Effects of Seasonality, Tree Species and Urban Green Space on Deciduous Leaf Litter Decomposition in Lithuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Leaf Litter Decomposition
2.3. Leaf Litter Quality
2.4. Statistical Data Analysis
3. Results
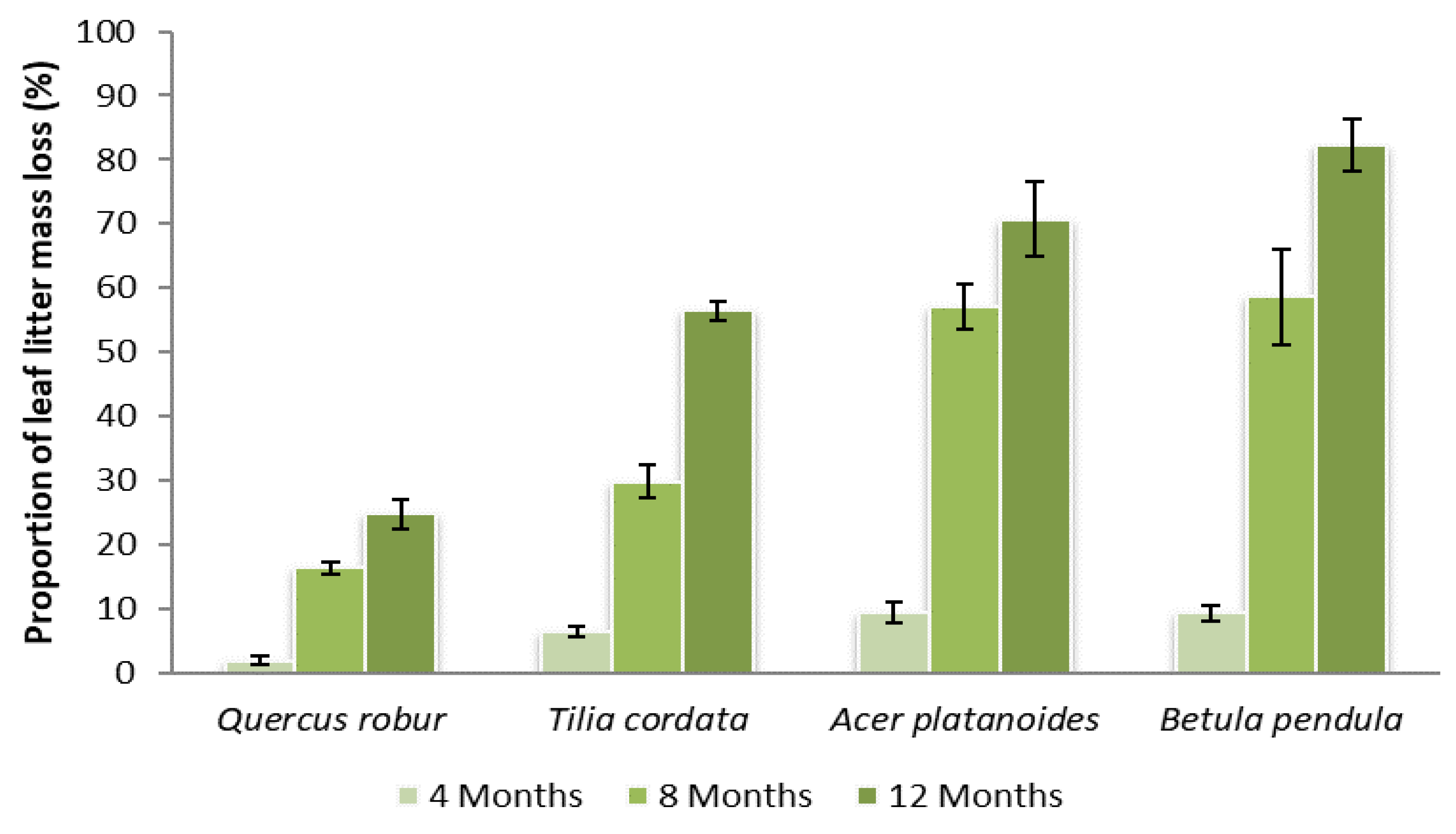
3.1. Leaf Litter Decomposition of Four Tree Species in Time
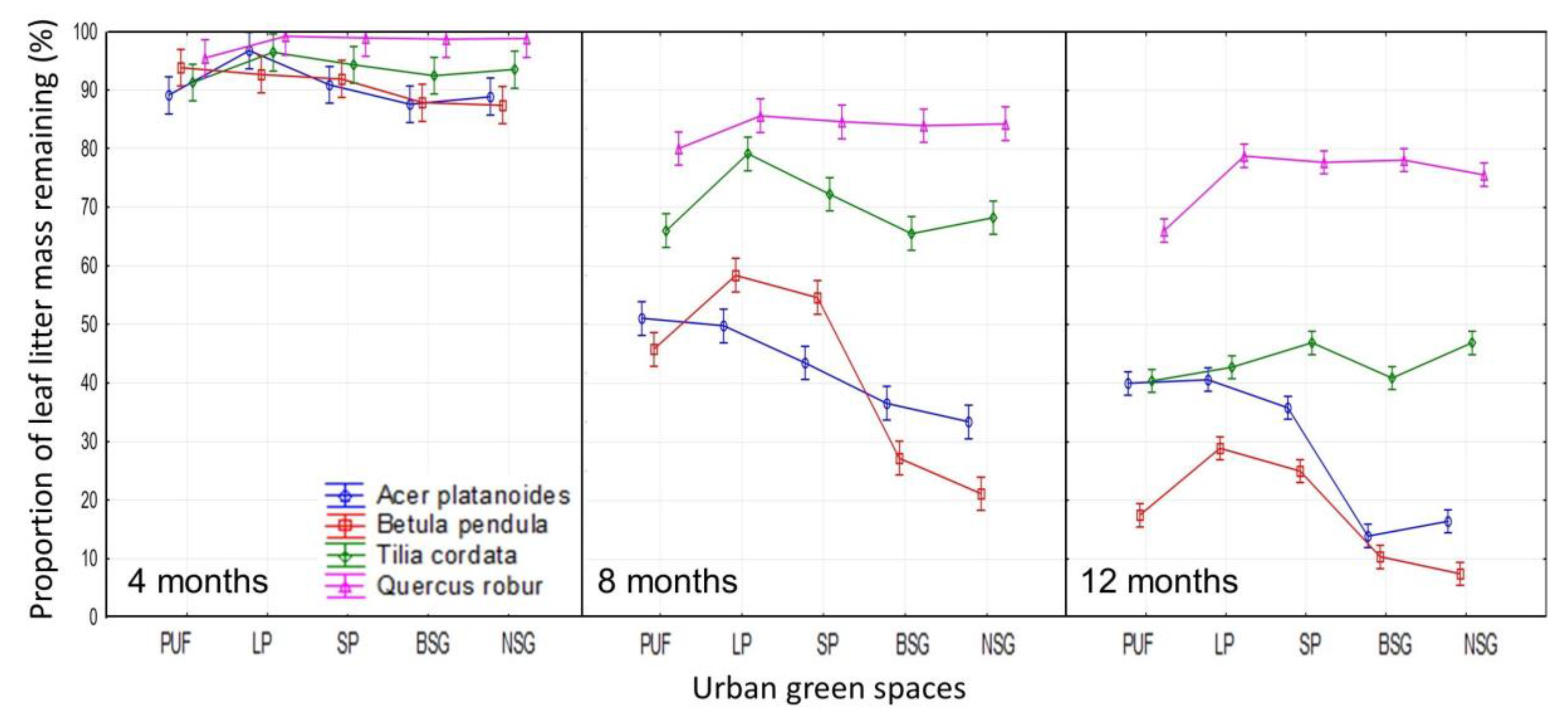
3.2. Influence of Different Urban Green Spaces on Tree Species Leaf Litter Decomposition
A. platanoides
B. pendula
T. cordata
Q. robur
3.3. Leaf Litter C:N, C:P, and N:P Ratios
4. Discussion
4.1. Leaf Litter Decomposition in Time and Space
4.2. Leaf Litter Decomposition in Different Urban Green Spaces
4.3. Leaf Litter C:N, C:P and N:P Ratio
4.4. Planning of Urban Green Space
Author Contributions
Funding
Conflicts of Interest
References
- Sachweh, M.; Rötzer, T. Climatic Change Effects on Phenological Phases in Southern Germany. In Proceedings of the 14th International Congress of Biometeorolgy, Ljubljana, Slovenia, 1–8 September 1996; pp. 226–233. [Google Scholar]
- Rötzer, T.; Sachweh, M. Climatic changes as reflected in phenological time series. Arboreta Phänologica 1995, 40, 17–23. [Google Scholar]
- Haines-Young, R.; Potschin, M. The links between biodiversity, ecosystem services and human well-being. Ecosyst. Ecol. New Synth. 2010, 1, 110–139. [Google Scholar]
- Walker, T.D. Functional and aesthetic uses of plants in design. In Plants in the Landscape, 2nd ed.; Carpenter, P.L., Walker, T.D., Eds.; WH Freeman: New York, NY, USA, 1990; pp. 152–178. [Google Scholar]
- Roy, S.; Byrne, J.; Pickering, C. A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban For. Urban Green. 2012, 11, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Fuller, R.A.; Gaston, K.J. The scaling of green space coverage in European cities. Biol. Lett. 2009, 5, 352–355. [Google Scholar] [CrossRef] [Green Version]
- Takács, Á.; Kiss, M.; Hof, A.; Tanács, E.; Gulyás, Á.; Kántor, N. Microclimate Modification by Urban Shade Trees–An Integrated Approach to Aid Ecosystem Service Based Decision-making. Procedia Environ. Sci. 2016, 32, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.; Hunt, B.; Walmsley, T. Trees in the Urban Landscape: Principles and Practice; Taylor & Francis: London, UK, 1995; p. 288. [Google Scholar]
