Quantifying Long-Term Urban Grassland Dynamics: Biotic Homogenization and Extinction Debts
Abstract
1. Introduction
2. Materials and Methods
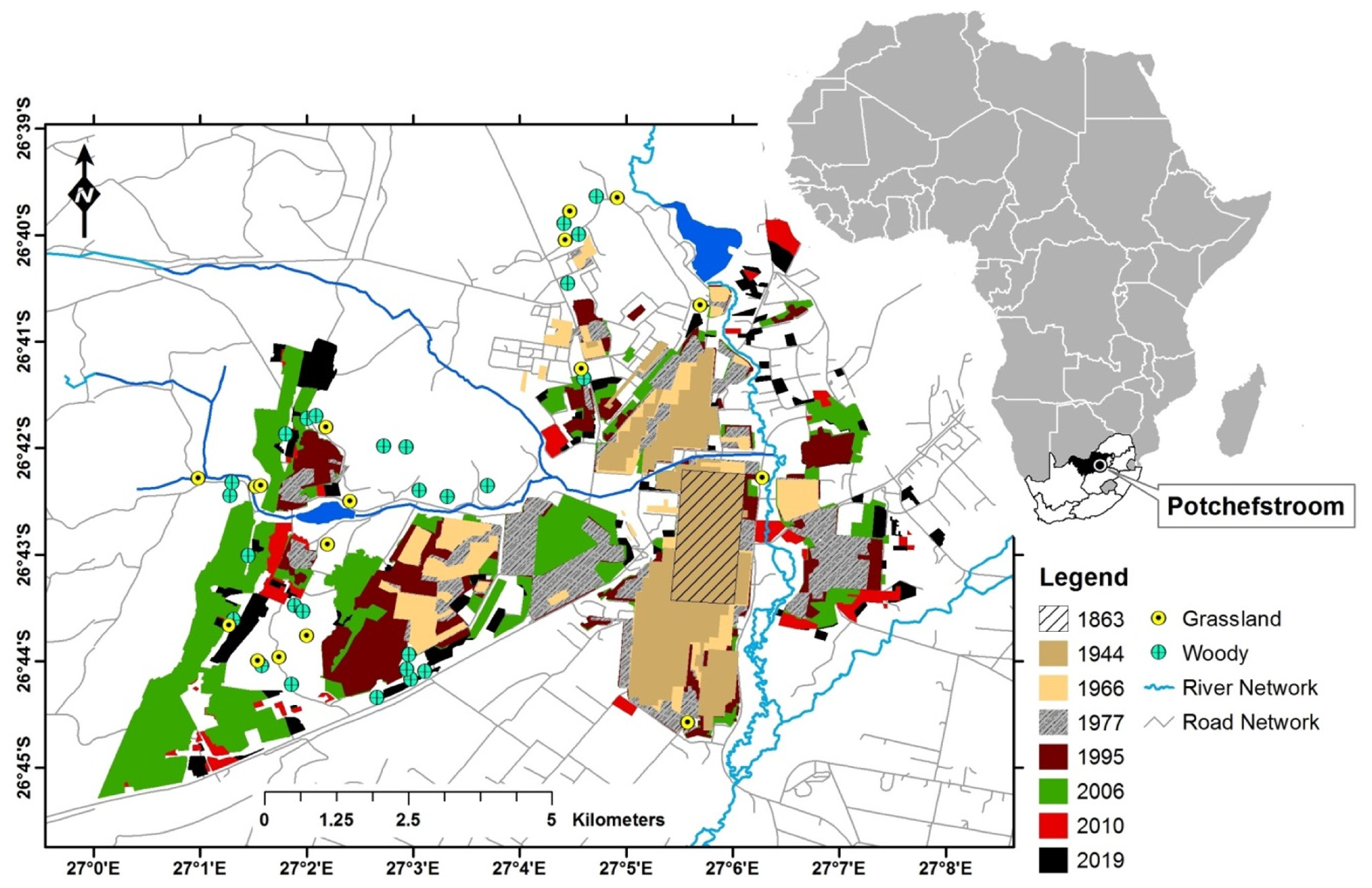
2.1. Study Area
2.2. Vegetation Sampling
2.3. Urban Landscape Measures
2.4. Data Analysis
3. Results
3.1. Little Evidence for Biotic Homogenization in Vegetation Communities from 1995 to 2019
3.2. Vegetation Patterns Changed Slightly between 1995 and 2019.
3.3. Time Lags Changed in Both Open and Woody Grassland Communities
3.4. Importance of Landscape Factors Driving these Patterns Changed
4. Discussion
4.1. Little Evidence for Biotic Homogenization in Vegetation Communities from 1995 to 2019
4.2. Vegetation Patterns Changed Slightly between 1995 and 2019
4.3. Time Lags Changed in Both Open and Woody Grassland Communities
4.4. The Importance of Landscape Factors Driving these Patterns Changed
4.5. Consequently, How will the Presence of Time Lags and Potential Extinction Debts Influence Conservation Strategies and Planning for Resilient and Sustainable Cities?
