Treatment Assessment of Road Runoff Water in Zones filled with ZVI, Activated Carbon and Mineral Materials
Abstract
:1. Introduction
2. Materials and Methods
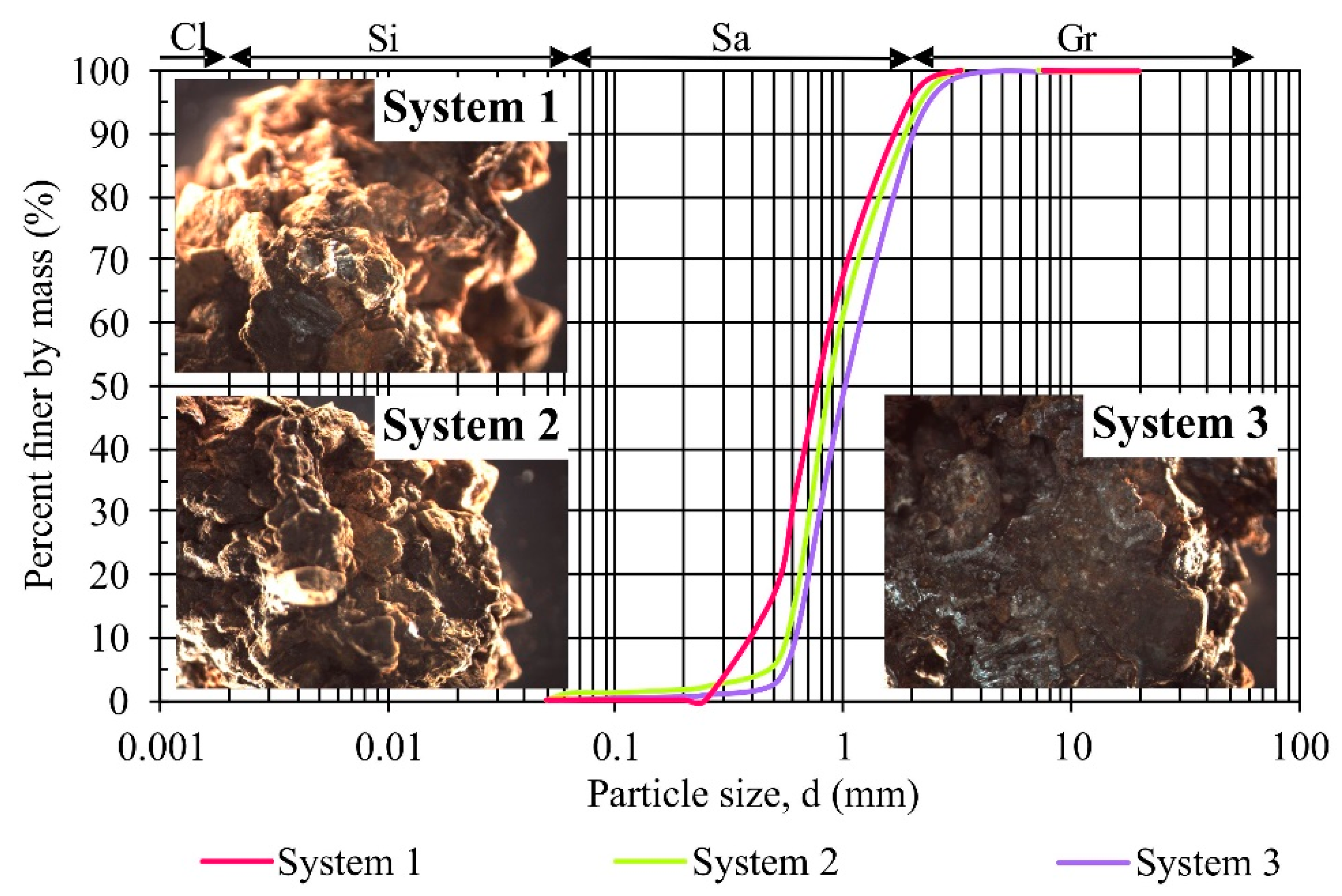
2.1. Pilot-Scale Tests
2.2. Statistical Analysis
3. Results and Discussion
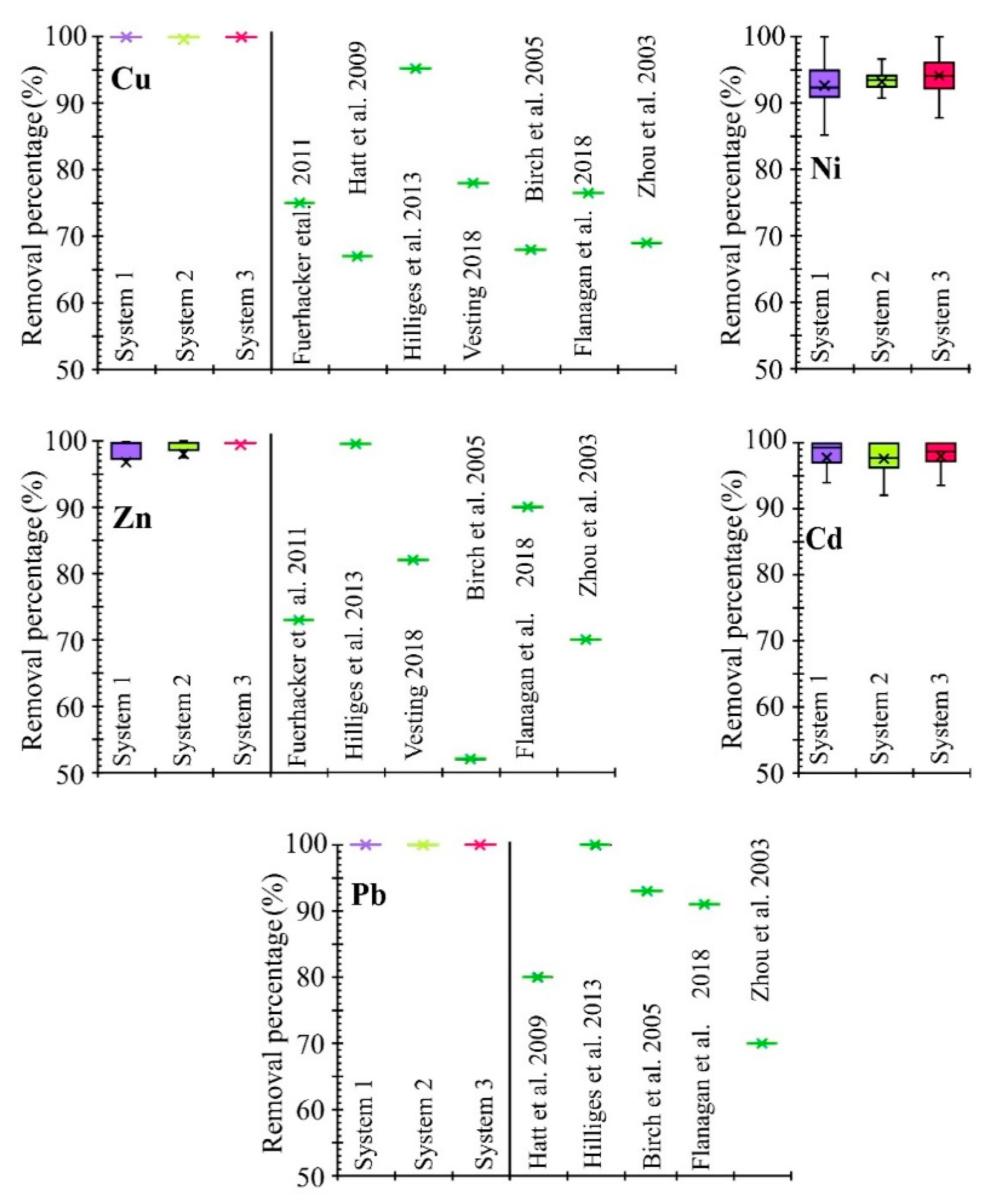
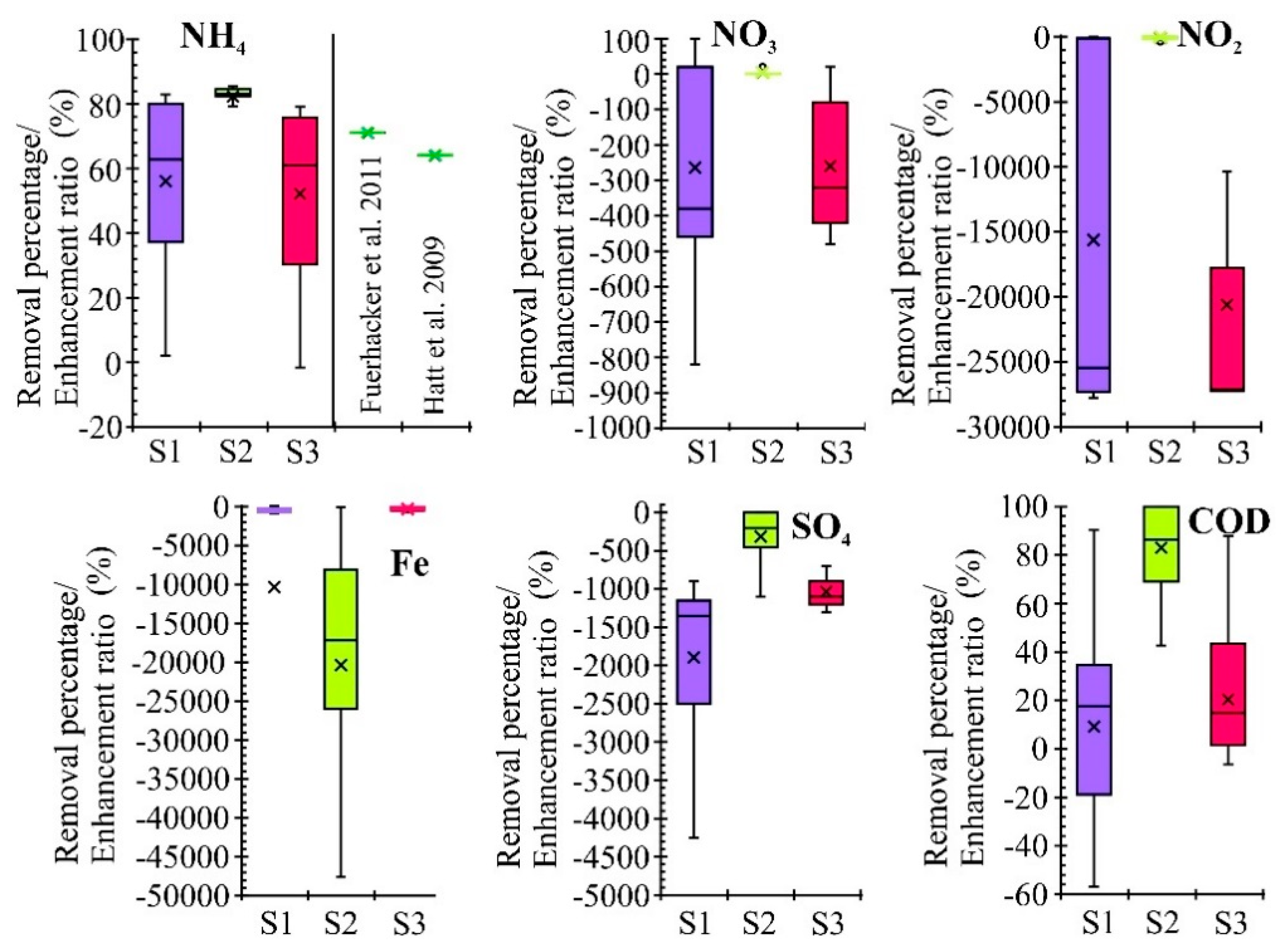
3.1. Characteristics of Removal Efficiency
