Tsetse Invasion as an Emerging Threat to Socioecological Resilience of Pastoral Communities in Karamoja, Uganda
Abstract
:1. Introduction
2. The Study Area
3. Research Methodology and Methods
3.1. Tsetse Entomological Survey
3.2. Participatory Assessment of Tsetse Prevalence in Karamoja
3.3. Cross-Sectional Survey
4. Research Analysis Implementation
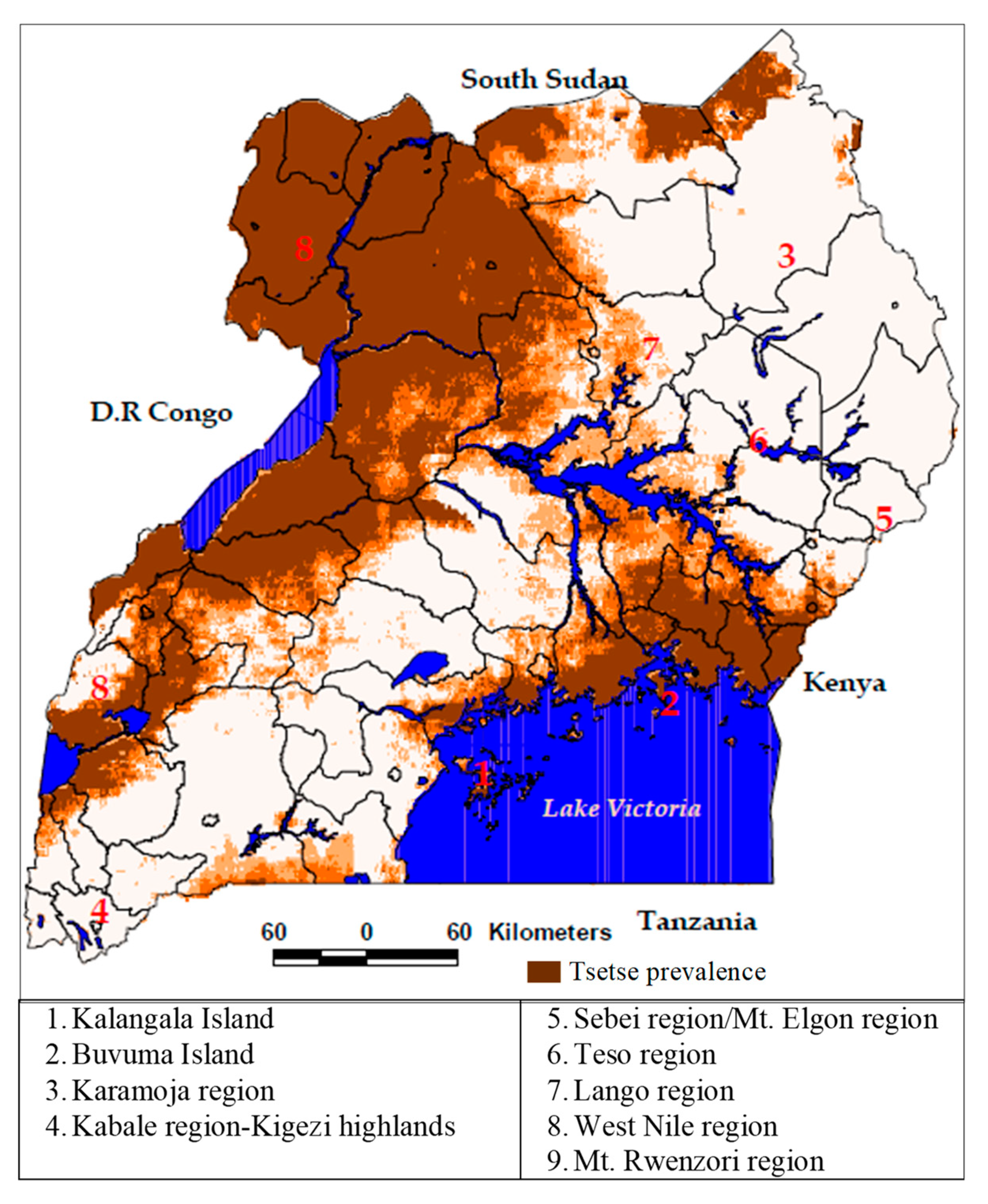
4.1. Tsetse Prevalence in Karamoja
4.2. Perceived Effect of Tsetse Invasion on Socioecological Resilience in Karamoja
- = Adaptive capacity index
- = Frequency of households that rate adaptation capacity as having no importance
- = Frequency of households that rate adaptation capacity as having low importance
- = Frequency of households that rate adaptation strategy as having moderate importance
- = Frequency of households that rate adaptation strategy as having high importance.
- = Absorptive capacity index
- = Frequency of households that rate absorption capacity as having no importance
- = Frequency of households that rate absorption capacity as having low importance
- = Frequency of households that rate absorption capacity as having moderate importance
- = Frequency of households that rate absorption capacity as having high importance.
- = Transformative capacity index
- = Frequency of households that rate transformative capacity as having no importance
- = Frequency of households that rate transformative capacity as having low importance
- = Frequency of households that rate transformative capacity as having moderate importance
- = Frequency of households that rate transformative capacity as having high importance.