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aronson, M.F.J.; Nilon, C.H.; Lepczyk, C.A.; Parker, T.S.; Warren, P.S.; Cilliers, S.S.; Goddard, M.A.; Hahs, A.K.; Herzog, C.; Katti, M.; et al. Hierarchical filters determine community assembly of urban species pools. Ecology 2016, 97, 2952–2963. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Olden, J.D. Biotic homogenization: A new research agenda for conservation biogeography. J. Biogeogr. 2006, 33, 2027–2039. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Zeeman, B.J.; McDonnell, M.J.; Kendal, D.; Morgan, J.W.; Schmidtlein, S. Biotic homogenization in an increasingly urbanized temperate grassland ecosystem. J. Veg. Sci. 2017, 28, 550–561. [Google Scholar] [CrossRef]
- Blouin, D.; Pellerin, S.; Poulin, M. Increase in non-native species richness leads to biotic homogenization in vacant lots of a highly urbanized landscape. Urban Ecosyst. 2019, 22, 879–892. [Google Scholar] [CrossRef]
- Olden, J.D.; Poff, N.L. Toward a mechanistic understanding and prediction of biotic homogenization. Am. Nat. 2003, 162, 442–460. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Tichý, L.; Danihelka, J.; Fajmon, K.; Hájek, O.; Kintrová, K.; Láníková, D.; Otýpková, Z.; Řehořek, V. Biotic homogenization of central european urban floras depends on residence time of alien species and habitat types. Biol. Conserv. 2012, 145, 179–184. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Aronson, M.F.J.; Williams, N.S.G.; Celesti-Grapow, L.; Cilliers, S.; Clarkson, B.D.; Dolan, R.W.; Hipp, A.; Klotz, S.; Kühn, I.; et al. Beta diversity of urban floras among European and non-European cities. Global Ecol. Biogeogr. 2014, 23, 769–779. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Danihelka, J.; Tichý, L.; Ricotta, C.; Kühn, I. Biotic homogenization of urban floras by alien species: The role of species turnover and richness differences. J. Veg. Sci. 2016, 27, 452–459. [Google Scholar] [CrossRef]
- Miller, J.R. Biodiversity conservation and the extinction of experience. Trends Ecol. Evol. 2005, 20, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef] [PubMed]
- Rhemtulla, J.M.; Mladenoff, D.J. Why history matters in landscape ecology. Landsc. Ecol. 2007, 22, 1–3. [Google Scholar] [CrossRef]
- Ramalho, C.E.; Hobbs, R.J. Time for a change: Dynamic urban ecology. Trends Ecol. Evol. 2012, 27, 179–188. [Google Scholar] [CrossRef]
- Du Toit, M.J.; Kotze, D.J.; Cilliers, S.S. Landscape history, time lags and drivers of change: Urban natural grassland remnants in Potchefstroom, South Africa. Landsc. Ecol. 2016, 31, 2133–2150. [Google Scholar] [CrossRef]
- Dupouey, J.L.; Dambrine, E.; Laffite, J.D.; Moares, C. Irreversible impact of past land use on forest soils and biodiversity. Ecology 2002, 83, 2978–2984. [Google Scholar] [CrossRef]
- Rhemtulla, J.M.; Mladenoff, D.J.; Clayton, M.K. Legacies of historical land use on regional forest composition and structure in Wisconsin, USA (mid-1800s-1930s-2000s). Ecol. Appl. 2009, 19, 1061–1078. [Google Scholar] [CrossRef]
- Burel, F. Time lags between spatial pattern changes and species distribution changes in dynamic landscapes. Landsc. Urban Plann. 1993, 24, 161–166. [Google Scholar] [CrossRef]
- Koyanagi, T.; Kusumoto, Y.; Yamamoto, S.; Okubo, S.; Iwasaki, N.; Takeuchi, K. Grassland plant functional groups exhibit distinct time-lags in response to historical landscape change. Plant Ecol. 2012, 213, 327–338. [Google Scholar] [CrossRef]