3.2. Statistical Analysis
3.2.1. ANOVA
3.2.2. Principal Component and Factor Analysis
Effect of Temperature on the Removal Processes
Effect of Hydraulic Conductivity on Removal Processes
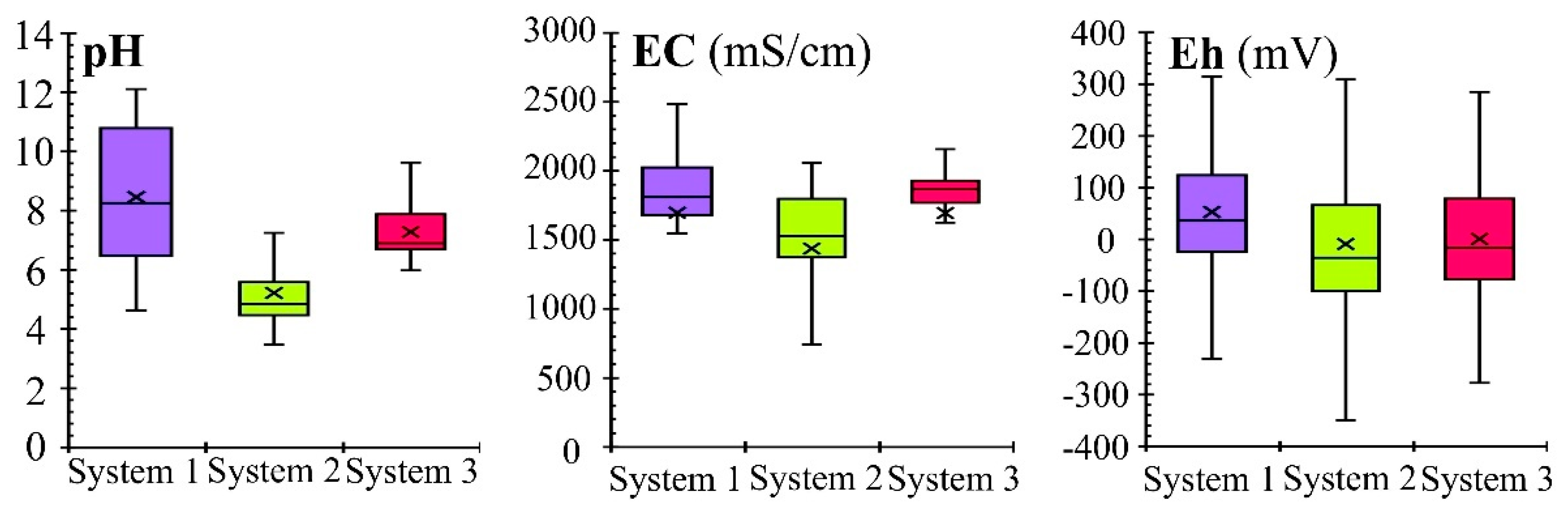
Effect of pH on the Removal Processes
Effect of Eh on the Removal Processes
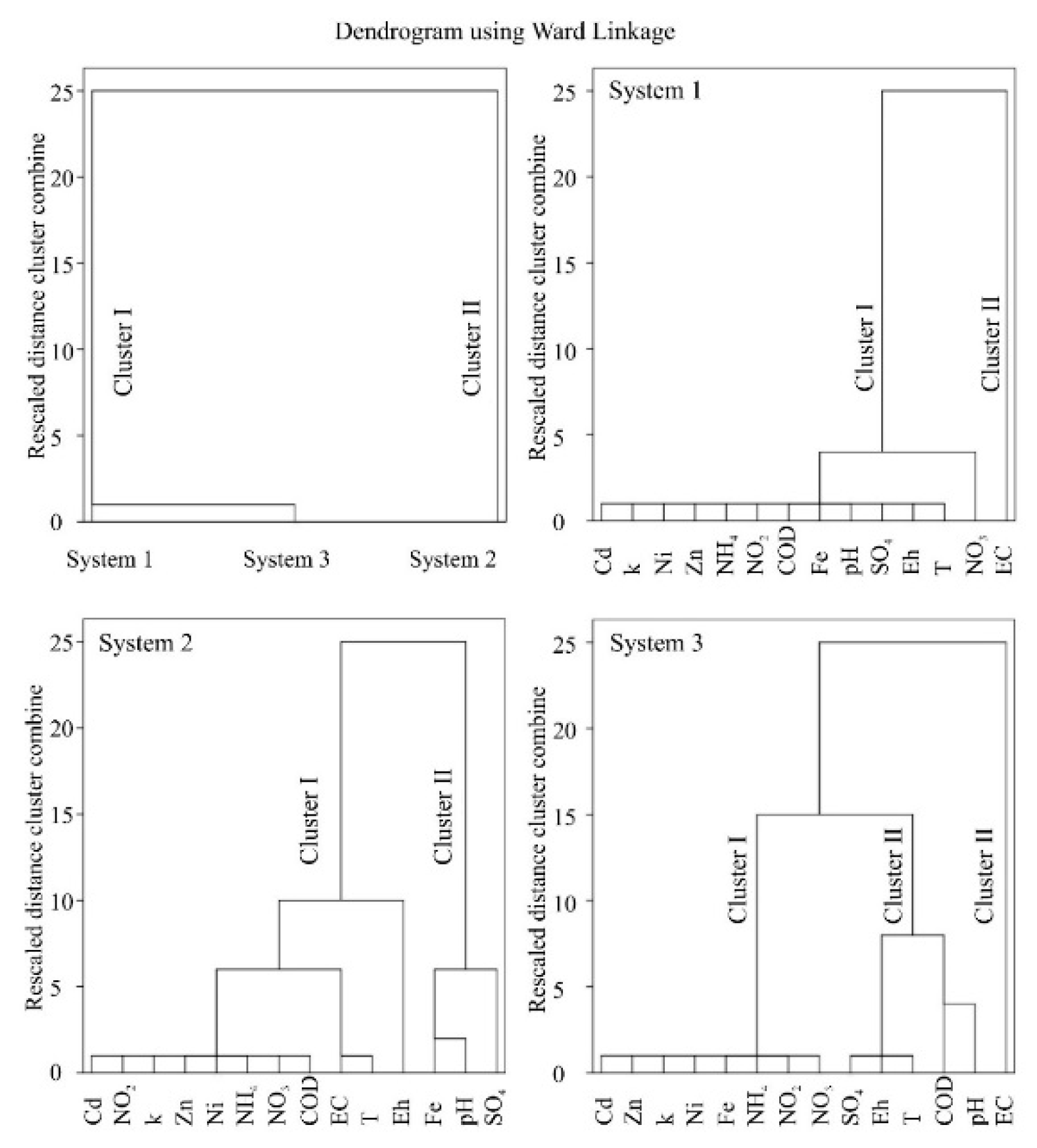
Cluster Analysis
4. Conclusions
- Proposed mixtures of reactive materials effectively remove dissolved heavy metals, primarily including Cu and Pb and, to a lesser extent, Zn, Cd and Ni. Statistically significant differences in the removal of Cd, Cu, Ni and Pb were not revealed for any of the selected mineral materials (SS, Z and LS). In addition, the system containing zeolite effectively removes ammonium ions.
