Removing Sr(II) and Cs(I) from the Aqueous Phase Using Basil Seed and Elucidating the Adsorption Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
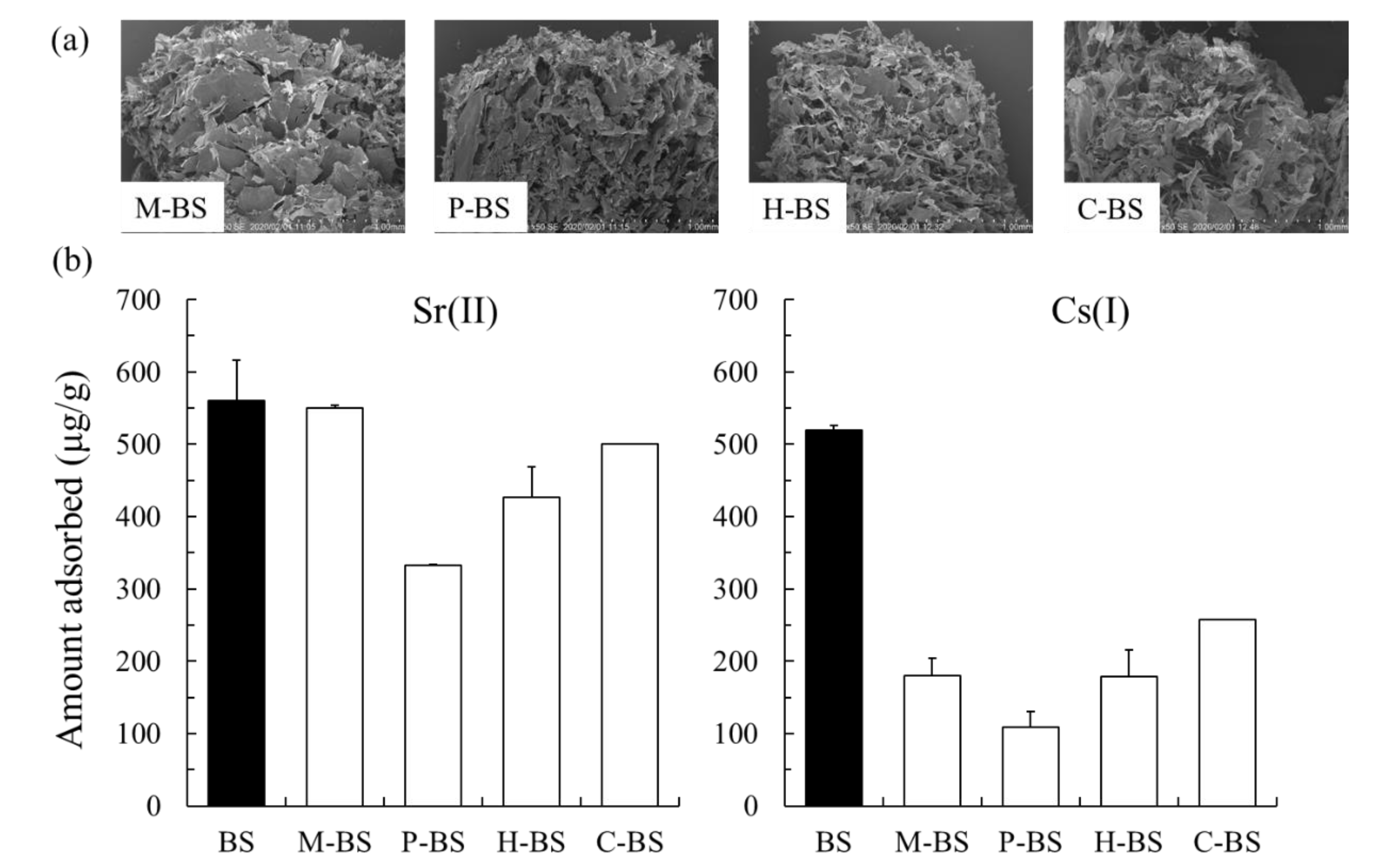
2.2. Adsorption Capability of Sr(II) And Cs(I) Using the BS, Calcined BS, And Enzyme-Treated BS
2.3. Adsorption Isotherms of Sr(II) And Cs(I) by the BS and Calcined BS
2.4. Effects of Contact Time And pH on the Adsorption of Sr(II) And Cs(I) by the BS and Calcined BS
3. Results and Discussion
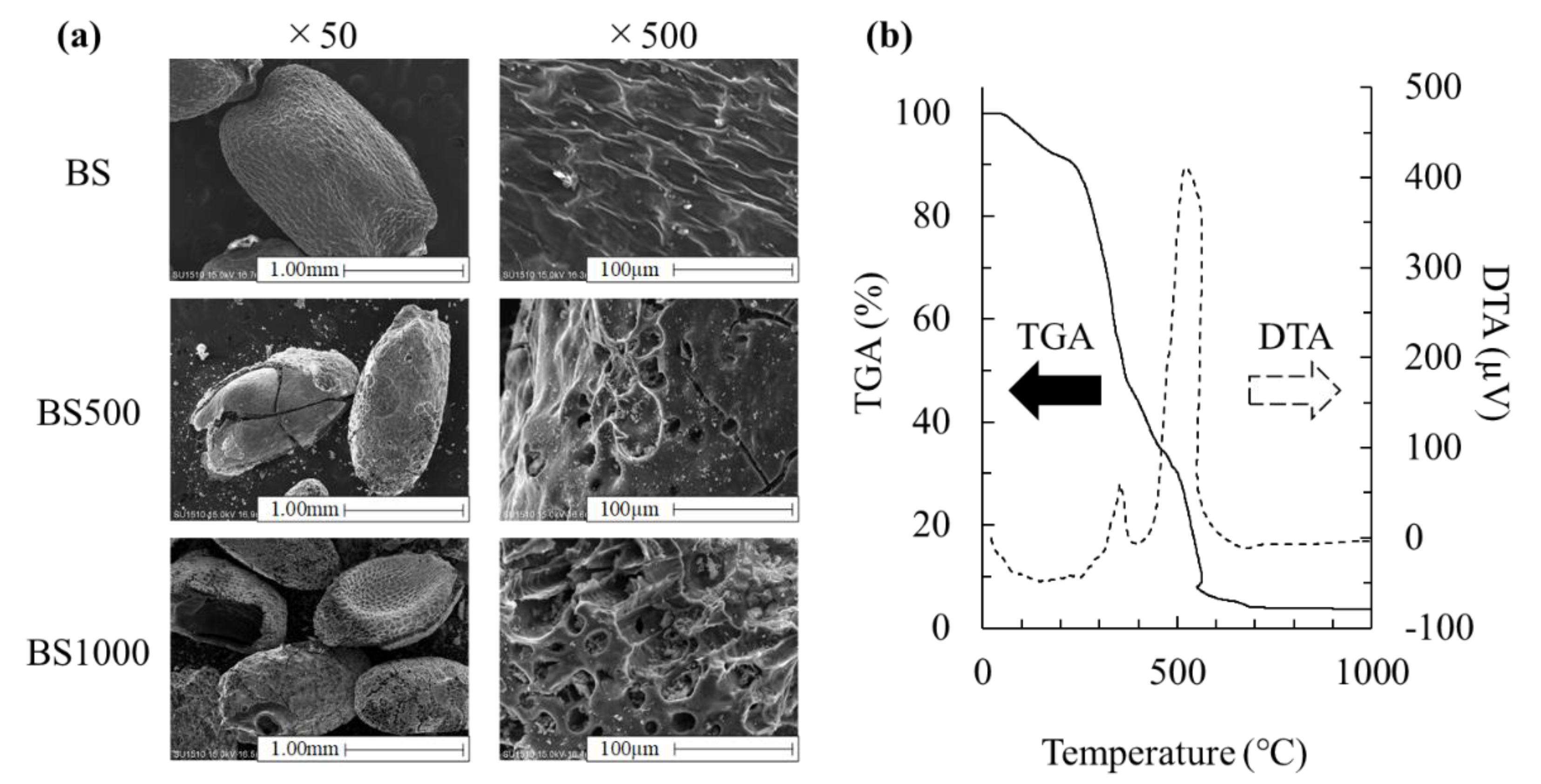
3.1. Effect of Calcination Treatment on Physicochemical Properties
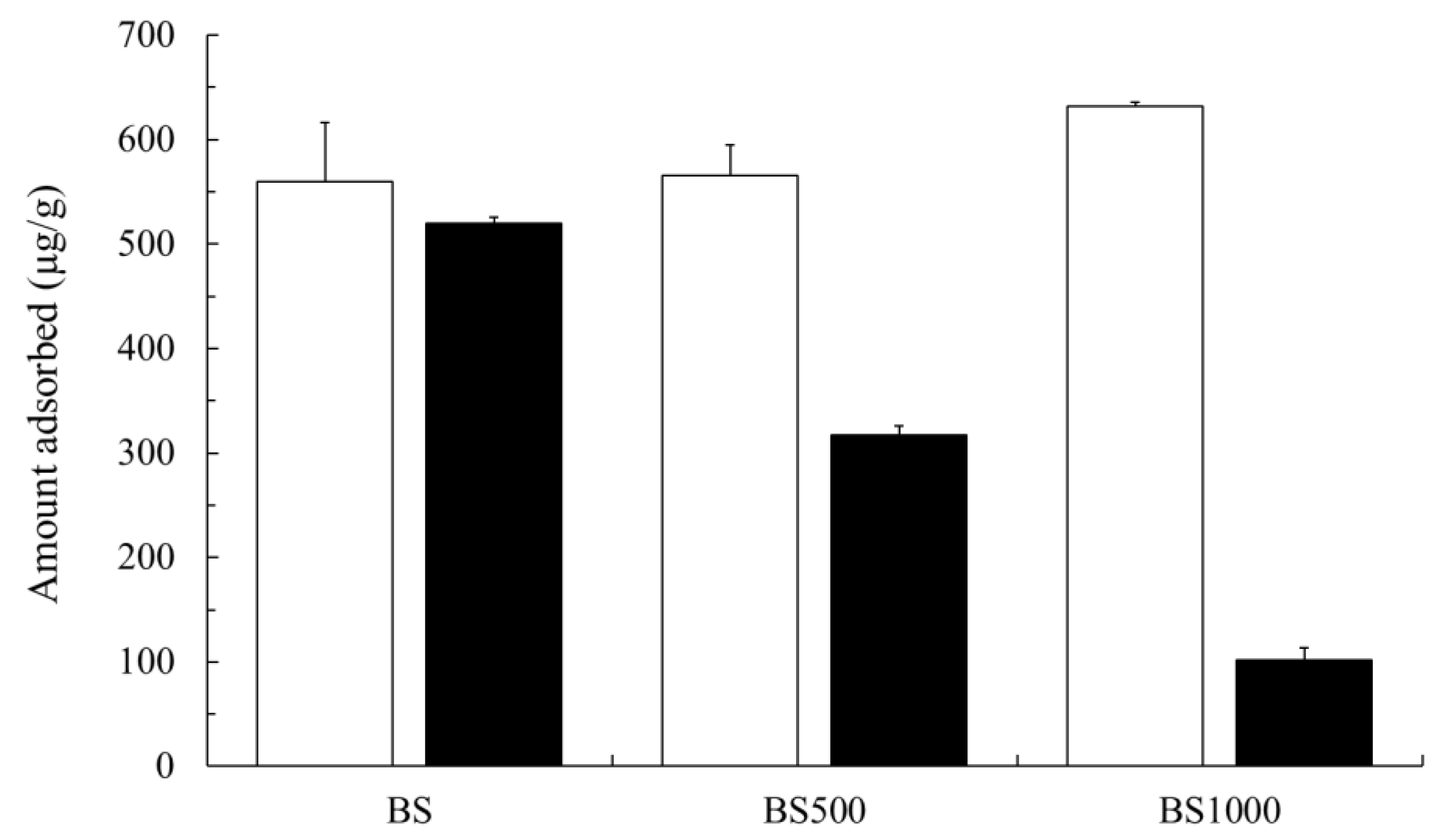
3.2. Adsorption Capability of Sr(II) And Cs(I)
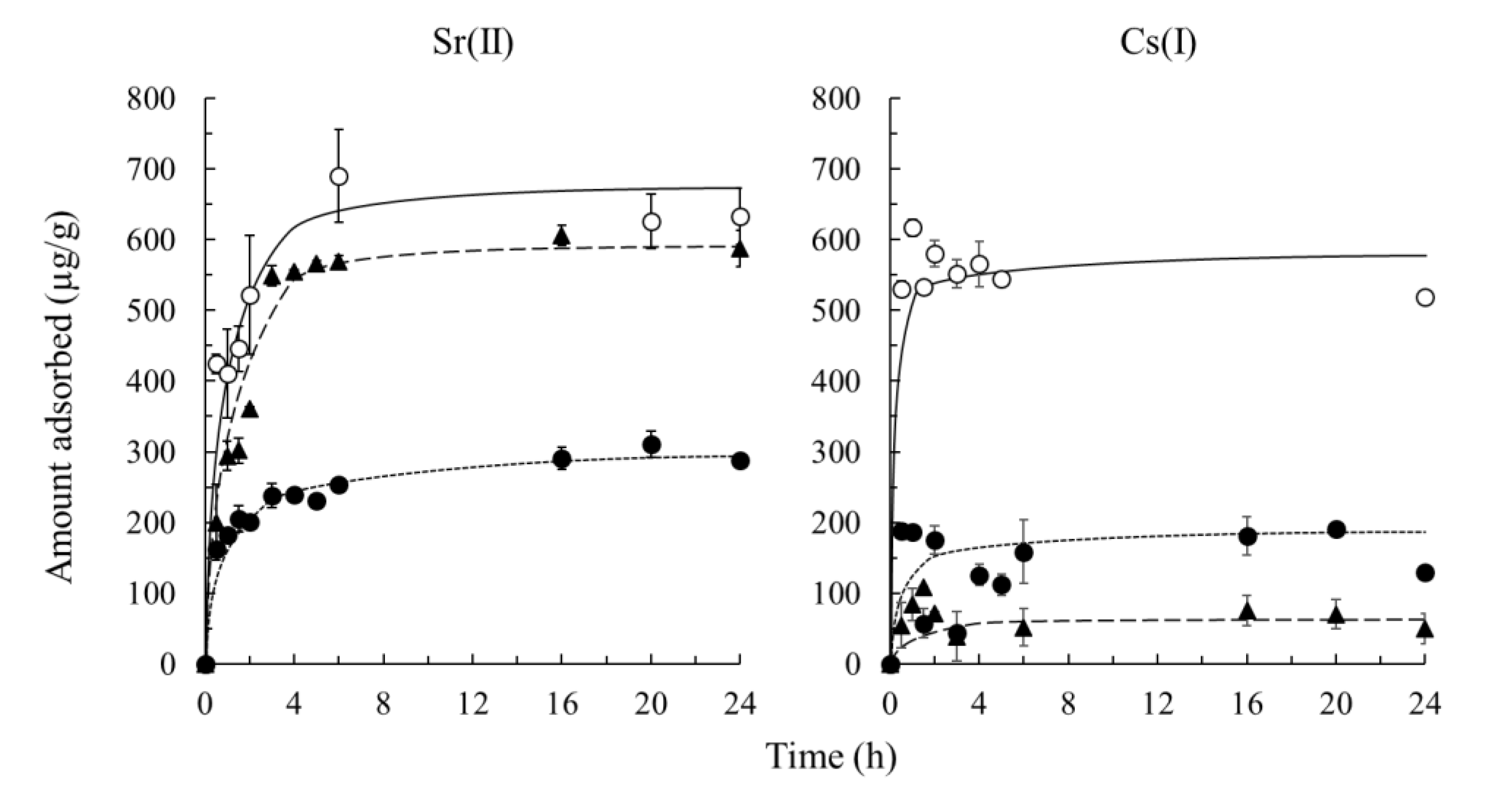
3.3. Adsorption Kinetics of Sr(II) And Cs(I)
3.4. Adsorption Isotherms of Sr(II) And Cs(I)
3.5. Effect of pH on the Adsorption of Sr(II) And Cs(I)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, J.L.; Zhuang, S.T.; Liu, Y. Metal hexacyanoferrates-based adsorbents for cesium removal. Coord. Chem. Rev. 2018, 374, 430–438. [Google Scholar] [CrossRef]
- Manolopoulou, M.; Vagena, E.; Stoulos, S.; Ioannodou, A.; Papastefanou, C. Radioiodine and radiocesium in Thessaloniki, North Greece due to the Fukushima nuclear accident. J. Environ. Radioact. 2011, 102, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Maji, S.; Bandyopadhyay, A.; Basu, S. Biosorption of cesium-137 and strontium-90 by mucilaginous seeds of Ocimum basilicum. Bioresour. Technol. 2007, 98, 2949–2952. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, A.; Zabihi, M.; Tahmasbi, M.; Bastami, T.R. Effect of adsorbents and chemical treatments on the removal of strontium from aqueous solution. J. Hazard. Mater. 2010, 182, 551–556. [Google Scholar] [CrossRef]