- Magnuson, J.J. Long-term ecological research and the invisible present. Bioscience 1990, 40, 495–501. [Google Scholar] [CrossRef]
- Lira, P.K.; de Souza Leite, M.; Metzger, J.P. Temporal lag in ecological responses to landscape change: Where are we now? Curr. Landsc. Ecol. Rep. 2019, 4, 70–82. [Google Scholar] [CrossRef]
- Tilman, D.; May, R.M.; Lehman, C.L.; Nowak, M.A. Habitat destruction and the extinction debt. Nature 1994, 371, 65–66. [Google Scholar] [CrossRef]
- Jamin, A.; Peintinger, M.; Gimmi, U.; Holderegger, R.; Bergamini, A. Evidence for a possible extinction debt in Swiss wetland specialist plants. Ecol. Evol. 2020, 00, 1–14. [Google Scholar]
- Helm, A.; Hanski, I.; Partel, M. Slow response of plant species richness to habitat loss and fragmentation. Ecol. Lett. 2006, 9, 72–77. [Google Scholar] [CrossRef]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Ockinger, E.; Partel, M.; Pino, J.; Roda, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef]
- Lindborg, R.; Eriksson, O. Historical landscape connectivity affects present plant species diversity. Ecology 2004, 85, 1840–1845. [Google Scholar] [CrossRef]
- Seto, K.C.; Guneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.A.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwartz, M.W.; et al. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef]
- Krauss, J.; Bommarco, R.; Guardiola, M.; Heikkinen, R.K.; Helm, A.; Kuussaari, M.; Lindborg, R.; Ockinger, E.; Partel, M.; Pino, J.; et al. Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol. Lett. 2010, 13, 597–605. [Google Scholar] [CrossRef]
- Hanski, I.; Ovaskainen, O. Extinction debt at extinction threshold. Conserv. Biol. 2002, 16, 666–673. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Morgan, J.W.; McDonnell, M.J.; McCarthy, M.A. Plant traits and local extinctions in natural grasslands along an urban-rural gradient. J. Ecol. 2005, 93, 1203–1213. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J. Extinction debt of cities and ways to minimise their realisation: A focus on Melbourne. Ecol. Manag. Restor. 2014, 15, 102–110. [Google Scholar] [CrossRef]
- Lunt, I.D. A protocol for integrated management, monitoring, and enhancement of degraded themeda triandra grasslands based on plantings of indicator species. Restor. Ecol. 2003, 11, 223–230. [Google Scholar] [CrossRef]
- Siebert, F.; Dreber, N. Forb ecology research in dry African savannas: Knowledge, gaps, and future perspectives. Ecol. Evol. 2019, 9, 7875–7891. [Google Scholar] [CrossRef]
- O’Connor, T.G. Influence of land use on plant community composition and diversity in highland sourveld grassland in the southern Drakensberg, South Africa. J. Appl. Ecol. 2005, 42, 975–988. [Google Scholar] [CrossRef]
- Zeeman, B.J.; Morgan, J.W. Increasing and declining native species in urban remnant grasslands respond differently to nitrogen addition and disturbance. Ann. Bot. 2018, 121, 691–697. [Google Scholar] [CrossRef]
- O’Connor, T.G.; Martindale, G.; Morris, C.D.; Short, A.; Witkowski, T.F.; Scott-Shaw, R. Influence of grazing management on plant diversity of highland sourveld grassland, Kwazulu-Natal, South Africa. Rangel. Ecol. Manag. 2011, 64, 196–207. [Google Scholar] [CrossRef]
- Dorrough, J.; Yen, A.; Turner, V.; Clark, S.G.; Crosthwaite, J.; Hirth, J.R. Livestock grazing management and biodiversity conservation in Australian temperate grassy landscapes. Aust. J. Agric. Res. 2004, 55. [Google Scholar] [CrossRef]