- Processes occurring during the flow of runoff water through filter beds resulted in the leaching of nitrogen oxides (S1 and S3), sulfates (all three systems) and iron (system 2) into the effluent solution. The concentration of nitrogen oxides and sulfates in the effluent solution from S1 and S3 and sulfate from S2 can be reduced by extending the residence time of runoff water in the treatment zones. However, this may result in increased iron release.
- The statistical analysis (ANOVA and Cluster Analysis) showed similarity in the treatment effectiveness of the filter beds containing calcareous materials (SS and LS). Significant differences between these two systems were observed for four out of the 14 analyzed parameters (Zn, NH4, SO4 and pH), which may be the deciding factor in choosing a cheaper material (silica spongolite) for future applications.
- During 11 months of the field pilot-scale tests, a decrease of hydraulic conductivity was detected by about two orders of magnitude for the filter beds containing i.a. silica spongolite (S1) and limestone (S2), and by one order of magnitude for systems containing i.a. zeolite (S3). This was due to the occurrence of iron corrosion process and the precipitation of iron hydroxides. However, the changes in hydraulic conductivity were statistically not significant; therefore, this factor did not affect the selection of the best mixture for future applications.
- The removal effectiveness of individual contaminants together with the results of the statistical analysis allow to consider ZVI/AC/Z as the most suitable mixture for large-scale application. However, due to clogging processes, it is necessary to consider the decrease of the ZVI content in the mixture and maintaining the treatment zone as fully saturated, e.g., by ensuring the level of the inlet and outlet of the runoff water above the filter bed level.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Helmreich, B.; Hilliges, R.; Schriewer, A.; Horn, H. Runoff pollutants of a highly trafficked urban road—Correlation analysis and seasonal influences. Chemosphere 2010, 80, 991–997. [Google Scholar] [CrossRef]
- Lundy, L.; Ellis, J.B.; Revitt, D.M. Risk prioritisation of stormwater pollutant sources. Water Res. 2012, 46, 6589–6600. [Google Scholar] [CrossRef]
- Mullaney, J.; Lucke, T. Practical review of pervious pavement designs. Clean–Soil Air Water 2014, 42, 111–124. [Google Scholar] [CrossRef]
- Revitt, D.M.; Lundy, L.; Coulon, F.; Fairley, M. The sources, impact and management of car park runoff pollution: A review. J. Environ. Manag. 2014, 146, 552–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.N.; Peak, B.M. Sources of heavy metals and polycyclic aromatic hydrocarbons in urban storm water runoff. Sci. Total Environ. 2006, 359, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Fuerhacker, M.; Haile, T.M.; Monai, B.; Mentler, A. Performance of a filtration system equipped with filter media for parking lot runoff treatment. Desalination 2011, 275, 118–125. [Google Scholar] [CrossRef]
- Alam, M.Z.; Anwar, A.F.; Heitz, A.; Sarker, D.C. Improving stormwater quality at source using catch basin inserts. J. Environ. Manag. 2018, 228, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Welker, A.; Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: Occurrence, influencing factors, and partitioning. Sci. Total Environ. 2016, 541, 895–919. [Google Scholar] [CrossRef] [PubMed]
- Kalmykova, Y.; Björklund, K.; Strömvall, A.M.; Blom, L. Partitioning of polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachates and stormwater. Water Res. 2013, 47, 1317–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markiewicz, A.; Björklund, K.; Eriksson, E.; Kalmykova, Y.; Strömvall, A.-M.; Siopi, A. Emissions of organic pollutants from traffic and roads: Priority pollutants selection and substance flow analysis. Sci. Total Environ. 2017, 580, 1162–1174. [Google Scholar] [CrossRef]
- Gavrić, S.; Leonhardt, G.; Marsalek, J.; Viklander, M. Processes improving urban stormwater quality in grass swales and filter strips: A review of research findings. Sci. Total Environ. 2019, 669, 431–447. [Google Scholar] [CrossRef]
- Maestre, A.; Pitt, R. The National Stormwater Quality Data-Base, Version 1.1 A Compilation and Analysis of NPDES Stormwater Monitoring Information; U.S. Environmental Protection Agency (EPA) Office of Water: Washington, DC, USA, 2005.