- Martell, E.A. Atmospheric aspects of 90Sr2+ fallout. Science 1959, 129, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Merceille, A.; Weinzaepfel, E.; Barre, Y.; Grandjean, A. The sorption behavior of synthetic sodium nonatitanate and Zeolite A for removing radioactive strontium from aqueous waste. Sep. Purif. Technol. 2012, 96, 81–88. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Alam, S.; Kawakita, H.; Ohto, K.; Inoue, K.; Harata, H. Adsorptive removal of Cs(I) from aqueous solution using polyphenols enriched biomass-based adsorbents. Chem. Eng. J. 2013, 231, 113–120. [Google Scholar] [CrossRef]
- Yoo, J.I.; Shinagawa, T.; Wood, J.P.; Linak, W.P.; Santoianni, D.A.; King, C.J.; Seo, Y.C.; Wendt, J.O.L. High-temperature sorption of cesium and strontium on dispersed kaolinite powders. Environ. Sci. Technol. 2005, 39, 5087–5094. [Google Scholar] [CrossRef]
- Liu, X.; Chen, G.R.; Lee, D.J.; Kawamoto, T.; Tanaka, H.; Chen, M.L.; Luo, Y.K. Adsorption removal of cesium from drinking waters: A mini review on use of biosorbents and other adsorbents. Bioresour. Technol. 2014, 160, 142–149. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Removal of Pb2+, As+, Cs+ and Sr2+ from aqueous solution by brewery’s waste biomass. J. Hazard. Mater. 2008, 151, 65–70. [Google Scholar] [CrossRef]
- Kajiyama, T.; Kokusen, H. Study of adsorption behavior of cesium and strontium ions with banana fiber adsorbent. J. Ion Exchange 2016, 27, 8–12. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wang, J.; Shi, J. Sr(II) removal from aqueous solution by Bacillus cereus biomass, equilibrium and kinetic studies. Adv. Mater. Res. 2011, 230–232, 1129–1132. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Ge, F. Equilibrium and kinetic studies of aqueous cesium(I) ions biosorption by Pseudomonas alcaligenes biomass as a low-cost natural biosorbent. Adv. Mater. Res. 2011, 171, 53–56. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Soeda, A.; Yamashiro, K.; Nakamura, T.; Saenjum, C.; Kawasaki, N. Removal of Sr(II) ions from aqueous solution by human hair treated with EDTA. Bioresour. Technol. Rep. 2020, 9, 100393. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Ueta, E.; Nakamura, T.; Kawasaki, N. Biomass potential of virgin and calcined tapioca (cassava starch) for the removal of Sr(II) and Cs(I) from aqueous solutions. Chem. Pharm. Bull. 2018, 66, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakdasri, W.; Ngamprasertsith, S.; Sooksai, S.; Noitang, S.; Sukaead, W.; Sawangkeaw, R. Defatted fiber produced from lemon Basil (Ocimum citriodorum Vis.) seed with supercritical CO2: Economic analysis. Ind. Crops Pro. 2019, 135, 188–195. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Ali Razavi, S.M. Functional properties and applications of basil seed gum: An overview. Food Hydro. 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Choi, J.Y.; Heo, S.; Bae, S.; Kim, J.; Moon, K.D. Discriminating the origin of basil seeds (Ocimum basilicum L.) using hyperspectral imaging analysis. LWT 2020, 118, 108715. [Google Scholar] [CrossRef]
- Azuma, J.; Sakamoto, M. Cellulosic hydrocolloid system present in seed of plants. Trends Glycosci. Glycotechnol. 2003, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Melo, J.S.; D’Souza, S.F. Removal of chromium by mucilaginous seeds of Ocimum basilicum. Biorecour. Technol. 2004, 92, 151–155. [Google Scholar]