- Hodges, J.A.; Price, J.N.; Nimmo, D.G.; Guja, L.K. Evidence for direct effects of fire-cues on germination of some perennial forbs common in grassy ecosystems. Austral Ecol. 2019, 44, 1271–1284. [Google Scholar] [CrossRef]
- Zaloumis, N.P.; Bond, W.J. Reforestation or conservation? The attributes of old growth grasslands in South Africa. Philos. Trans. R Soc. Lond B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Burns, C.E.; Collins, S.L.; Smith, M.D. Plant community response to loss of large herbivores: Comparing consequences in a south african and a North American grassland. Biodivers. Conserv. 2009, 18, 2327–2342. [Google Scholar] [CrossRef]
- Van den Bergh, G. Die tweede Potchefstroom, opmeting en besetting 1841-60. S. Afr. J. Surv. Mapp. 1992, 21, 167–178. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; Strelitzia 19; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- South Africa. National environmental management: Biodiversity act (10/2004): National list of ecosystems that are threatened and in need of protection (notice 1624). Proc. R47 Gov. Gaz. 2011, 34809, 3. [Google Scholar]
- North West Department of Agriculture, Conservation, Environment and Rural Development. North West Province Biodiversity Conservation Assessment Technical Report Version 1; North West Department of Agriculture, Conservation, Environment and Rural Development: Mmbatho, South Africa, 2009. [Google Scholar]
- Cilliers, S.S.; Van Wyk, E.; Bredenkamp, G.J. Urban nature conservation: Vegetation of natural areas in the Potchefstroom municipal area, North West Province, South Africa. Koedoe 1999, 42, 1–30. [Google Scholar] [CrossRef][Green Version]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Plant Ecology; John Wiley and Sons: New York, NY, USA, 1974. [Google Scholar]
- Bredenkamp, G.J.; Theron, G.K. A synecological account of the Suikerbosrand Nature Reserve 1. The phytosociology of the Witwatersrand geological system. Bothalia 1978, 12, 513–529. [Google Scholar] [CrossRef]
- Eckhardt, H.; Van Rooyen, N.; Bredenkamp, G. Use of Braun-Blanquet data for the assessment of veld condition and grazing capacity in grassland. Afr. J. Range Forage Sci. 1993, 10, 41–46. [Google Scholar] [CrossRef]
- Louw, W.J. An Ecological Account of the Vegetation of the Potchefstroom Area; Memoirs of the Botanical Survey of South Africa, No. 24; Government Printer: Pretoria, South Africa, 1951.
- Bredenkamp, G.J.; Joubert, A.F.; Bezuidenhout, H. A reconnaissance survey of the plains of the Potchefstroom-Parys-Fochville area. S. Afr. J. Bot. 1989, 55, 199–206. [Google Scholar] [CrossRef]
- Bezuidenhout, H.; Bredenkamp, G.J.; Theron, G.K. A classification of the vegetation of the western Transvaal dolomite and chert grassland, South Africa. S. Afr. J. Bot. 1994, 60, 152–161. [Google Scholar] [CrossRef][Green Version]
- Cilliers, S.S.; Bredenkamp, G.J. Ruderal and degraded natural vegetation on vacant lots in the Potchefstroom Municipal Area, North West Province, South Africa. S. Afr. J. Bot. 1999, 65, 163–173. [Google Scholar] [CrossRef][Green Version]
- ESRI. Arcgis, Version 10.0; Environmental Systems Research Institude: Redlands, CA, USA, 2010. [Google Scholar]
- Hawth’s Analysis Tools Version 3.27. Available online: http://www.spatialecology.com/htools (accessed on 11 February 2019).