- Ruark, G.A.; Mader, D.L.; Tattar, T.A. The influence of soil compaction and aeration on the root growth and vigour of trees-a literature review. Part 1. Arboric. J. 1982, 6, 251–265. [Google Scholar] [CrossRef]
- Pauleit, S.; Jones, N.; Garcia-Martin, G.; Garcia-Valdecantos, J.L.; Rivière, L.M.; Vidal-Beaudet, L.; Bodson, M.; Randrup, T.B. Tree establishment practice in towns and cities–Results from a European survey. Urban For. Urban Green. 2002, 1, 83–96. [Google Scholar] [CrossRef]
- Enloe, H.A.; Lockaby, B.G.; Zipperer, W.C.; Somers, G.L. Urbanization effects on leaf litter decomposition, foliar nutrient dynamics and aboveground net primary productivity in the subtropics. Urban Ecosyst 2015, 18, 1285–1303. [Google Scholar] [CrossRef]
- Vaidelys, T.; Straigytė, L. Length of grow season for native tree species in different green spaces. Žemės Moksl. 2017, 24, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Boyero, L.; Pearson, R.G.; Gessner, M.O.; Barmuta, L.A.; Ferreira, V.; Graça, M.A.S.; Dudgeon, D.; Boulton, A.J.; Callisto, M.; Chauvet, E.; et al. A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol. Lett. 2011, 14, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbie, S.E.; Baker, L.A.; Buyarski, C.; Nidzgorski, D.; Finlay, J.C. Decomposition of tree leaf litter on pavement: Implications for urban water quality. Urban Ecosyst. 2014, 17, 369–385. [Google Scholar] [CrossRef] [Green Version]
- García-Palacios, P.; McKie, B.G.; Handa, I.T.; Frainer, A.; Hättenschwiler, S. The importance of litter traits and decomposers for litter decomposition: A comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol. 2016, 30, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Waring, R.; Schlesinger, W. Decomposition and Forest Soil Development; Academic Press: Orlando, FL, USA, 1985; pp. 181–208. [Google Scholar]
- Couteaux, M.-M.; Bottner, P.; Berg, B. Litter decomposition, climate and liter quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Sariyildiz, T.; Anderson, J.M. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Aubert, M.; Margerie, P.; Trap, J.; Bureau, F. Aboveground–belowground relationships in temperate forests: Plant litter composes and microbiota orchestrates. Forest Ecol. Manag. 2010, 259, 563–572. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Howe, K.; Parkhurst, D.F.; Pouyat, R.V. Variation in quality and decomposability of red oak leaf litter along an urban-rural gradient. Biol. Fertil. Soils 1999, 30, 258–268. [Google Scholar] [CrossRef]