5. Results
5.1. Tsetse Spatial Distribution and Prevalence
5.2. Historical Patterns and Dispersal Routes of Tsetse in the Karamoja Sub-Region
5.3. Perceived Socioecological Effects of Tsetse in Karamoja
6. Discussion
6.1. Tsetse Prevalence and Distribution Patterns
6.2. Socioecological Effects of Tsetse Invasion in Karamoja
6.3. Implications of the Assessment on Socioecological Practices of Tsetse Control
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cecchi, G.; Mattioli, R.C.; Slingenbergh, J.; De La Rocque, S. Land cover and tsetse fly distributions in sub-Saharan Africa. Med. Vet. Entomol. 2008, 22, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Robinson, T.P. Tsetse distribution. In The Trypanosomiases; Maudlin, I., Holmes, P.H., Miles, M.A., Eds.; CABI: Wallingford, UK, 2004. [Google Scholar]
- Saarman, N.; Burak, M.; Opiro, R.; Hyseni, C.; Echodu, R.; Dion, K.; Opiyo, E.A.; Dunn, A.W.; Amatulli, G.; Aksoy, S.; et al. A spatial genetics approach to inform vector control of tsetse flies (Glossina fuscipes fuscipes) in Northern Uganda. Ecol. Evol. 2018, 8, 5336–5354. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Malele, I.; Abd-Alla, A.M.; Njiokou, F. Blood feeding tsetse flies as hosts and vectors of mammals-pre-adapted African Trypanosoma: Current and expected research directions. BMC Microbiol. 2018, 18, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Echodu, R.; Sistrom, M.; Bateta, R.; Murilla, G.; Okedi, L.; Aksoy, S.; Enyioha, C.; Enyaru, J.; Opiyo, E.; Gibson, W.; et al. Genetic diversity and population structure of Trypanosoma brucei in Uganda: Implications for the epidemiology of sleeping sickness and Nagana. PLoS Negl. Trop. Dis. 2015, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Stefaniak, J.; Smuszkiewicz, P.; Van Esbroeck, M.; Geysen, D.; Clerinx, J. Outcome of acute East African trypanosomiasis in a Polish traveller treated with pentamidine. BMC Infect. Dis. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mwanakasale, V.; Songolo, P.; Babaniyi, O.; Simarro, P. Clinical presentation of human African trypanosomiasis in Zambia is linked to the existence of strains of Trypanosoma brucei rhodesiense with varied virulence: Two case reports. J. Med. Case Rep. 2014, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Steverding, D. The history of African trypanosomiasis. Parasites Vectors 2008, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Muhanguzi, D.; Mugenyi, A.; Bigirwa, G.; Kamusiime, M.; Kitibwa, A.; Akurut, G.G.; Ochwo, S.; Amanyire, W.; Okech, S.G.; Hattendorf, J.; et al. African animal trypanosomiasis as a constraint to livestock health and production in Karamoja region: A detailed qualitative and quantitative assessment. BMC Veter. Res. 2017, 13, 355. [Google Scholar] [CrossRef] [Green Version]
- Mugenyi, A.W. Spatial Distribution of Tsetse (Diptera: Glossinidae) within the Trypanosoma brucei rhodesiense focus of Uganda. Ph.D. Thesis, The University of Edinburg, Edinburg, UK, 2015; p. 250. Available online: http://coctu.go.ug/T&T%20draft%20Policy_2014.pdf (accessed on 10 March 2019).
- Shaw, A.P.M.; Torr, S.J.; Waiswa, C.; Cecchi, G.; Wint, G.R.W.; Mattioli, R.C.; Robinson, T.P. Estimating the costs of tsetse control options: An example for Uganda. Prev. Veter. Med. 2013, 110, 290–303. [Google Scholar] [CrossRef]
- Kame-Ngasse, G.I.; Njiokou, F.; Melachio-Tanekou, T.T.; Farikou, O.; Simo, G.; Geiger, A. Prevalence of symbionts and trypanosome infections in tsetse flies of two villages of the “Faro and Déo” division of the Adamawa Region of Cameroon. BMC Microbiol. 2018, 18, 83–91. [Google Scholar] [CrossRef]
- Ilemobade, A.A. Tsetse and trypanosomosis in Africa: The challenges, the opportunities. Onderstepoort J. Veter. Res. 2009, 76, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Krätli, S.; Huelsebusch, C.; Brooks, S.; Kaufmann, B. Pastoralism: A critical asset for food security under global climate change. Anim. Front. 2013, 3, 42–50. [Google Scholar] [CrossRef]
- Chitanga, S.; Marcotty, T.; Namangala, B.; Van den Bossche, P.; Van Den Abbeele, J.; Delespaux, V. High prevalence of drug resistance in animal trypanosomes without a history of drug exposure. PLoS Negl. Trop. Dis. 2011, 5, e1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easter, T.S.; Killion, A.K.; Carter, N.H. Climate change, cattle, and the challenge of sustainability in a telecoupled system in Africa. Ecol. Soc. 2018, 23, 10. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, B.A.; Echaubard, P.; de Garine-Wichatitsky, M.; Ramirez, B. Vector-borne disease and climate change adaptation in African dryland social-ecological systems. Infect. Dis. Poverty 2019, 8, 36. [Google Scholar] [CrossRef] [Green Version]
- Barrett, M.P. Veterinary link to drug resistance in human African trypanosomiasis? Lancet 2001, 358, 603–604. [Google Scholar] [CrossRef]
- Ministry of Agriculture Animal Industry and Fisheries. National Policy for the Eradication of Tsetse Flies and Elimination of Trypanosomosis; Entebbe, Uganda, 2014; p. 14. Available online: http://coctu.go.ug/T&T%20draft%20Policy_2014.pdf (accessed on 18 August 2019).