- Kayhanian, M.; Fruchtman, B.D.; Gulliver, J.S.; Montanaro, C.; Ranieri, E.; Wuertz, S. Review of highway runoff characteristics: Comparative analysis and universal implications. Water Res. 2012, 46, 6609–6624. [Google Scholar] [CrossRef]
- Griffiths, L.N.; Mitsch, W.J. Removal of nutrients from urban stormwater runoff by storm-pulsed and seasonally pulsed created wetlands in the subtropics. Ecol. Eng. 2017, 108, 414–424. [Google Scholar] [CrossRef]
- Duan, S.; Mayer, P.M.; Kaushal, S.S.; Wessel, B.M.; Johnson, T. Regenerative stormwater conveyance (RSC) for reducing nutrients in urban stormwater runoff depends upon carbon quantity and quality. Sci. Total Environ. 2019, 652, 134–146. [Google Scholar] [CrossRef]
- Novotny, E.V.; Murphy, D.; Stefan, H.G. Increase of urban lake salinity by road deicing salt. Sci. Total Environ. 2008, 406, 131–144. [Google Scholar] [CrossRef]
- Collins, S.J.; Russell, R.W. Toxicity of road salt to Nova Scotia amphibians. Environ. Pollut. 2009, 157, 320–324. [Google Scholar] [CrossRef]
- Fronczyk, J.; Radziemska, M.; Dynowski, P.; Mazur, Z.; Bazydło, M. Quality of water in the road drainage systems in the Warsaw agglomeration, Poland. Water 2016, 8, 429. [Google Scholar] [CrossRef] [Green Version]
- Gunawardana, C.; Goonetilleke, A.; Egodawatta, P.; Dawes, L.; Kokot, S. Source characterisation of road dust based on chemical and mineralogical composition. Chemosphere 2012, 87, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Pratt, C. A review of source control of urban stormwater runoff. Water Environ. Manag. 1995, 9, 132–139. [Google Scholar] [CrossRef]
- Norrström, A.C. Metal mobility by de-icing salt from an infiltration trench for highway runoff. Appl. Geochem. 2005, 20, 1907–1919. [Google Scholar] [CrossRef]
- Fach, S.; Dierkes, C. On-site infiltration of road runoff using pervious pavements with subjacent infiltration trenches as source control strategy. Water Sci. Technol. 2011, 64, 1388–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, E.; Baun, A.; Scholes, L.; Ledin, A.; Ahlman, S.; Revitt, M.; Noutsopoulos, C.; Mikkelsen, P.S. Selected stormwater priority pollutants—A European perspective. Sci. Total Environ. 2007, 383, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Hilliges, R.; Schriewer, A.; Helmreich, B. A three-stage treatment system for highly polluted urban road runoff. J. Environ. Manag. 2013, 128, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bogacz, A.; Woźniczka, P.; Burszta-Adamiak, E.; Kolasińska, K. Methods of enhancing water retention in urban areas. Sci. Rev. Eng. Env. Sci. 2013, 59, 27–35. (In Polish) [Google Scholar]
- Matusiewicz, W.; Wrzesiński, G. Drainage of the depression area in a small urban catchment. Acta Sci. Pol. Archit. 2018, 17, 131–144. (In Polish) [Google Scholar]
- Weiss, P.T.; LeFevre, G.; Gulliver, J.S. Contamination of Soil and Groundwater Due to Stormwater Infiltration Practices. A Literature Review; Minnesota Pollution Control Agency: Saint Paul, MN, USA, 2008. [Google Scholar]
- Zhou, W.; Beck, B.F.; Green, E.T. Evaluation of a peat filtration system for treating highway runoff in a karst setting. Environ. Geol. 2003, 44, 187–202. [Google Scholar] [CrossRef]
- Birch, G.F.; Fayeli, F.S.; Matthai, C. Efficiency of an infiltration basin in removing contaminants from urban storm water. Environ. Monit. Assess. 2005, 101, 145–155. [Google Scholar]
- Karczmarczyk, A.; Bus, A. Removal of phosphorus using suspended reactive filters (SRFs)—Efficiency and potential applications. Water Sci. Technol. 2017, 76, 1104–1111. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, Y.; Xu, Z.; Doherty, L.; Liu, R. Highway runoff treatment by hybrid adsorptive media-baffled subsurface flow constructed wetland. Ecol. Eng. 2016, 91, 231–239. [Google Scholar] [CrossRef]
- Reddy, K.R.; Kumar, G. Permeable reactive filter systems for the treatment of urban stormwater runoff with mixed pollutants. Geotech. Front. 2017, 2017, 508–517. [Google Scholar]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Treatment performance of gravel filter media: Implications for design and application of stormwater infiltration systems. Water Res. 2007, 41, 2513–2524. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.M.; Wanielista, M.P.; Chang, N.-B.; Xuan, Z.; Harris, W.G. Nutrient removal using biosorption activated media: Preliminary biogeochemical assessment of an innovative stormwater infiltration basin. Sci. Total Environ. 2012, 432, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.; Branchu, P.; Boudahmane, L.; Caupos, E.; Demare, D.; Deshayes, S.; Dubois, P.; Meffray, L.; Partibane, C.; Saad, M.; et al. Field performance of two biofiltration systems treating micropollutants from road runoff. Water Res. 2018, 145, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Vesting, A. Entwicklung und Evaluation Eines Dezentralen Behandlungssystems zum Rückhalt von Organischen Spurenstoffen und Schwermetallen aus Verkehrsflächenabflüssen; Schriftenreihe Siedlungswasserwirtschaft Bochum: Bochum, Germany, 2018; p. 74. [Google Scholar]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. 2007, 133, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Capturing phosphates with iron enhanced sand filtration. Water Res. 2012, 46, 3032–3042. [Google Scholar] [CrossRef]