- Taylor, B.R.; Jones, H.G. Litter decomposition under snow cover in a balsam fir forest. Can. J. Bot. 1990, 68, 112–120. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen limitation of decomposition and decay: How can it occur? Glob. Chang. Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Cornelissen, J.H.C.; Vendramini, F.; Cabido, M.; Castellanos, A. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 2000, 218, 21–30. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Huang, Y. Comparisons of litterfall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. Forest Ecol. Manag. 2008, 255, 1210–1218. [Google Scholar] [CrossRef]
- Janusauskaite, D.; Straigyte, L. Leaf litter decomposition differences between alien and native maple species. Baltic. For. 2011, 17, 189–196. [Google Scholar]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Martínez-Yrízar, A.; Núñez, S.; Búrquez, A. Leaf litter decomposition in a southern Sonoran Desert ecosystem, northwestern Mexico: Effects of habitat and litter quality. Acta Oecol. 2007, 32, 291–300. [Google Scholar] [CrossRef]
- Månsson, K.F.; Falkengren-Grerup, U. The effect of nitrogen deposition on nitrification, carbon and nitrogen mineralisation and litter C:N ratios in oak (Quercus robur L.) forests. For. Ecol. Manag. 2003, 179, 455–467. [Google Scholar] [CrossRef]
- Bärlocher, F. Leaf Mass Loss Estimated by Litter Bag Technique. In Methods to Study Litter Decomposition: A Practical Guide; Graça, M.A.S., Bärlocher, F., Gessner, M.O., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 37–42. [Google Scholar]
- Crossley, D.A., Jr.; Hoglund, M.P. A Litter-Bag Method for the Study of Microarthropods Inhabiting Leaf Litter. Ecology 1962, 43, 571–573. [Google Scholar] [CrossRef]
- McKight, P.E.; Najab, J. Kruskal-Wallis Test. In The Corsini Encyclopedia of Psychology; Weiner, I.B., Craighead, W.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Gotelli, N.; Ellison, A. A Primer of Ecological Statistics, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2013. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Jacob, M.; Weland, N.; Platner, C.; Schaefer, M.; Leuschner, C.; Thomas, F.M. Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol. Biochem. 2009, 41, 2122–2130. [Google Scholar] [CrossRef]