- Franco, J.R.; Simarro, P.P.; Diarra, A.; Jannin, J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014, 6, 257. [Google Scholar] [CrossRef]
- Fèvre, E.M.; Coleman, P.G.; Welburn, S.C.; Maudlin, I. Reanalyzing the 1900–1920 sleeping sickness epidemic in Uganda. Emerg. Infect. Dis. 2004, 10, 567–573. [Google Scholar] [CrossRef] [Green Version]
- Fèvre, E.M.; Coleman, P.G.; Odiit, M.; Magona, J.W.; Welburn, S.C.; Woolhouse, M.E.J. The origins of a new Trypanosoma brucei rhodesiense sleeping sickness outbreak in eastern Uganda. Lancet 2001, 358, 625–628. [Google Scholar] [CrossRef]
- Thumbi, S.M.; Jung’a, J.O.; Mosi, R.O.; McOdimba, F.A. Spatial distribution of African animal trypanosomiasis in Suba and Teso districts in Western Kenya. BMC Res. Notes 2010, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Rutto, J.J.; Karuga, J.W. Temporal and spatial epidemiology of sleeping sickness and use of geographical information system (GIS) in Kenya. J. Vector Borne Dis. 2009, 46, 18. [Google Scholar] [PubMed]
- Berrang-Ford, L.; Odiit, M.; Maiso, F.; Waltner-Toews, D.; McDermott, J. Sleeping sickness in Uganda: Revisiting current and historical distributions. Afr. Health Sci. 2006, 6, 223–231. [Google Scholar] [CrossRef]
- Wanyama, O. Tsetse Flies Trigger Migration in Kaabong. URN. 2014. Available online: https://ugandaradionetwork.com/story/tsetse-flies-trigger-migration-in-kaabong (accessed on 18 August 2019).
- Garmestani, A.; Craig, R.K.; Gilissen, H.K.; McDonald, J.; Soininen, N.; van Doorn-Hoekveld, W.; van Rijswick, H.F. The role of social-ecological resilience in coastal zone management: A comparative law approach to three coastal nations. Front. Ecol. Evol. 2019, 7, 410. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.D.; Williams, B.K. Resilience and resource management. Environ. Manag. 2015, 56, 1416–1427. [Google Scholar] [CrossRef] [Green Version]
- Scoones, I.; Dzingirai, V.; Anderson, N.; MacLeod, E.; Mangwanya, L.; Matawa, F.; Murwira, A.; Nyakupinda, L.; Shereni, W.; Welburn, S.C. People, Patches, and Parasites: The Case of Trypanosomiasis in Zimbabwe. Hum. Ecol. 2017, 45, 643–654. [Google Scholar] [CrossRef] [Green Version]
- Wamwiri, F.N.; Changasi, R.E. Tsetse flies (Glossina) as vectors of human African trypanosomiasis: A review. Biomed. Res. Int. 2016, 8, 6201350. [Google Scholar] [CrossRef] [Green Version]
- Tchouomene-Labou, J.; Nana-Djeunga, H.; Simo, G.; Njitchouang, G.R.; Cuny, G.; Asonganyi, T.; Njiokou, F. Spatial and temporal variations relevant to tsetse control in the Bipindi focus of southern Cameroon. Parasites Vectors 2013, 6, 193. [Google Scholar] [CrossRef] [Green Version]
- Wacher, T.J.; Milligan, P.J.M.; Rawlings, P.; Snow, W.F. Tsetse–trypanosomiasis challenge to village N’Dama cattle in The Gambia: Field assessments of spatial and temporal patterns of tsetse–cattle contact and the risk of trypanosomiasis infection. Parasitology 1994, 109, 149–162. [Google Scholar] [CrossRef]
- Schneider, D.I.; Saarman, N.; Onyango, M.G.; Hyseni, C.; Opiro, R.; Echodu, R.; O’Neill, M.; Bloch, D.; Vigneron, A.; Johnson, T.J.; et al. Spatio-temporal distribution of Spiroplasma infections in the tsetse fly (Glossina fuscipes fuscipes) in northern Uganda. PLoS Negl. Trop. Dis. 2019, 13, e0007340. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, A.A.; Basu, N.; Kahn, L.H.; Morin, X.K.; Echaubard, P.; Wilcox, B.A.; Beasley, V.R. Transdisciplinary and social-ecological health frameworks—Novel approaches to emerging parasitic and vector-borne diseases. Parasite Epidemiol. Control 2019, 4, e00084. [Google Scholar] [CrossRef]
- Colwell, D.D.; Dantas-Torres, F.; Otranto, D. Vector-borne parasitic zoonoses: Emerging scenarios and new perspectives. Veter. Parasitol. 2011, 182, 14–21. [Google Scholar] [CrossRef]
- De Vos, A.; Cumming, G.S.; Cumming, D.H.; Ament, J.M.; Baum, J.; Clements, H.S.; Grewar, J.D.; Maciejewski, K.; Moore, C. Pathogens, disease, and the social-ecological resilience of protected areas. Ecol. Soc. 2016, 21, 20. [Google Scholar] [CrossRef] [Green Version]
- Egeru, A.; Wasonga, O.; MacOpiyo, L.; Mburu, J.; Tabuti, J.R.; Majaliwa, M.G. Piospheric influence on forage species composition and abundance in semi-arid Karamoja sub-region, Uganda. Pastoralism 2015, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Egeru, A.; Wasonga, O.; Kyagulanyi, J.; Majaliwa, G.M.; MacOpiyo, L.; Mburu, J. Spatio-temporal dynamics of forage and land cover changes in Karamoja sub-region, Uganda. Pastoralism 2014, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Agriculture, Animal Industry and Fisheries and Uganda Bureau of Statistics. The National Livestock Census: A Summary Report of the National Livestock Census; Kampala, Uganda, 2009; p. 273. Available online: https://www.ubos.org/wp-content/uploads/publications/05_2019THE_NATIONAL_LIVESTOCK_CENSUS_REPORT_2008.pdf (accessed on 9 February 2020).