- Erickson, A.J.; Gulliver, J.S.; Weiss, P.T. Phosphate removal from agricultural tile drainage with iron enhanced sand. Water 2017, 9, 672. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Nutrients removal from urban stormwater by different filter materials. Water Air Soil Pollut. 2014, 225, 1778. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-Term Performance of Zero-Valent Iron Permeable Reactive Barriers: A Critical Review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, J. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef]
- Arora, M.; Snape, I.; Stevens, G.W. The effect of temperature on toluene sorption by granular activated carbon and its use in permeable reactive barriers in cold regions. Cold Reg. Sci. Technol. 2011, 66, 12–16. [Google Scholar] [CrossRef]
- Pawluk, K.; Fronczyk, J.; Garbulewski, K. Reactivity of nano zero-valent iron in permeable reactive barriers. Pol. J. Chem. Technol. 2015, 17, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Pawluk, K.; Fronczyk, J.; Garbulewski, K. Experimental development of contaminants removal from multicomponent solutions—Column test. Desalin. Water Treat. 2019, 144, 89–98. [Google Scholar] [CrossRef]
- Pawluk, K.; Fronczyk, J. Evaluation of single and multilayered reactive zones for heavy metals removal from stormwater. Environ. Technol. 2015, 36, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Fronczyk, J.; Mumford, K.A. The impact of temperature on the removal of inorganic contaminants typical of urban stormwater. Appl. Sci. 2019, 9, 1273. [Google Scholar] [CrossRef] [Green Version]
- Caré, S.; Crane, R.; Calabrò, P.S.; Ghauch, A.; Temgoua, E.; Noubactep, C. Modeling the permeability loss of metallic iron water filtration systems. Clean—Soil Air Water 2013, 41, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Fronczyk, J. Artificial road runoff water treatment by a pilot-scale horizontal permeable treatment zone. Ecol. Eng. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Majewski, G.; Przewoźniczuk, W.; Kleniewska, M. Precipitation at the meteorological station in Ursynów WULS—SGGW in 1960–2009. Sci. Rev. Eng. Env. Sci. 2010, 2, 3–22. (In Polish) [Google Scholar]
- Landau, S.; Everitt, B. A Handbook of Statistical Analysis Using SPSS, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Kalaycı, Ş. SPSS Uygulamalı çok Değişkenli Istatistik Teknikleri, 1st ed.; Asil Yayın Dağıtım: Çankaya, Turkey, 2009. (In Turkish) [Google Scholar]
- Jolliffe, I. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Brtnický, M.; Pecina, V.; Hladký, J.; Radziemska, M.; Koudelková, Z.; Klimánek, M.; Richtera, L.; Adamcová, D.; Elbl, J.; Galiová, M.V.; et al. Assessment of phytotoxicity, environmental and health risks of historical urban park soils. Chemosphere 2019, 220, 678–686. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fideli, L.S. Using Multivariate Statistics, 4th ed.; Ally and Bacon: Boston, FL, USA, 2001; pp. 256–267. [Google Scholar]
- Prakash, M.M.; Dagaonkar, A. Application of cluster analysis to physco-chemical parameters of Munj Sagar Talab, Dhar (Madhya Pradesh, India). Recent Res. Sci. Technol. 2011, 3, 41–50. [Google Scholar]
- Tiri, A.; Lahbari, N.; Boudoukha, A. Assessment of the quality of water by hierarchical cluster and variance analyses of the Koudiat Medouar Watershed, East Algeria. Appl. Water Sci. 2017, 7, 4197–4206. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.H.; Davis, A.P.; Needelman, B.A. Nitrogen removal from urban stormwater runoff through layered bioretention columns. Water Environ. Res. 2007, 79, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- LeFevre, G.H.; Paus, K.H.; Natarajan, P.; Gulliver, J.S.; Novak, P.J.; Hozalski, R.M. Review of dissolved pollutants in urban storm water and their removal and fate in bioretention cells. J. Environ. Eng. 2015, 141, 04014050. [Google Scholar] [CrossRef]
- Roehl, K.E.; Meggyes, T.; Simon, F.G.; Stewart, D.I. (Eds.) Long-Term Performance of Permeable Reactive Barriers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Suponik, T.; Lutyński, M. In-situ treatment of groundwater contaminated with underground coal gasification products. Arch. Min. Sci. 2013, 58, 1263–1278. [Google Scholar]
- Luo, P.; Bailey, E.H.; Mooney, S.J. Quantification of changes in zero valent iron morphology using X-ray computed tomography. J. Environ. Sci. 2013, 25, 2344–2351. [Google Scholar] [CrossRef]
- Noubactep, C.; Temgoua, E.; Rahman, M.A. Designing iron-amended biosand filters for decentralized safe drinking water provision. Clean—Soil Air Water 2012, 40, 798–807. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Karmakar, S.; Salama, H.; Gactha-Bandjun, N.; Btatkeu, K.B.D.; Noubactep, C. Optimising the design of Fe0-based filtration systems for water treatment: The suitability of porous iron composites. J. Appl. Solut. Chem. Model. 2013, 2, 165–177. [Google Scholar]
- Claytor, R.A.; Schueler, T.R. Design of Storfm Water Filtering Systems; Chesapeake Research Consortium and U.S. Environmental Protection Agency: Washington, DC, USA, 1996.