- Cotrufo, F.M.; Ineson, P.; Derek Roberts, J. Decomposition of birch leaf litters with varying C-to-N ratios. Soil Biol. Biochem. 1995, 27, 1219–1221. [Google Scholar] [CrossRef]
- Dorendorf, J.; Wilken, A.; Eschenbach, A.; Jensen, K. Urban-induced changes in tree leaf litter accelerate decomposition. Ecol. Process. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Siddique, M.R.H.; Rahman, M.S.; Hossain, M.Z.; Hasan, M.M. Nutrient dynamics associated with leaf litter decomposition of three agroforestry tree species (Azadirachta indica, Dalbergia sissoo, and Melia azedarach) of Bangladesh. J. For. Res. 2011, 22, 577. [Google Scholar] [CrossRef]
- Imberger, S.J.; Walsh, C.J.; Grace, M.R. More microbial activity, not abrasive flow or shredder abundance, accelerates breakdown of labile leaf litter in urban streams. J. North Am. Benthol. Soc. 2008, 27, 549–561. [Google Scholar] [CrossRef]
- Straigytė, L.; Vaidelys, T.; Manton, M. Impact of urban green spaces, native tree species and seasons on soil pH in Kaunas, Lithuania. Baltic For. 2019, 25, 257–262. [Google Scholar]
- Pouyat, R.V.; Carreiro, M.M. Controls on mass loss and nitrogen dynamics of oak leaf litter along an urban-rural land-use gradient. Oecologia 2003, 135, 288–298. [Google Scholar] [CrossRef]
- Pouyat, R.V.; McDonnell, M.J.; Pickett, S.T. Litter decomposition and nitrogen mineralization in oak stands along an urban-rural land use gradient. Urban Ecosyst. 1997, 1, 117–131. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M.; Rawlik, K.; Kaźmierowski, C. Slope exposure and forest stand type as crucial factors determining the decomposition rate of herbaceous litter on a reclaimed spoil heap. Catena 2019, 175, 219–227. [Google Scholar] [CrossRef]
- Blair, J.M.; Crossley, D., Jr. Litter decomposition, nitrogen dynamics and litter microarthropods in a southern Appalachian hardwood forest 8 years following clearcutting. J. Appl. Ecol. 1988, 683–698. [Google Scholar] [CrossRef]
- Heneghan, L.; Coleman, D.C.; Zou, X.; Crossley, D., Jr.; Haines, B. Soil microarthropod contributions to decomposition dynamics: Tropical–temperate comparisons of a single substrate. Ecology 1999, 80, 1873–1882. [Google Scholar]