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Rooney, T.P.; Wiegmann, S.M.; Rogers, D.A.; Waller, D.M. Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv. Biol. 2004, 18, 787–798. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Armentano, T.V.; Sah, J.P.; Ross, M.S.; Jones, D.T.; Cooley, H.C.; Smith, C.S. Rapid responses of vegetation to hydrological changes in Taylor Slough, Everglades National Park, Florida, USA. Hydrobiologia 2006, 569, 293–309. [Google Scholar] [CrossRef]
- Gaston, K.J. Abundances and range sizes: Measuring rarity. In Rarity; Springer: Berlin/Heidelberg, Germany, 1994; pp. 22–56. [Google Scholar]
- Hallett, L.M.; Jones, S.K.; MacDonald, A.A.M.; Jones, M.B.; Flynn, D.F.B.; Ripplinger, J.; Slaughter, P.; Gries, C.; Collins, S.L.; Poisot, T. Codyn: An R package of community dynamics metrics. Methods Ecol. Evol. 2016, 7, 1146–1151. [Google Scholar] [CrossRef]
- Xu, Y.; Wan, D.; Xiao, Z.; Wu, H.; Jiang, M. Spatio-temporal dynamics of seedling communities are determined by seed input and habitat filtering in a subtropical montane forest. For. Ecol. Manag. 2019, 449, 117475. [Google Scholar] [CrossRef]
- McGeoch, M.A.; Gaston, K.J. Occupancy frequency distributions: Patterns, artefacts and mechanisms. Biol. Rev. 2002, 77, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Glenn, S.M. A hierarchical analysis of species’ abundance patterns in grassland vegetation. Am. Nat. 1990, 135, 633–648. [Google Scholar] [CrossRef]
- Jacobs, S.M.; Naiman, R.J. Large african herbivores decrease herbaceous plant biomass while increasing plant species richness in a semi-arid savanna toposequence. J. Arid Environ. 2008, 72, 891–903. [Google Scholar] [CrossRef]
- Van Coller, H.; Siebert, F.; Siebert, S.J. Herbaceous species diversity patterns across various treatments of herbivory and fire along the sodic zone of the Nkuhlu exclosures, Kruger National Park. Koedoe 2013, 55. [Google Scholar] [CrossRef]
- Archibald, S.; Bond, W.J.; Stock, W.D.; Fairbanks, D.H.K. Shaping the landscape: Fire-grazer interactions in an African savanna. Ecol. Appl. 2005, 15, 96–109. [Google Scholar] [CrossRef]
- Kendal, D.; Zeeman, B.J.; Ikin, K.; Lunt, I.D.; McDonnell, M.J.; Farrar, A.; Pearce, L.M.; Morgan, J.W. The importance of small urban reserves for plant conservation. Biol. Conserv. 2017, 213, 146–153. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Morgan, J.W.; McCarthy, M.A.; McDonnell, M.J. Local extinction of grassland plants: The landscape matrix is more important than patch attributes. Ecology 2006, 87, 3000–3006. [Google Scholar] [CrossRef]
- O’Connor, T.G. Local extinction in perennial grasslands: A life-history approach. Am. Nat. 1991, 137, 753–773. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Primack, R.B.; Devictor, V.; Corlett, R.T.; Cumming, G.S.; Loyola, R.; Maas, B.; Pejchar, L. How does habitat fragmentation affect biodiversity? A controversial question at the core of conservation biology. Biol. Conserv. 2019, 232, 271–273. [Google Scholar] [CrossRef]
- Middleton, E.L.; Bever, J.D.; Schultz, P.A. The effect of restoration methods on the quality of the restoration and resistance to invasion by exotics. Restor. Ecol. 2010, 18, 181–187. [Google Scholar] [CrossRef]
- Prober, S.M.; Thiele, K.R.; Lunt, I.D.; Koen, T.B. Restoring ecological function in temperate grassy woodlands: Manipulating soil nutrients, exotic annuals and native perennial grasses through carbon supplements and spring burns. J. Appl. Ecol. 2005, 42, 1073–1085. [Google Scholar] [CrossRef]
- McCain, K.N.S.; Baer, S.G.; Blair, J.M.; Wilson, G.W.T. Dominant grasses suppress local diversity in restored tallgrass prairie. Restor. Ecol. 2010, 18, 40–49. [Google Scholar] [CrossRef]
- Johnson, D.P.; Catford, J.A.; Driscoll, D.A.; Gibbons, P.; Fraser, L. Seed addition and biomass removal key to restoring native forbs in degraded temperate grassland. Appl. Veg. Sci. 2018, 21, 219–228. [Google Scholar] [CrossRef]
- Zamin, T.J.; Jolly, A.; Sinclair, S.; Morgan, J.W.; Moore, J.L.; Souza, L. Enhancing plant diversity in a novel grassland using seed addition. J. Appl. Ecol. 2018, 55, 215–224. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban For. Urban Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Kildisheva, O.A.; Erickson, T.E.; Madsen, M.D.; Dixon, K.W.; Merritt, D.J. Seed germination and dormancy traits of forbs and shrubs important for restoration of North American dryland ecosystems. Plant Biol. 2019, 21, 458–469. [Google Scholar] [CrossRef]
- Pilon, N.A.L.; Assis, G.B.; Souza, F.M.; Durigan, G. Native remnants can be sources of plants and topsoil to restore dry and wet cerrado grasslands. Restor. Ecol. 2018, 27, 569–580. [Google Scholar] [CrossRef]
- Volis, S.; Deng, T. Importance of a single population demographic census as a first step of threatened species conservation planning. Biodivers. Conserv. 2019, 29, 527–543. [Google Scholar] [CrossRef]
- Red List of South African Plants. Available online: http://redlist.sanbi.org/ (accessed on 30 January 2020).