- Fronczyk, J.; Radziemska, M.; Mazur, Z. Copper removal from contaminated groundwater using natural and engineered limestone sand in permeable reactive barriers. Fresenius Environ. Bull. 2015, 24, 228–234. [Google Scholar]
- Hair, J.F.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Factorial Analysis. Multivariate Data Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1998. [Google Scholar]
- Sharma, S. Applied Multivariate Techniques, 1st ed.; John Wiley & Sons Inc.: New York, NY, USA, 1996. [Google Scholar]
- Suzuki, T.; Moribe, M.; Oyama, Y.; Niinae, M. Mechanism of nitrate reduction by zero-valent iron: Equilibrium and kinetics studies. Chem. Eng. J. 2012, 183, 271–277. [Google Scholar] [CrossRef]
- Hossain, M.; Mahmud, M.; Parvez, M.; Cho, H.M. Impact of current density, operating time and pH of textile wastewater treatment by electrocoagulation process. Environ. Eng. Res. 2013, 18, 157–161. [Google Scholar] [CrossRef]
- Suthersan, S.S.; Horst, J.; Schnobrich, M.; Welty, N.; McDonough, J. Remediation Engineering: Design Concepts, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Kuo, J. Practical Design Calculations for Groundwater and Soil Remediation, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Reardon, E.J. Zerovalent irons: Styles of corrosion and inorganic control on hydrogen pressure buildup. Environ. Sci. Technol. 2005, 39, 7311–7317. [Google Scholar] [CrossRef]
- Brennan, R.B. Chemical Amendment of Dairy Slurry for the Control of Phosphorus in Runoff from Grassland. Ph.D. Thesis, National University of Ireland Galway, Galway, Ireland, 2011. [Google Scholar]
- Kunz, A.; Mukhtar, S. Hydrophobic membrane technology for ammonia extraction from wastewaters. Eng. Agríc. 2016, 36, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Yong, R.N.; Mulligan, C.N. Natural Attenuation of Contaminants in Soil, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]

| Reference | Type of Infiltration System (Country) | Filter Materials | Contaminants Monitored |
|---|---|---|---|
| Zhou et al. [28] | Permeable treatment wall (USA) | Gabion filled with stone followed by peat filter | PAHs, heavy metals (Cu, Pb, Zn), TPH, TSS, TDS, TVS |
| Birch et al. [29] | Infiltration basin (Australia) | Zeolite and coarse, pure quartzitic sand mixture (1:6 by volume) | NO3, TKN, TP, fecal coliforms, trace metals (Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn), organochlorines, PCBs, PAHs, methylnaphthalene, oil, grease |
| Hatt et al. [33] | Biofiltration basins (Australia) | (1) 80% sandy loam, 10% vermiculite, 10% perlite (by volume) (2) 80% sandy loam, 10% compost, 10% hardwood mulch (by volume) | TSS, TP, filterable reactive phosphorus, TN, NH4, NOx, dissolved organic nitrogen, Cu, Cd, Pb, Zn, Mn |
| Fuerhacker et al. [6] | 3 chambers filled with filter materials (Austria) | Vermiculite, zeolite, activated carbon, gravel | PAHs, mineral oil, TOC, TSS, NH4, chlorides, total and dissolved heavy metals (Cd, Cu, Zn) |
| O’Reilly i in. [34] | Infiltration basin amended using biosorption activated media (USA) | Tire crumb, silt and clay and sand mixture (1.0:1.9:4.1 by volume) | Nutrients (NO3, NH4, PO4-P) |
| Hilliges et al. [24] | System containing: (1) gutter system, (2) grit chamber and (3) filter unit (Germany) | Filter unit (stage 3 of treatment system) consisted of lignite coke (activated lignite) | Heavy metals (Zn, Cu, Pb), Na, TOC |
| Flanagan et al. [35] | Linear biofilters and vegetative filter strip (France) | Silt loam topsoil and 0-4 mm limestone mixture (1:1.5 by volume) | TSS, OC, nutrients (N and P forms), trace metals and major elements (Al, As, Ba, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Pb, Si, Sr, Ti, V, Zn), C10-C40 hydrocarbons, PAHs, bisphenol-A, alkylphenols, phthalates |
| Vesting [36] | “FiltaPex” system consisting of a sedimentation chamber and a layer of filter mats (Germany) | Filter mats filled with mixture of activated granular Al2O3 and Fe2O3, aerated concrete and activated carbon | Ca, Na, Cl, P, Fe, Cu, Zn, hydrocarbons, PAHs, MTBE |
| Total Mass of Reactive Materials (kg) | System 1 | System 2 | System 3 |
|---|---|---|---|
| 75 kg of a Mixture of Reactive Materials in a Weight Proportion of 1:1:1 | |||
| ZVI/AC/SS * | ZVI/AC/Z ** | ZVI/AC/LS *** | |
