Brazil, 2008. [Google Scholar]
- Marinho-Soriano, E. Historical context of commercial exploitation of seaweeds in Brazil. J. Appl. Phycol. 2017, 29, 665–671. [Google Scholar] [CrossRef]
- Estado Mundial, E.l. De La Pesca Y La Acuicultura 2018; FAO: Rome, Italy, 2018; ISBN 978-92-5-130688-8. [Google Scholar]
- de Oliveira Fernandes, F.; de Oliveira, S.R.; Klein, V.; do Amaral Carneiro, M.A.; Colepicolo, P.; Marinho-Soriano, E. Effect of fertilization pulses on the production of Gracilaria birdiae seedlings under laboratory and field conditions. J. Appl. Phycol. 2017, 29, 695–705. [Google Scholar] [CrossRef]
- Baghel, R.S.; Reddy, C.; Jha, B. Characterization of agarophytic seaweeds from the biorefinery context. Bioresour. Technol. 2014, 159, 280–285. [Google Scholar] [CrossRef]
- Hargreaves, P.I.; Barcelos, C.A.; Da Costa, A.C.A.; Pereira, N. Production of ethanol 3G from Kappaphycus alvarezii: Evaluation of different process strategies. Bioresour. Technol. 2013, 134, 257–263. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, N.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Jiang, R.; Ingle, K.N.; Golberg, A. Macroalgae (seaweed) for liquid transportation biofuel production: What is next? Algal. Res. 2016, 14, 48–57. [Google Scholar] [CrossRef]
- De Castro, G.M.C.; Benevides, N.M.B.; Cabral, M.C.; Miranda, R.D.S.; Rocha, M.V.P.; Araújo, M.L.H.; Filho, E.G. Optimized acid hydrolysis of the polysaccharides from the seaweed Solieria filiformis (Kützing) P.W. Gabrielson for bioethanol production. Acta Sci. Boil. Sci. 2017, 39, 423. [Google Scholar] [CrossRef] [Green Version]
- Adeniyi, O.M.; Azimov, U.; Burluka, A. Algae biofuel: Current status and future applications. Renew. Sustain. Energy Rev. 2018, 90, 316–335. [Google Scholar] [CrossRef]
- Khambhaty, Y.; Mody, K.; Gandhi, M.R.; Thampy, S.; Maiti, P.; Brahmbhatt, H.; Eswaran, K.; Ghosh, P.K. Kappaphycus alvarezii as a source of bioethanol. Bioresour. Technol. 2012, 103, 180–185. [Google Scholar] [CrossRef]
- Shukla, R.; Kumar, M.; Chakraborty, S.; Gupta, R.; Kumar, S.; Sahoo, D.; Kuhad, R.C. Process development for the production of bioethanol from waste algal biomass of Gracilaria verrucosa. Bioresour. Technol. 2016, 220, 584–589. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, S.; Angelidaki, I.; Holdt, S.L.; Karakashev, D.B.; Morales, M.A.; Das, D. Recent developments on biofuels production from microalgae and macroalgae. Renew. Sustain. Energy Rev. 2016, 65, 235–249. [Google Scholar] [CrossRef]
- Organization of Ukrainian Nationalists. World Population Prospects 2017; Population Division, Department of Economic and Social Affairs, OUN: New York, NY, USA, 2017; Volume 91. [Google Scholar]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- United Nations Intergovernmental Organization. Mulheres Conquistam Independência Financeira com Projeto de Maricultura No RN. Available online: https://nacoesunidas.org/mulheres-conquistam-independencia-financeira-com-projeto-de-maricultura-no-rn/ (accessed on 1 January 2020).