- Uganda Bureau of Statistics. National Population and Housing Census 2014—Main Report; Uganda Bureau of Statistics: Kampala, Uganda, 2016; p. 104. Available online: https://unstats.un.org/unsd/demographic/sources/census/wphc/Uganda/UGA-2016–05-23.pdf (accessed on 9 February 2020).
- Uganda Investment Authority. Karamoja Investment Profile 2016; Uganda Investment Authority: Kampala, Uganda, 2016; p. 32. Available online: https://www.ugandainvest.go.ug/wp-content/uploads/2016/04/uia-Karamoja-profile.pdf (accessed on 12 March 2017).
- Williams, B.; Dransfield, R.; Brightwell, R. Monitoring tsetse populations. The intrinsic variability of trap catches of Glossina pallidipes at Nguruman, Kenya. Med. Vet. Entomol. 1990, 4, 167–179. [Google Scholar] [CrossRef]
- Pollock, J.N. Vol.1: Tsetse Biology, Systematics and Distribution, Techniques. In Training Manual for Tsetse Control Personnel; FAO: Rome, Italy, 1982; p. 274. Available online: http://www.fao.org/3/a-p5178e.pdf (accessed on 9 February 2020).
- Uganda Bureau of Statistics (UBOS). Uganda National Household Survey 2012/2013; UBOS: Kampala, Uganda, 2014; p. 230. Available online: https://www.ubos.org/wp-content/uploads/publications/04_20182012_13_UNHS_Final_Report.pdf (accessed on 9 February 2020).
- Egeru, A. Climate Risk Management Climate risk management information, sources and responses in a pastoral region in East Africa. Clim. Risk Manag. 2016, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Galiè, A.; Teufel, N.; Girard, A.W.; Baltenweck, I.; Dominguez-Salas, P.; Price, M.J.; Jones, R.; Lukuyu, B.; Korir, L.; Raskind, I.; et al. Women’s empowerment, food security and nutrition of pastoral communities in Tanzania. Glob. Food Secur. 2019, 23, 125–134. [Google Scholar] [CrossRef]
- Holling, C.S. Resilience and stability of ecological systems. Ann. Rev. Ecol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Bollettino, V.; Alcayna, T.; Dy, P.; Vinck, P. Introduction to socio-ecological resilience. In Oxford Research Encyclopedia of Natural Hazard Science; Oxford University Press: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Carpenter, S.; Walker, B.; Anderies, J.M.; Abel, N. From metaphor to measurement: Resilience of what to what? Ecosystems 2001, 4, 765–781. [Google Scholar] [CrossRef]
- Heijman, W.; Hagelaar, G.; van der Heide, M. Rural resilience as a new development concept. In EU Bioeconomy Economics and Policies; Palgrave Macmillan: London, UK, 2019; Volume 2, pp. 195–211. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Carpenter, S.R.; Kofinas, G.P.; Folke, C.; Abel, N.; Clark, W.C.; Olsson, P.; Smith, D.M.S.; Walker, B.; Young, O.R.; et al. Ecosystem stewardship: Sustainability strategies for a rapidly changing planet. Trends Ecol. Evol. 2010, 25, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Folke, C. Resilience: The emergence of a perspective for social–ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Walker, B.; Carpenter, S.; Anderies, J.; Abel, N.; Cumming, G.; Janssen, M.; Lebel, L.; Norberg, J.; Peterson, G.D.; Pritchard, R. Resilience management in social-ecological systems: A working hypothesis for a participatory approach. Conserv. Ecol. 2002, 6, 14. Available online: http://www.consecol.org/vol6/iss1/art14 (accessed on 19 February 2020).