| Treatment zone area (m2) | 0.13 | 0.13 | 0.13 |
| Layers of treatment system (starting from the top) |
|
|
|
| Runoff surface (m2) | 3 | 3 | 3 |
| System | Average k Value (±SD) (m/s) | Median k Value (m/s) | Initial k Value (m/s) | End k Value (m/s) |
|---|---|---|---|---|
| S1 | 1.71 × 10−4 (±0.00024) | 5.99 × 10−5 | 9.64 × 10−4 | 3.22 × 10−5 |
| S2 | 1.83 × 10−4 (±0.00029) | 5.99 × 10−5 | 1.09 × 10−3 | 3.27 × 10−5 |
| S3 | 1.59 × 10−4 (±0.00018) | 1.03 × 10−4 | 8.49 × 10−4 | 5.04 × 10−5 |
| Variable | Sum of Squares | df 1 | Mean Square | F 2 | Sig. |
|---|---|---|---|---|---|
| Cd | 0.000 | 2 | 0.000 | 0.088 | 0.916 |
| Ni | 0.001 | 2 | 0.001 | 1.136 | 0.326 |
| Zn | 0.012 | 2 | 0.006 | 6.084 | 0.003 |
| COD 3 | 292.673 | 2 | 146.336 | 69.085 | 0.000 |
| NH4 | 1.171 | 2 | 0.585 | 18.850 | 0.000 |
| NO3 | 38.857 | 2 | 19.429 | 23.781 | 0.000 |
| NO2 | 2.848 | 2 | 1.424 | 38.807 | 0.000 |
| Fe | 249.472 | 2 | 124.736 | 7.657 | 0.001 |
| SO4 | 15,495.763 | 2 | 7747.882 | 43.197 | 0.000 |
| pH | 200.915 | 2 | 100.458 | 55.316 | 0.000 |
| EC 4 | 1,355,197.007 | 2 | 677,598.504 | 1.760 | 0.178 |
| Eh 5 | 81,037.964 | 2 | 40,518.982 | 4.329 | 0.016 |
| T 6 | 8.188 | 2 | 4.094 | 0.151 | 0.860 |
| k 7 | 0.000 | 2 | 0.000 | 0.075 | 0.928 |
| Dependent Variable | (I) System | (J) System | Mean Difference (I-J) | SE | Sig. | Dependent Variable | (I) System | (J) System | Mean Difference (I-J) | SE | Sig. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | 1 | 2 | 0.0196 * | 0.008 | 0.018 | Fe | 1 | 2 | −6.74 * | 2.28 | 0.004 |
| 3 | 0.0269 * | 0.008 | 0.001 | 3 | 2.00 | 2.27 | 0.379 | ||||
| 2 | 1 | −0.0196 * | 0.008 | 0.018 | 2 | 1 | 6.74 * | 2.29 | 0.004 | ||
| 3 | 0.0072 | 0.008 | 0.371 | 3 | 8.75 * | 2.29 | 0.000 | ||||
| 3 | 1 | −0.026 * | 0.008 | 0.001 | 3 | 1 | −2.00 | 2.27 | 0.379 | ||
| 2 | −0.007 | 0.008 | 0.371 | 2 | −8.75 * | 2.29 | 0.000 | ||||
| COD | 1 | 2 | 4.07 * | 0.37 | 0.000 | SO4 | 1 | 2 | 32.20 * | 3.41 | 0.000 |
| 3 | 0.60 | 0.36 | 0.104 | 3 | 17.12 * | 3.38 | 0.000 | ||||
| 2 | 1 | −4.07 * | 0.37 | 0.000 | 2 | 1 | −32.20 * | 3.41 | 0.000 | ||
| 3 | −3.47 * | 0.37 | 0.000 | 3 | −15.07 * | 3.41 | 0.000 | ||||
| 3 | 1 | −0.607 | 0.36 | 0.104 | 3 | 1 | −17.12 * | 3.38 | 0.000 | ||
| 2 | 3.47 * | 0.37 | 0.000 | 2 | 15.07 * | 3.41 | 0.000 | ||||
| NH4 | 1 | 2 | −0.039 | 0.04 | 0.376 | pH | 1 | 2 | 3.39 * | 0.34 | 0.000 |
| 3 | 0.220 * | 0.04 | 0.000 | 3 | 0.73 * | 0.34 | 0.034 | ||||
| 2 | 1 | 0.039 | 0.04 | 0.376 | 2 | 1 | −3.39 * | 0.35 | 0.000 | ||
| 3 | 0.260 * | 0.04 | 0.000 | 3 | −2.65 * | 0.35 | 0.000 | ||||
| 3 | 1 | −0.220 * | 0.04 | 0.000 | 3 | 1 | −0.73 * | 0.34 | 0.034 | ||
| 2 | −0.260 * | 0.04 | 0.000 | 2 | 2.65 * | 0.35 | 0.000 | ||||
| NO3 | 1 | 2 | 1.41 * | 0.23 | 0.000 | Eh | 1 | 2 | 71.84 * | 24.91 | 0.005 |
| 3 | 0.098 | 0.23 | 0.671 | 3 | 28.48 | 24.70 | 0.252 | ||||
| 2 | 1 | −1.41 * | 0.23 | 0.000 | 2 | 1 | −71.84 * | 24.91 | 0.005 | ||
| 3 | −1.32 * | 0.23 | 0.000 | 3 | −43.35 | 24.91 | 0.085 | ||||
| 3 | 1 | −0.098 | 0.23 | 0.671 | 3 | 1 | −28.48 | 24.70 | 0.252 | ||
| 2 | 1.320 * | 0.23 | 0.000 | 2 | 43.35 | 24.91 | 0.085 | ||||
| NO2 | 1 | 2 | 0.311 * | 0.04 | 0.000 | T | 1 | 2 | −0.043 | 1.34 | 0.974 |
| 3 | −0.100 * | 0.04 | 0.044 | 3 | 0.58 | 1.32 | 0.665 | ||||
| 2 | 1 | −0.311 * | 0.04 | 0.000 | 2 | 1 | 0.04 | 1.34 | 0.974 | ||
| 3 | −0.411 * | 0.04 | 0.000 | 3 | 0.62 | 1.34 | 0.644 | ||||
| 3 | 1 | 0.100 * | 0.04 | 0.044 | 3 | 1 | −0.57 | 1.32 | 0.665 | ||
| 2 | 0.411 * | 0.04 | 0.000 | 2 | −0.62 | 1.34 | 0.644 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fronczyk, J.; Markowska-Lech, K.; Bilgin, A. Treatment Assessment of Road Runoff Water in Zones filled with ZVI, Activated Carbon and Mineral Materials. Sustainability 2020, 12, 873. https://doi.org/10.3390/su12030873
Fronczyk J, Markowska-Lech K, Bilgin A. Treatment Assessment of Road Runoff Water in Zones filled with ZVI, Activated Carbon and Mineral Materials. Sustainability. 2020; 12(3):873. https://doi.org/10.3390/su12030873
Chicago/Turabian StyleFronczyk, Joanna, Katarzyna Markowska-Lech, and Ayla Bilgin. 2020. "Treatment Assessment of Road Runoff Water in Zones filled with ZVI, Activated Carbon and Mineral Materials" Sustainability 12, no. 3: 873. https://doi.org/10.3390/su12030873