- Faria, P.C.C.; Orfao, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical identification of surface groups. J. Adv. Catal. Sci. Technol. 1966, 16, 179–274. [Google Scholar]
- Yokoyama, R.; Hayashi, S.; Nakanishi, M.; Takada, J. NO3-N adsorption property of Ca-containing charcoal. J. Jpn. Soc. Water Environ. 2008, 31, 47–52. [Google Scholar] [CrossRef]
- DzoyemL, J.P.; McGaw, J.; Kuete, V.; Bakowsky, U. Anti-inflammatory and Anti-nociceptive Activities of African Medicinal Spices and Vegetables. In Medicinal spices and vegetables from Africa: therapeutic potential against metabolic, inflammatory, infectious and systemic diseases; Academic Press: Amsterdam, The Netherlands, 2017; pp. 239–270. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Jang, J.; Mirana, W.; Divine, S.D.; Nawaz, M.; Shahzad, A.; Woo, S.H.; Lee, D.S. Rice straw-based biochar beads for the removal of radioactive strontium from aqueous solution. Sci. Total Environ. 2018, 615, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Kogej, A.; Pavko, A. Comparison of Rhizopus nigricans in a pelleted growth from with some other types of waste microbial biomass as biosorbents for metal ions. World J. Microbiol. Biotechnol. 2001, 17, 677–685. [Google Scholar] [CrossRef]
- Khandaker, S.; Kuba, T.; Kamida, S.; Uchikawa, Y. Adsorption of cesium from aqueous solution by raw and concentrated nitric acid-modified bamboo charcoal. J. Environ. Chem. Eng. 2017, 5, 1456–1464. [Google Scholar] [CrossRef]