- Leslie, P.; McCabe, J.T.; Bollig, M.; Greiner, C.; Fratkin, E.; Galaty, J.G.; Homewood, K.; Lansing, S.; Lu, F.; Moran, E.F.; et al. Response diversity and resilience in social-ecological systems. Curr. Anthropol. 2013, 54, 114–143. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.W.; Berkes, F. Applying resilience thinking to questions of policy for pastoralist systems: Lessons from the Gabra of northern Kenya. Hum. Ecol. 2010, 38, 335–350. [Google Scholar] [CrossRef]
- Cumming, G.S.; Barnes, G.; Perz, S.; Schmink, M.; Sieving, K.E.; Southworth, J.; Binford, M.; Holt, R.D.; Stickler, C.; Van Holt, T. An exploratory framework for the empirical measurement of resilience. Ecosystems 2005, 8, 975–987. [Google Scholar] [CrossRef]
- Cutter, S.L.; Barnes, L.; Berry, M.; Burton, C.; Evans, E.; Tate, E.; Webb, J. A place-based model for understanding community resilience to natural disasters. Glob. Environ. Chang. 2008, 18, 598–606. [Google Scholar] [CrossRef]
- Béné, C.; Wood, R.G.; Newsham, A.; Davies, M. Resilience: New utopia or new tyranny? Reflection about the potentials and limits of the concept of resilience in relation to vulnerability reduction programmes. IDS Work. Pap. 2012, 2012, 1–61. [Google Scholar] [CrossRef]
- Opiyo, F.; Mureithi, S.; Manzano, J.N.A.; Pitaud, T. An Indicator Framework for Measuring Pastoralists’ Resilience to Drought in the Horn of Africa. Sci. Environ. 2018, 32, 52–68. [Google Scholar]
- Adger, W.N. Vulnerability. Glob. Environ. Chang. 2006, 16, 268–281. [Google Scholar] [CrossRef]
- Walker, B.; Holling, C.S.; Carpenter, S.R.; Kinzig, A. Resilience, Adaptability and Transformability in Social-Ecological Systems. Available online: https://www.jstor.org/stable/26267673 (accessed on 18 February 2020).
- Uddin, M.N.; Bokelmann, W.; Entsminger, J.S. Factors affecting farmers’ adaptation strategies to environmental degradation and climate change effects: A farm level study in Bangladesh. Climate 2014, 2, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Wint, W. Kilometre resolution tsetse fly distribution maps for the Lake Victoria basin and West Africa. In Report to the Joint Food and Agriculture Organization of the United Nations/International Atomic Energy Agency Programme; Food and Agriculture Organization: Rome, Italy, 2001. [Google Scholar]
- Munang’andu, H.M.; Siamudaala, V.; Munyeme, M.; Nalubamba, K.S. A review of ecological factors associated with the epidemiology of wildlife trypanosomiasis in the Luangwa and Zambezi valley ecosystems of Zambia. Interdiscip. Perspect. Infect. Dis. 2012, 372523, 13. [Google Scholar] [CrossRef]
- Messina, J.P.; Moore, N.J.; DeVisser, M.H.; McCord, P.F.; Walker, E.D. Climate change and risk projection: Dynamic spatial models of tsetse and African trypanosomiasis in Kenya. Ann. Assoc. Am. Geogr. 2012, 102, 1038–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, N.; Messina, J. A landscape and climate data logistic model of tsetse distribution in Kenya. PLoS ONE 2010, 5, e11809. [Google Scholar] [CrossRef]
- Ngonyoka, A.; Gwakisa, P.S.; Estes, A.B.; Nnko, H.J.; Hudson, P.J.; Cattadori, I.M. Variation of tsetse fly abundance in relation to habitat and host presence in the Maasai Steppe, Tanzania. J. Vector Ecol. 2017, 42, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Mugerwa, S.; Kayiwa, S.; Egeru, A. Status of livestock water sources in Karamoja sub-region, Uganda. Res. Environ. 2014, 4, 58–66. [Google Scholar] [CrossRef]
- Meyer, A.; Holt, H.R.; Selby, R.; Guitian, J. Past and ongoing tsetse and animal trypanosomiasis control operations in five African countries: A systematic review. PLoS Negl. Trop. Dis. 2016, 10, e0005247. [Google Scholar] [CrossRef] [Green Version]
- Loewenberg, S. Breaking the cycle: Drought and hunger in Kenya. Lancet 2014, 383, 1025–1028. [Google Scholar] [CrossRef]
- Akwango, D.; Obaa, B.B.; Turyahabwe, N.; Baguma, Y.; Egeru, A. Effect of drought early warning system on household food security in Karamoja subregion, Uganda. Agric. Food Secur. 2017, 6, 43. [Google Scholar] [CrossRef]
- Abdalla, M.; Hastings, A.; Chadwick, D.R.; Jones, D.L.; Evans, C.D.; Jones, M.B.; Rees, R.M.; Smith, P. Critical review of the impacts of grazing intensity on soil organic carbon storage and other soil quality indicators in extensively managed grasslands. Agric. Ecosyst. Environ. 2018, 253, 62–81. [Google Scholar] [CrossRef] [Green Version]
- Kgosikoma, O.E.; Mogotsi, K. Understanding the causes of bush encroachment in Africa: The key to effective management of savanna grasslands. Trop. Grassl.-Forrajes Trop. 2013, 1, 215–219. [Google Scholar] [CrossRef]
- Alinovi, L.; D’errico, M.; Mane, E.; Romano, D. Livelihoods Strategies and Household Resilience to Food Insecurity: An Empirical Analysis to Kenya; European Report on Development: Rome, Italy, 2010; pp. 1–52. Available online: http://www.technicalconsortium.org/wp-content/uploads/2014/05/Livelihoods-Strategies_Household-Res.pdf (accessed on 16 December 2019).
- Craft, T. Enabling Resilience for Pastoral Communities in Ethiopia. Prime Impact and Results Report; Mercy Corps: Portland, OR, USA, 2019; p. 16. Available online: https://www.mercycorps.org/sites/default/files/mc_prime_impact_report_FINAL_March2019.pdf (accessed on 16 December 2019).