- Nath, S.S.; Bolte, J.P.; Ross, L.G.; Aguilar-Manjarrez, J. Applications of geographical information systems (GIS) for spatial decision support in aquaculture. Aquac. Eng. 2000, 23, 233–278. [Google Scholar] [CrossRef]
- Salam, M.; Ross, L.G.; Beveridge, C. A comparison of development opportunities for crab and shrimp aquaculture in southwestern Bangladesh, using GIS modelling. Aquaculture 2003, 220, 477–494. [Google Scholar] [CrossRef]
- Liu, Y.; Saitoh, S.-I.; Radiarta, I.N.; Isada, T.; Hirawake, T.; Mizuta, H.; Yasui, H. Improvement of an aquaculture site-selection model for Japanese kelp (Saccharinajaponica) in southern Hokkaido, Japan: An application for the impacts of climate events. ICES J. Mar. Sci. 2013, 70, 1460–1470. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, F.E.S.; Moura, E.A.; Marinho-Soriano, E. Use of geographic information systems (GIS) to identify adequate sites for cultivation of the seaweed Gracilaria birdiae in Rio Grande do Norte, Northeastern Brazil. Rev. Bras. Farm. 2012, 22, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Radiarta, I.N.; Saitoh, S.-I.; Miyazono, A. GIS-based multi-criteria evaluation models for identifying suitable sites for Japanese scallop (Mizuhopecten yessoensis) aquaculture in Funka Bay, southwestern Hokkaido, Japan. Aquaculture 2008, 284, 127–135. [Google Scholar] [CrossRef]
- Muehe Ceará, D. Die Guaporé-Expedition (1933–1935); Böhlau Verlag: Köln, Germany, 2015; pp. 52–63. [Google Scholar]
- Ministério do Meio Ambiente. ÁREAS PRIORITÁRIAS PARA CONSERVAÇÃO, USO SUSTENTÁVEL E REPARTIÇÃO DE BENEFÍCIOS DA BIODIVERSIDADE BRASILEIRA; MMA: Brasilia, Brazil, 2008; ISBN 9788577380763. [Google Scholar]
- Carneiro, M.A.A.; Marinho-Soriano, E.; Plastino, E.M. Phenology of an agarophyte Gracilaria birdiae Plastino and E.C. Oliveira (Gracilariales, Rhodophyta) in Northeastern Brazil. Rev. Bras. Farmacogn. 2011, 21, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Vital, H.; Da Silveira, I.M.; Tabosa, W.F.; Lima, Z.M.C.; Lima-Filho, F.P.; De Souza, F.E.S.; Chaves, M.D.S.; Pimenta, F.M.; Gomes, M.P. Beaches of Rio Grande do Norte. In Climate Change and Sea Level Rise in South Florida; Springer Science and Business Media LLC: Berlin, Germany, 2016; Volume 17, pp. 201–229. [Google Scholar]
- Instituto De Desenvolvimento Econômico E Meio Ambiente. Perfil do Rio Grande do Norte; IDEMA: Natal, Brasil, 2014. [Google Scholar]
- VITAL, H.; Silveira, I.M.d.S.; Tabosa, W.F.; Lima, Z.M.C.; Lima-Filho, F.P.; Souza, F.E.S.; Chaves, M.d.S.; Pimenta, F.M.; Gomes, M.P. Brazilian Beach Systems, 1st ed.; Springer International: Berlin, Germany, 2016. [Google Scholar]
- Nimer, E. Climatologia do Brasil, 2nd ed.; Departamento de Recursos Naturais e Estudos Ambientais, IBGE: Rio de janeiro, Brazil, 1989; ISBN 8524002824. [Google Scholar]
- Asokan, A.; Anitha, J. Change detection techniques for remote sensing applications: A survey. Earth Sci. Inform. 2019, 12, 143–160. [Google Scholar] [CrossRef]
- The National Aeronautics and Space Administration. Landsat 7 Science Data Users Handbook; Landsat Project Science Office, NASA: Greenbelt, MA, USA, 2011.
- Chacon, M.; Carrillo, O.; Argotty, F.; Fung, E.; Imbach, P.; Islas, G.; Larios, E.; Lopez, D.; Maldonado, V.; Medellin, C.; et al. El Estado del Monitoreo Forestal en Latinoamérica y el Caribe: Tipos de Iniciativas y uso de Tecnologías; CATIE: Turrialba, Cartago, Costa Rica, 2016; ISBN 9789977577098. [Google Scholar]
- Junior, C.I.; Gregorio, L.M.; Francisco, M.; Hugo, S. Multitemporal Analysis of Forest Cover Using the Methodology of Spatial Remote Sensing and GIS in the Sub-Basin of the Coroico river—Caranavi Province in the Years 1989–2014. J. Selva Andina Res. Soc. 2018. Available online: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S2072-92942018000100003 (accessed on 1 January 2020).