- Dahiya, S.; Tripathi, R.M.; Hedge, A.G. Biosorption of heavy metals and radionuclide from aqueous solutions by pre-treated arca shell biomass. J. Hazard. Mater. 2008, 150, 376–386. [Google Scholar] [CrossRef]
- Jalali-Rad, R.; Ghafourian, H.; Asef, Y.; Dalir, S.T.; Sahafipour, M.H.; Gharanjik, B.M. Biosorption of cesium by native and chemically modified biomass of marine algal: introduce the new biosorbents for biotechnology applications. J. Hazard. Mater. 2004, B116, 125–134. [Google Scholar] [CrossRef]
- Pangeni, B.; Paudyal, H.; Inoue, K.; Kawakita, H.; Ohto, K.; Gurung, M.; Alam, S. Development of low cost adsorbents from agricultural waste biomass for the removal of Sr(II) and Cs(I) from water. Waste Biomass Valor. 2014, 5, 1019–1028. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffie, Kunglia Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption process. Pro. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chairat, M.; Rattanaphani, S.; Bremner, J.B.; Rattanaphani, V. An adsorption and kinetic study of lac dyeing on silk. Dyes Pigments 2005, 64, 231–241. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, T.; Li, P.; Jiang, R.; Wu, S.; Nie, H.; Wang, Y. Phosphorus recovery from biogas fermentation liquid by Ca-Mg loaded biochar. J. Environ. Sci. 2015, 29, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.T. Over the adsorption in solution. J. Phy. Chem. 1906, 57, 385–471. [Google Scholar]
- Abe, I.; Hayashi, K.; Kitagawa, M. Studies on the adsorption of surfactants on activated carbons. I. Adsorption of nonionic surfactants. Yukagaku 1976, 25, 145–150. [Google Scholar]




| Adsorbents | Enzyme | pH | Temperature (°C) |
|---|---|---|---|
| M-BS | Mannanase BGM | 5.0 | 50–70 |
| P-BS | Pectinase G | 4.0 | 50 |