- Fenta, M.; Jordaan, A.; Melka, Y. Vulnerability of Southern Afar pastoralists to climate variability and change, Ethiopia. Jàmbá J. Dis. Risk Stud. 2019, 11, 1–8. [Google Scholar] [CrossRef]
- Muricho, D.N.; Otieno, D.J.; Oluoch-Kosura, W.; Jirström, M. Building pastoralists’ resilience to shocks for sustainable disaster risk mitigation: Lessons from West Pokot County, Kenya. Int. J. Dis. Risk Reduct. 2019, 34, 429–435. [Google Scholar] [CrossRef]
- Little, P.D.; McPeak, J.G. Resilience and Pastoralism in Africa south of the Sahara, with a Particular Focus on the Horn of Africa and the Sahel, West Africa. Available online: http://www.vivo.colostate.edu/lccrsp/reports/2020resilienceconfpaper09.pdf (accessed on 10 February 2020).
- Birhanu, Z.; Ambelu, A.; Berhanu, N.; Tesfaye, A.; Woldemichael, K. Understanding resilience dimensions and adaptive strategies to the impact of recurrent droughts in Borana Zone, Oromia Region, Ethiopia: A grounded theory approach. Int. J. Environ. Res. Public Health 2017, 14, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekuyie, M.; Jordaan, A.; Melka, Y. Understanding resilience of pastoralists to climate change and variability in the Southern Afar Region, Ethiopia. Clim. Risk Manag. 2018, 20, 64–77. [Google Scholar] [CrossRef]
- Ambelu, A.; Birhanu, Z.; Tesfaye, A.; Berhanu, N.; Muhumuza, C.; Kassahun, W.; Daba, T.; Woldemichael, K. Intervention pathways towards improving the resilience of pastoralists: A study from Borana communities, southern Ethiopia. Weather Clim. Extrem. 2017, 17, 7–16. [Google Scholar] [CrossRef]
- Selby, R.; Bardosh, K.; Picozzi, K.; Waiswa, C.; Welburn, S.C. Cattle movements and trypanosomes: Restocking efforts and the spread of Trypanosoma brucei rhodesiense sleeping sickness in post-conflict Uganda. Parasites Vectors 2013, 6, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bardosh, K.L.; Ryan, S.J.; Ebi, K.; Welburn, S.; Singer, B. Addressing vulnerability, building resilience: Community-based adaptation to vector-borne diseases in the context of global change. Infect. Dis. Poverty 2017, 6, 166. [Google Scholar] [CrossRef] [Green Version]
- Dicko, A.H.; Percoma, L.; Sow, A.; Adam, Y.; Mahama, C.; Sidibé, I.; Dayo, G.K.; Thévenon, S.; Fonta, W.; Sanfo, S.; et al. A spatio-temporal model of African animal trypanosomosis risk. PLoS Negl. Trop. Dis. 2015, 9, e0003921. [Google Scholar] [CrossRef] [Green Version]
- Dick, M.; Rous, A.M.; Nguyen, V.M.; Cooke, S.J. Necessary but challenging: Multiple disciplinary approaches to solving conservation problems. Facets 2016, 1, 67–82. [Google Scholar] [CrossRef]
- Paolisso, M.; Prell, C.; Johnson, K.J.; Needelman, B.; Khan, I.M.; Hubacek, K. Enhancing socio-ecological resilience in coastal regions through collaborative science, knowledge exchange and social networks: A case study of the Deal Island Peninsula, USA. Socio-Ecol. Pract. Res. 2019, 1, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Assmuth, T.; Chen, X.; Degeling, C.; Haahtela, T.; Irvine, K.N.; Keune, H.; Kock, R.; Rantala, S.; Rüegg, S.; Vikström, S. Integrative concepts and practices of health in transdisciplinary social ecology. Socio-Ecol. Pract. Res. 2019, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mwaseba, D.L.; Kigoda, K.J. Knowledge, attitude, and practices about tsetse control among communities neighbouring Serengeti National Park, Tanzania. Heliyon 2017, 3, e00324. [Google Scholar] [CrossRef] [Green Version]
- Percoma, L.; Sow, A.; Pagabeleguem, S.; Dicko, A.H.; Serdebéogo, O.; Ouédraogo, M.; Rayaissé, J.B.; Bouyer, J.; Belem, A.M.; Sidibé, I. Impact of an integrated control campaign on tsetse populations in Burkina Faso. Parasites Vectors 2018, 11, 270. [Google Scholar] [CrossRef]
- Grant, C. Politics of Knowledge: Whose Knowledge Matters in Trypanosomiasis Policy Making in Zambia? STEPS Centre: Brighton, UK, 2014; p. 35. Available online: https://opendocs.ids.ac.uk/opendocs/bitstream/handle/20.500.12413/6701/Tsetse-wp2.pdf?sequence=1&isAllowed=y (accessed on 16 December 2019).