- Española de Teledetección; Maldonado, F.D.; Dos Santos, J.R.; De Carvalho, y.V.C. Caracterización de cambios e influencia climática sobre la cobertura vegetal en el semiárido brasileño mediante Análisis de Componentes Principales (ACP). Available online: http://www.aet.org.es/?q=revista13-3 (accessed on 1 January 2020).
- de Andrade Neto, J.C. Produção potencial de etanol a partir de Macroalga Gracilaria birdiae cultivada; UFRN: Natal, Brazil, 2015. [Google Scholar]
- IBGE Weblet Importer. IBGE, Rio do Fogo. Available online: https://cidades.ibge.gov.br/brasil/rn/rio-do-fogo/panorama (accessed on 14 October 2019).
- Da Silva, R.R.; Bacha, C.J.C. Polígonos de Voronoi como alternativa aos problemas das áreas mínimas comparáveis: Uma análise das mudanças populacionais na região norte do Brasil. Revista Brasileira de Estudos de População 2011, 28, 133–151. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, A.; Marinho-Soriano, E. Cultivation of the red seaweed Gracilaria birdiae (Gracilariales, Rhodophyta) in tropical waters of northeast Brazil. Biomass Bioenergy 2010, 34, 1813–1817. [Google Scholar] [CrossRef]
- Gelli, V.C. Desenvolvimento Ordenado e Potencial da Produção da Macroalga Kappaphycus Alvarezii no Estado de São Paulo Para Extração de Biofertilizante; UNICAMP: Campinas, Brazil, 2019. [Google Scholar]
- Ferreira, D.M. Extração de Agar de Algas Vermelhas do género Gracilaria; Instituto Superior de Engenharia de Coimbra: Coimbra, Portugal, 2015. [Google Scholar]
- IBGE censo 2010 | resultados. IBGE, censo2010. Available online: https://censo2010.ibge.gov.br/apps/mapa/ (accessed on 14 October 2019).
- IBGE Censo Demográfico 2010. IBGE, Censo Demográfico 2010. Available online: http://www.ibge.gov.br/home/estatistica/%0Apopulacao/censo2010/caracteristicas_da_populacao/%0Aresultados_do_universo.pdf (accessed on 14 October 2019).
- dos Santos Júnior, J.; Rebouças, L.d.O.S.; Figueiredo, J.P.d.V.; Bessa Júnior, A.P.; Maia, I.d.S. Viabilidade econômica de um produto a base de algas em uma associação de maricultoras do litoral Norte Potiguar. Pubvet 2017, 11, 313–339.14. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Distance to: | Weights | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NATURAL Beds | Seaward | Market | Districts | Estuary | Road | Main City | Urban Areas | |||
| Distance to: | natural beds | 1 | 0.0351 | |||||||
| seaward | 3 | 1 | 0.0423 | |||||||
| market | 5 | 5 | 1 | 0.1949 | ||||||
| districts | 3 | 3 | 1/5 | 1 | 0.0599 | |||||
| estuary | 5 | 5 | 1/3 | 5 | 1 | 0.1333 | ||||
| road | 1 | 3 | 1/3 | 1 | 1/3 | 1 | 0.0502 | |||
| main city | 5 | 5 | 1 | 5 | 3 | 7 | 1 | 0.2357 | ||
| urban areas | 3 | 3 | 3 | 3 | 3 | 7 | 1 | 1 | 0.2486 | |
| Weights | 1/9 | 1/7 | 1/5 | 1/3 | 1 | 3 | 5 | 7 | ||
| Extremely | Very strong | Strongly | Moderately | Equally | Moderately | Strongly | Extremely | |||
| Levels | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
 | ||||||||||
| Less important | More important | |||||||||
| Districts | Locality | Area Vulnerability (ha) | Total Area (ha) * | |||
|---|---|---|---|---|---|---|
| Low | Medium | High | Total | |||
| Touros | Touros | 0 | 64.89 | 150.57 | 215.46 | 216.88 |