| H-BS | Hemicellulase | 4.5 | 45 |
| C-BS | Cellulase A | 4.5 | 50–60 |
| Adsorbents | pHpzc | Surface Functional Groups (mmol/g) | Specific Surface Area (m2/g) | Pore Volume (cc/g) | |||
|---|---|---|---|---|---|---|---|
| Acidic | Basic | Micro | Meso | Macro | |||
| BS | 5.59 | 0.050 | 0.605 | 0.265 | N/A* | N/A* | N/A* |
| BS500 | 9.85 | 0.006 | 0.127 | 0.937 | N/A* | 0.004 | 0.006 |
| BS1000 | 0.006 | 0.006 | 0.187 | 0.187 | N/A* | 0.019 | 0.005 |
| Samples | Adsorbents | Adsorption Capability (mg/g) | pH | Temp.(°C) | Initial Concentration (mg/L) | Contact Time (h) | Adsorbent (g/L) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sr(II) | Paprika biochar | 0.02 | - | 25 | 1 | 24 | 1 | [26] |
| Spent mushroom biochar | 0.04 | - | 25 | 1 | 24 | 1 | [26] | |
| Rice straw biochar | 0.05 | - | 25 | 1 | 24 | 1 | [26] | |
| Brewery’s waste biomass | 7.91 | 4 | 30 | Approximately 700 | 3 | 2 | [10] | |
| Rhizopus nigricans | 24.5 | 5 | 22 ± 1 | 1000 | 2 | - | [27] | |
| BS | 0.63 | - | 25 | 1 | 24 | 1 | This study | |
| Cs(I) | Brewery’s waste biomass | 10.1 | 4 | 30 | Approximately 700 | 3 | 2 | [10] |
| Arca shell biomass | 4.00 | 5.5 | 25 ± 2 | 10–500 | 3 | 5 | [29] | |
| Bamboo charcoal | 0.17 | - | 20 | 800 | 6 | 1 | [28] | |
| Gracilaria corticata | 14.6 | 5.5 | 30 | 20–500 | 3 | 2 | [30] | |
| BS | 0.52 | - | 25 | 1 | 24 | 1 | This study |
| Samples | Adsorbents | qe,exp | Pseudo-First Order Model | Pseudo-Second Order Model | ||||
|---|---|---|---|---|---|---|---|---|
| k1 (1/h) | qe,cal (mg/g) | r | k2 (g/mg/h) | qe,cal (mg/g) | r | |||
| Sr(II) | BS | 632.5 | 2.4 × 10−1 | 293.0 | 0.961 | 1.2 × 10−3 | 674.9 | 0.986 |