- Heneghan, L.; Rauschenberg, C.; Fatemi, F.; Workman, M. European buckthorn (Rhamnus cathartica) and its effects on some ecosystem properties in an urban woodland. Ecol. Restor. 2004, 22, 275–280. [Google Scholar] [CrossRef]
- Straigyté, L.; Zalkauskas, R. Effect of climate variability on Quercus rubra phenotype and spread in Lithuanian forests. Dendrobiology 2012, 67, 79–85. [Google Scholar]
- Straigytė, L.; Jurkšienė, G.; Armolaitis, K. Decomposition of Oak and Maple Leaf Litters: Comparative Study of Native and Alien Species. Sustain. Dev. For. 2009, 4, 196–200. [Google Scholar]
- Jonsson, M.; Wardle, D.A. Context dependency of litter-mixing effects on decomposition and nutrient release across a long-term chronosequence. Oikos 2008, 117, 1674–1682. [Google Scholar] [CrossRef]
- Kurka, A.M.; Starr, M.; Heikinheimo, M.; Salkinoja-Salonen, M. Decomposition of cellulose strips in relation to climate, litterfall nitrogen, phosphorus and C/N ratio in natural boreal forests. Plant Soil 2000, 219, 91–101. [Google Scholar] [CrossRef]
- Templer, P.H.; Toll, J.W.; Hutyra, L.R.; Raciti, S.M. Nitrogen and carbon export from urban areas through removal and export of litterfall. Environ. Pollut. 2015, 197, 256–261. [Google Scholar] [CrossRef]
- Scalenghe, R.; Marsan, F.A. The anthropogenic sealing of soils in urban areas. Landsc. Urban Plan. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 2003, 137, 578–586. [Google Scholar] [CrossRef]
- Varnagirytė-Kabašinskienė, I.; Hagen-Thorn, A.; Armolaitis, K. Comparative study of litterfall in different deciduous species plantations. Miškininkystė 2005, 1, 30–36. [Google Scholar]


| Urban Green Spaces | Exposure (Months) | Acer Platanoides | Betula Pendula | Tilia Cordata | Quercus Robur |
|---|---|---|---|---|---|
| Peri-urban forests | 4 | 0.55 (±0.07) | 0.31 (±0.13) | 0.44 (±0.11) | 0.23 (±0.09) |
| 8 | 2.45 (±0.08) | 2.71 (±0.03) | 1.70 (±0.07) | 1.00 (±0.05) | |
| 12 | 3.01 (±0.02) | 4.13 (±0.04) | 2.98 (±0.03) | 1.70 (±0.11) | |
| Large urban parks | 4 | 0.17 (±0.05) | 0.37 (±0.14) | 0.18 (±0.07) | 0.04 (±0.02) |
| 8 | 2.51 (±0.06) | 2.08 (±0.13) | 1.04 (±0.10) | 0.72 (±0.04) | |
| 12 | 2.97 (±0.03) | 3.56 (±0.07) | 2.86 (±0.04) | 1.06 (±0.08) | |
| Small urban parks | 4 | 0.46 (±0.11) | 0.41 (±0.05) | 0.29 (±0.12) | 0.06 (±0.02) |
| 8 | 2.82 (±0.07) | 2.27 (±0.08) | 1.39 (±0.04) | 0.77 (±0.08) | |