| Touros | Touros | 0 | 25.65 | 36.63 | 62.28 | 62.05 |
| Touros | Touros | 0 | 45.72 | 22.32 | 68.04 | 68.07 |
| Touros | Touros | 2.34 | 22.05 | 13.86 | 38.25 | 37.35 |
| Rio do Fogo | Rio do Fogo | 38.61 | 52.83 | 37.44 | 128.88 | 128.90 |
| Rio do Fogo | Rio do Fogo | 8.55 | 73.44 | 62.1 | 144.09 | 144.54 |
| Maxaranguape | Maxaranguape | 0 | 156.33 | 33.93 | 190.26 | 190.90 |
| Maxaranguape | Maxaranguape | 147.78 | 155.07 | 85.59 | 388.44 | 390.80 |
| Maxaranguape | Maxaranguape | 0 | 0 | 18.09 | 18.09 | 18.67 |
| Ceara-Mirim | Ceara-Mirim | 15.84 | 54.18 | 71.28 | 141.3 | 140.28 |
| Extremoz | Extremoz | 9.9 | 27.72 | 0 | 47.16 | 47.56 |
| Extremoz | Extremoz | 16.47 | 75.96 | 0 | 92.43 | 93.30 |
| Natal | Natal | 0 | 1.35 | 0 | 1.35 | 1.20 |
| Natal | Natal | 0 | 16.56 | 0 | 16.56 | 16.85 |
| Natal | Natal | 5.67 | 48.51 | 0 | 54.18 | 53.55 |
| Parnamirim | Parnamirim | 81 | 131.31 | 27.54 | 284.76 | 284.57 |
| Nísia Floresta | Nísia Floresta | 0 | 33.93 | 15.12 | 49.05 | 49.33 |
| Tibau do Sul | Tibau do Sul | 0 | 14.31 | 27.45 | 41.76 | 42.70 |
| Tibau do Sul | Tibau do Sul | 7.11 | 118.89 | 0 | 126 | 125.08 |
| Baia Formosa | Baia Formosa | 14.4 | 104.85 | 0 | 119.25 | 119.63 |
| Total (ha) | 347.67 | 1.223.55 | 601.92 | 2.227.59 | 2.232.20 | |
| Scenario 1 * | Scenario 2** | Scenario 3*** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 20% | 50% | 100% | 20% | 50% | 100% | 20% | 50% | 100% | ||
| Area (ha) | 120.38 | 300.96 | 601.92 | 365.09 | 912.73 | 1825.47 | 445.18 | 1113.79 | 2227.59 | |
| Fresh biomass (t/y) | 10,545 | 26,364 | 52,728 | 31,981 | 79,955 | 159,911 | 38,997 | 97,568 | 195,136 | |
| Dry biomass (t/y) | 1054 | 2636 | 5272 | 3198 | 7995 | 15,991 | 3899 | 9756 | 19,513 | |
| Bioethanol (kl/y) | 113.89 | 284.73 | 569.46 | 345.40 | 863.52 | 1727.04 | 421.18 | 1,053.73 | 2107.48 | |
| Biofertilizer (l/y) | 5272 | 13,182 | 26,364 | 15,990 | 39,977 | 79,955 | 19,498 | 48,784 | 97,568 | |
| Agar | 22% | 2320 | 5800 | 11,600 | 7036 | 17,590 | 35,180 | 8579 | 21,464 | 42,930 |
| (t/y) | 47% | 4956 | 12,391 | 24,782 | 15,031 | 37,578 | 75,158 | 18,328 | 45,856 | 91,714 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, H.M.M.d.Q.; Rosa, L.P.; Souza, F.E.S.d.; Silva, N.F.d.; Cabral, M.C.; Teixeira, D.I.A. Seaweed Production Potential in the Brazilian Northeast: A Study on the Eastern Coast of the State of Rio Grande do Norte, RN, Brazil. Sustainability 2020, 12, 780. https://doi.org/10.3390/su12030780
Andrade HMMdQ, Rosa LP, Souza FESd, Silva NFd, Cabral MC, Teixeira DIA. Seaweed Production Potential in the Brazilian Northeast: A Study on the Eastern Coast of the State of Rio Grande do Norte, RN, Brazil. Sustainability. 2020; 12(3):780. https://doi.org/10.3390/su12030780
Chicago/Turabian StyleAndrade, Herika Mylena Medeiros de Queiroz, Luiz Pinguelli Rosa, Flavo Elano Soares de Souza, Neilton Fidelis da Silva, Maulori Curié Cabral, and Dárlio Inácio Alves Teixeira. 2020. "Seaweed Production Potential in the Brazilian Northeast: A Study on the Eastern Coast of the State of Rio Grande do Norte, RN, Brazil" Sustainability 12, no. 3: 780. https://doi.org/10.3390/su12030780