| BS500 | 287.6 | 1.9 × 10−1 | 114.8 | 0.917 | 4.1 × 10−3 | 305.1 | 0.998 | |
| BS1000 | 587.3 | 6.4 × 10−1 | 547.1 | 0.956 | 1.8 × 10−3 | 621.9 | 0.998 | |
| Cs(I) | BS | 519.4 | 4.8 × 10−3 | 88.0 | 0.050 | −2.4 × 10−2 | 519.6 | 1.000 |
| BS500 | 191.7 | 5.4 × 10−2 | 10.9 | 0.459 | −1.5 × 10−2 | 151.8 | 0.972 | |
| BS1000 | 49.2 | 3.6 × 10−3 | 47.0 | 0.090 | −7.8 × 10−2 | 58.3 | 0.970 | |
| Samples | Adsorbents | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | r | logKF | 1/n | r | ||
| Sr(II) | BS | 441.6 | 5.6 × 10−3 | 0.962 | 1.3 | 1.5 | 0.966 |
| BS500 | 838.4 | 8.0 × 10−4 | 1.000 | 0.8 | 1.3 | 0.998 | |
| BS1000 | 825.0 | 1.1 × 10−3 | 0.989 | 1.1 | 0.9 | 0.990 | |
| Cs(I) | BS | 948.0 | 1.6 × 10−3 | 0.996 | 1.1 | 1.1 | 0.997 |
| BS500 | 421.0 | 1.0 × 10−3 | 0.986 | 1.1 | 0.3 | 0.981 | |
| BS1000 | 12.1 | 1.4 × 10−3 | 0.964 | 2.6 | 3.7 × 10−6 | 0.957 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uematsu, Y.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removing Sr(II) and Cs(I) from the Aqueous Phase Using Basil Seed and Elucidating the Adsorption Mechanism. Sustainability 2020, 12, 2895. https://doi.org/10.3390/su12072895
Uematsu Y, Ogata F, Saenjum C, Nakamura T, Kawasaki N. Removing Sr(II) and Cs(I) from the Aqueous Phase Using Basil Seed and Elucidating the Adsorption Mechanism. Sustainability. 2020; 12(7):2895. https://doi.org/10.3390/su12072895
Chicago/Turabian StyleUematsu, Yugo, Fumihiko Ogata, Chalermpong Saenjum, Takehiro Nakamura, and Naohito Kawasaki. 2020. "Removing Sr(II) and Cs(I) from the Aqueous Phase Using Basil Seed and Elucidating the Adsorption Mechanism" Sustainability 12, no. 7: 2895. https://doi.org/10.3390/su12072895