- Vale, G.A.; Hall, D.R.; Chamisa, A.; Torr, S.J. Towards an early warning system for Rhodesian sleeping sickness in savannah areas: Man-like traps for tsetse flies. PLoS Negl. Trop. Dis. 2012, 6, e1978. [Google Scholar] [CrossRef] [Green Version]
- Sindato, C.; Malele, I.I.; Mwalimu, C.; Nyingilili, H.S.; Kaboya, S.; Kombe, E. Seasonal epidemiological variation of human African trypanosomiasis in Babati District, Tanzania. Tanzania J. Health Res. 2007, 9, 136–139. Available online: https://www.ajol.info/index.php/thrb/article/viewFile/14317/2681 (accessed on 18 December 2019). [CrossRef]
- Nnko, H.J.; Ngonyoka, A.; Salekwa, L.; Estes, A.B.; Hudson, P.J.; Gwakisa, P.S.; Cattadori, I.M. Seasonal variation of tsetse fly species abundance and prevalence of trypanosomes in the Maasai Steppe, Tanzania. J. Vector Ecol. 2017, 42, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukaw, Y.S.; Abdelrahman, M.M.; Mohammed, Y.O.; Ochi, E.B.; Elrayah, I.E. Factors influencing seasonal abundance of Glossina fuscipes fuscipes (Glossina: Glossinidae) in Kajo-Keji County, South Sudan. Curr. Res. J. Biol. Sci. 2014, 6, 222–228. [Google Scholar] [CrossRef]
- Rutto, J.J.; Osano, O.; Thuranira, E.G.; Kurgat, R.K.; Odenyo, V.A.O. Socio-economic and cultural determinants of human African trypanosomiasis at the Kenya–Uganda transboundary. PLoS Negl. Trop. Dis. 2013, 7, e2186. [Google Scholar] [CrossRef] [Green Version]
- Shereni, W.; Anderson, N.E.; Nyakupinda, L.; Cecchi, G. Spatial distribution and trypanosome infection of tsetse flies in the sleeping sickness focus of Zimbabwe in Hurungwe District. Parasites Vectors 2016, 9, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]











| Altitude | Kaabong | Kotido | SE | P-value |
|---|---|---|---|---|
| Parameter | ||||
| Tsetse Males | 20.698 a | 1.033 b | 4.12903 | 0.0101 |
| Tsetse Females | 23.893 a | 3.1 b | 4.13741 | 0.0008 |
| Tsetse Total | 44.59 a | 4.13 b | 8.13339 | 0.0026 |
| Tsetse FTD | 14.864 a | 1.378 b | 2.71113 | 0.0026 |
| Colonial Period through 1950s | Post-Colonial Period 1962–Early1970s | 1970s–Early 2000s | Mid-2000s to Post–Disarmament Period |
|---|---|---|---|
 |  |  |  |
| Colonial administration was established in Karamoja with several effects including the well-established subdivision of cultural groupings in Karamoja. Tsetse was recognised as a problem in the region and a control camp was established at Lolelia. A camp was also located on the fertile lands of Lokapir and Naperetom. This group used to kill wildlife, including elephants, as trophies. This was perhaps the worst time for wildlife and livestock. Cattle were herded and confined to the east of Karamoja. | Post-colonial government recognised the tsetse menace in the sub-region. A tsetse control officer called Ayela had been posted to Karamoja who established a camp in Kotido and parts of Kaabong closer to Kidepo Valley. Control mechanisms, including a gazette of communities in one location/camps and mobilising able-bodied men to cut and burn all bushes and trees. Much vegetation was cut, transforming woodlands into open grasslands. Evidence of these remnant tree trunks can be observed at the Kidepo Valley. In addition, herbicides that killed everything including bees (non-selective) were used in the eradication efforts. At the fall of the Obote I government in the early 1970s, Karamojong acquired guns after overrunning the Moroto barracks. Light weapons made entry into the life of the pastoral community in this region. Ayela was killed by Amin’s soldiers, and this marked the end of the tsetse eradication efforts in the region. | Ayela, a re-known tsetse eradication officer, had been killed in the early 1970s. The Karamojong were now armed, and the rapid proliferation of light weapons became more prominent in the sub-region. Governance systems collapsed with regards to public authority and administration. Intense livestock rustling and wildlife poaching became prominent. Wildlife were confined into the Kidepo National Park, which became more like a refuge centre. This period also represented a period of obscurity of the Karamoja sub-region from government investment, and Karamoja was viewed as a problem to the neighbouring communities in Teso, Lango, Sebei and Acholi. | In the mid-2000s approximately 2003, a disarmament exercise by the Government of Uganda was initiated. First, there was a peaceful disarmament involving voluntary declaration and return of guns to the Uganda People’s Defense Forces (UPDF). Failure to achieve success and continued counter raids, forced the Government of Uganda to launch a forceful disarmament exercise. Upon the disarmament, the UPDF then provided security to communities and herders. This marked the return of normalcy and ‘peace’ in the region. The wildlife that had hitherto been confined to Kidepo Valley National Park slowly started to return to graze in the outskirts of the National Park, especially during the dry season. With the return of the wildlife, the reinvasion of tsetse was first reported in Kaabong and Kotido. As the wildlife continues to routinely return to the grazing and waterholes outside the park, the prevalence of tsetse is also intensifying. |