| 12 | 3.21 (±0.04) | 3.75 (±0.03) | 2.66 (±0.05) | 1.11 (±0.07) | |
| Broad street greeneries | 4 | 0.62 (±0.09) | 0.61 (±0.06) | 0.38 (±0.08) | 0.07 (±0.02) |
| 8 | 3.17 (±0.02) | 3.64 (±0.06) | 1.72 (±0.10) | 0.80 (±0.04) | |
| 12 | 4.30 (±0.03) | 4.48 (±0.02) | 2.96 (±0.03) | 1.10 (±0.03) | |
| Narrow street greeneries | 4 | 0.56 (±0.06) | 0.63 (±0.04) | 0.33 (±0.06) | 0.06 (±0.01) |
| 8 | 3.33 (±0.03) | 3.94 (±0.04) | 1.59 (±0.12) | 0.79 (±0.10) | |
| 12 | 4.18 (±0.02) | 4.63 (±0.02) | 2.66 (±0.06) | 1.22 (±0.06) |
| Tree Species | PUF | LP | SP | BSG | NSG |
|---|---|---|---|---|---|
| C:N (g g−1) | |||||
| Acer platanoides | 45.2 ± 1.8 | 43.8 ± 0.4 | 43.0 ± 0.3 | 40.4 ± 1.7 | 39.2 ± 0.2 |
| Betula pendula | 42.0 ± 3.7 | 58.0 ± 0.1 | 41.3 ± 3.8 | 59.2 ± 1.9 | 53.0 ± 0.4 |
| Tilia cordata | 32.0 ± 0.4 | 35.2 ± 0.3 | 30.8 ± 0.2 | 31.0 ± 0.2 | 30.0 ± 2.6 |
| Quercus robur | 40.5 ± 1.9 | 39.4 ± 0.1 | 40.3 ± 0.9 | 36.3 ± 0.8 | 38.5 ± 0.8 |
| C:P (g g−1) | |||||
| Acer platanoides | 809 ± 64 | 498 ± 3 | 426 ± 8 | 537 ± 33 | 487 ± 26 |
| Betula pendula | 632 ± 29 | 240 ± 11 | 256 ± 18 | 244 ± 12 | 342 ± 9 |
| Tilia cordata | 550 ± 14 | 480 ± 12 | 401 ± 20 | 484 ± 9 | 590 ± 66 |
| Quercus robur | 777 ± 17 | 583 ± 17 | 329 ± 20 | 525 ± 22 | 872 ± 10 |
| N:P (g g−1) | |||||
| Acer platanoides | 17.9 ± 1.1 | 11.4 ± 0.3 | 9.9 ± 0.2 | 13.3 ± 0.5 | 12.4 ± 1.0 |
| Betula pendula | 15.4 ± 2.1 | 4.1 ± 0.03 | 6.5 ± 1.8 | 4.1 ± 0.1 | 6.5 ± 0.4 |
| Tilia cordata | 17.2 ± 0.5 | 13.7 ± 0.3 | 13 ± 1.0 | 15.5 ± 0.3 | 19.6 ± 0.9 |
| Quercus robur | 19.2 ± 0.6 | 14.8 ± 0.7 | 8.1 ± 0.6 | 14.4 ± 0.5 | 22.7 ± 0.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaidelys, T.; Straigytė, L.; Manton, M. Effects of Seasonality, Tree Species and Urban Green Space on Deciduous Leaf Litter Decomposition in Lithuania. Sustainability 2020, 12, 2210. https://doi.org/10.3390/su12062210
Vaidelys T, Straigytė L, Manton M. Effects of Seasonality, Tree Species and Urban Green Space on Deciduous Leaf Litter Decomposition in Lithuania. Sustainability. 2020; 12(6):2210. https://doi.org/10.3390/su12062210
Chicago/Turabian StyleVaidelys, Tadas, Lina Straigytė, and Michael Manton. 2020. "Effects of Seasonality, Tree Species and Urban Green Space on Deciduous Leaf Litter Decomposition in Lithuania" Sustainability 12, no. 6: 2210. https://doi.org/10.3390/su12062210