- Germishuizen, G.; Meyer, N.L.; Steenkamp, Y.; Keith, M. A Checklist of South African Plants; Southern African Botanical Diversity Network Reoprt, No. 41; SABONET: Pretoria, South African, 2006. [Google Scholar]





| SIMPER (Bray-Curtis Similarity Index) | ANOSIM | |||
|---|---|---|---|---|
| 1995 | 2012 | 2019 | ||
| Open Grasslands | ||||
| All species | 26% | 25% | 22% | R = 0.023 (p = 0.176) |
| Indigenous species | 26% | 25% | 22% | R = 0.023 (p = 0.186) |
| Exotic Species | 3% | 3% | 6% | R = 0.018 (p = 0.208) |
| % Plots with no exotic species | 44% | 56% | 44% | |
| Woody Grasslands | ||||
| All species | 27% | 26% | 26% | R = 0.085 (p = 0.001) |
| Indigenous species | 25% | 26% | 26% | R = 0.06 (p = 0.013) |
| Exotic Species | 45% | 21% | 21% | R = 0.139 (p = 0.001) |
| % Plots with no exotic species | 0% | 8% | 8% | |
| One-way ANOVA | Post hoc Tukey Unequal N HSD | |
|---|---|---|
| Woody ISR | F(2,69) = 1.602, p = 0.209 | - |
| Woody DSR | F(2,69) = 0.688, p = 0.506 | - |
| Woody forb_SR | F(2,79) = 0.889, p = 0.415 | - |
| Woody grass_SR | F(2,79) = 0.526, p = 0.593 | - |
| Grassland ISR | F(2,45) = 2.840, p = 0.069 | - |
| Grassland DSR | F(2,45) = 2.987, p = 0.061 | - |
| Grassland forb_SR | F(2,51) = 7.078, p = 0.002 | G95 vs. G12, G95 vs. G19 |
| Grassland grass_SR | F(2,51) = 2.664, p = 0.079 | - |
| Urban Landscape Measures | Time Lag in Years | |||||||
|---|---|---|---|---|---|---|---|---|
| Open grassland | ||||||||
| ISR | 1995 | +ALT | +AGE | -PN | +RNDN | 1 | ||
| 2012 | +ALT | +AGE | +RNDN | +CD | +H’ | 2 | ||
| 2019 | +ALT | +AGE | -PN | -RNDN | 9 | |||
| DSR | 1995 | -PN | 25 | |||||
| 2012 | +RNDN | 6 | ||||||
| 2019 | -PN | 9 | ||||||
| Woody grassland | ||||||||
| ISR | 1995 | -ALT | -PN | -RNDN | -CD | -H’ | 25 | |
| 2012 | -ALT | +AGE | -RNDN | +CD | 18 | |||
| 2019 | -CD | 49 | ||||||
| DSR | 1995 | -ALT | -RNDN | -H’ | 34 | |||
| 2012 | -ALT | -PN | 42 | |||||
| 2019 | PN | H | 9 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Toit, M.J.; Kotze, D.J.; Cilliers, S.S. Quantifying Long-Term Urban Grassland Dynamics: Biotic Homogenization and Extinction Debts. Sustainability 2020, 12, 1989. https://doi.org/10.3390/su12051989
du Toit MJ, Kotze DJ, Cilliers SS. Quantifying Long-Term Urban Grassland Dynamics: Biotic Homogenization and Extinction Debts. Sustainability. 2020; 12(5):1989. https://doi.org/10.3390/su12051989
Chicago/Turabian Styledu Toit, Marié J., D. Johan Kotze, and Sarel S. Cilliers. 2020. "Quantifying Long-Term Urban Grassland Dynamics: Biotic Homogenization and Extinction Debts" Sustainability 12, no. 5: 1989. https://doi.org/10.3390/su12051989
APA Styledu Toit, M. J., Kotze, D. J., & Cilliers, S. S. (2020). Quantifying Long-Term Urban Grassland Dynamics: Biotic Homogenization and Extinction Debts. Sustainability, 12(5), 1989. https://doi.org/10.3390/su12051989