| Livestock | Livestock Owned | Livestock Sold | ||
|---|---|---|---|---|
| Affected by Tsetse (N = 76) | Not Affected by Tsetse (N = 75) | Affected by Tsetse (N = 76) | Not Affected by Tsetse (N = 75) | |
| Cattle | 5.8 ± 5.5 | 5.8 ± 7.7 | 1.3 ± 1.9 | 1.1 ± 1.6 |
| Sheep | 20.6 ± 84.5 | 6.8 ± 12.7 | 2.4 ± 4.5 | 1.1 ± 2.4 |
| Donkeys | 0.8 ± 1.9 | 0.7 ± 1.3 | 0.1 ± 0.5 | 0.1 ± 0.9 |
| Goats | 11.3 ± 21.3 | 8.3 ± 9.6 | 2.7 ± 3.4 | 1.7 ± 2.5 |
| Absorptive Capacity | High | Medium | Low | No | ABCI | Rank |
|---|---|---|---|---|---|---|
| Diversified economic activities | 14 | 54 | 7 | 1 | 157 | 1 |
| Access to financial services | 13 | 29 | 18 | 16 | 115 | 2 |
| Livestock off-take | 2 | 35 | 26 | 13 | 102 | 3.5 |
| Bush burning | 11 | 23 | 23 | 18 | 102 | 3.5 |
| Seeking loan facility | 4 | 35 | 17 | 20 | 99 | 5 |
| Adaptive Capacity | High | Medium | Low | No | ADCI | Rank |
|---|---|---|---|---|---|---|
| Access to drugs | 59 | 13 | 4 | 0 | 207 | 1 |
| Deployment of paravets (CAHWs) | 52 | 20 | 4 | 0 | 200 | 2 |
| Access to information and early warning systems | 25 | 36 | 12 | 3 | 159 | 3 |
| Diversification of livestock | 17 | 42 | 11 | 6 | 146 | 4 |
| Temporary migration | 2 | 11 | 36 | 27 | 64 | 5 |
| Transformative Capacity | High | Medium | Low | No | TRCI | Rank |
|---|---|---|---|---|---|---|
| Tsetse control infrastructure | 38 | 29 | 8 | 1 | 180 | 1 |
| Access to extension services (entomologists) | 34 | 25 | 14 | 3 | 166 | 2 |
| Sedentary level | 6 | 39 | 27 | 4 | 123 | 3 |
| Urbanization | 1 | 11 | 35 | 29 | 60 | 4 |
| Factor | Comp1 | Comp2 | Comp3 | Comp4 | Comp5 | Comp6 | Unexplained |
|---|---|---|---|---|---|---|---|
| Livestock off-take | 0.1338 | −0.2675 | −0.2449 | 0.4227 | 0.3244 | −0.2339 | 0.2871 |
| Access to financial services | −0.3083 | 0.3402 | 0.3808 | 0.2425 | 0.1411 | 0.2371 | |
| Seeking a loan facility | −0.3816 | 0.3184 | 0.3436 | −0.0556 | 0.1755 | 0.1939 | |
| Diversified economic activities | 0.3189 | 0.3586 | −0.1308 | 0.1810 | −0.1907 | 0.2019 | 0.2768 |
| Bush burning | 0.3650 | 0.1085 | −0.1384 | 0.3779 | 0.4266 | ||
| Diversification of livestock | 0.2767 | −0.1351 | 0.2258 | 0.4052 | 0.5365 | 0.2222 | |
| Temporary migration | 0.0929 | 0.1782 | −0.4880 | 0.2928 | −0.2090 | 0.2488 | 0.3173 |
| Deployment of paravets (CAHWs) * | 0.3971 | 0.2630 | 0.1318 | −0.1775 | 0.3587 | ||
| Access to information and early warning systems | 0.3327 | −0.3271 | 0.1804 | 0.2127 | −0.1220 | 0.0741 | 0.3053 |
| Access to drugs | 0.1950 | −0.3140 | 0.1980 | 0.2766 | −0.2799 | −0.4527 | 0.249 |
| Tsetse control infrastructure | 0.2034 | 0.4264 | 0.2428 | 0.2544 | −0.2356 | −0.2063 | 0.2206 |
| Urbanization | −0.1928 | −0.1585 | −0.2920 | 0.4505 | −0.1497 | 0.1398 | 0.3682 |
| Sedentary level | 0.1662 | 0.2266 | −0.2594 | 0.1437 | 0.6075 | −0.2235 | 0.2373 |
| Access to central extension services (entomologists) | −0.0939 | 0.5299 | 0.2290 | 0.1693 | 0.1096 | −0.0854 | 0.2581 |
| Variable | 25th Percentile | 50th Percentile | 75th Percentile | Mean | Std. Dev. (Mean) | Min | Max |
|---|---|---|---|---|---|---|---|
| Resilience Index | 1.0802 | 2.2285 | 3.0526 | 2.052675 | 1.304063 | −0.5905 | 4.8386 |
| Rescaled Resilience Index | 0.3077 | 0.5192 | 0.6710 | 0.4868532 | 0.2401987 | 0 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egeru, A.; Opio, J.; Siya, A.; Barasa, B.; Magaya, J.P.; Namaalwa, J.J. Tsetse Invasion as an Emerging Threat to Socioecological Resilience of Pastoral Communities in Karamoja, Uganda. Sustainability 2020, 12, 1599. https://doi.org/10.3390/su12041599
Egeru A, Opio J, Siya A, Barasa B, Magaya JP, Namaalwa JJ. Tsetse Invasion as an Emerging Threat to Socioecological Resilience of Pastoral Communities in Karamoja, Uganda. Sustainability. 2020; 12(4):1599. https://doi.org/10.3390/su12041599
Chicago/Turabian StyleEgeru, Anthony, Joseph Opio, Aggrey Siya, Bernard Barasa, John Paul Magaya, and Justine J. Namaalwa. 2020. "Tsetse Invasion as an Emerging Threat to Socioecological Resilience of Pastoral Communities in Karamoja, Uganda" Sustainability 12, no. 4: 1599. https://doi.org/10.3390/su12041599





