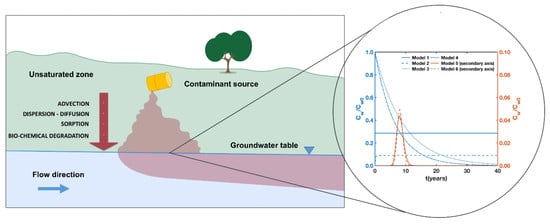
4.1. Predicted Concentration over the Time According to the Different Models
In order to evaluate the differences among the solutions of Models I-VI, the dimensionless concentrations C
w/C
w0 have been calculated at a certain spatial location (z = 5 m) as a function of time, fixing a single contaminant, Trichloroethylene (TCE), soil type (sand), source thickness (L
1 = 2 m). The results of the ratio between C
w/C
w0 are sketched in
Figure 2.
As shown in
Figure 2, the value of the initial dimensionless concentration and the shape of the curve are influenced by source hypotheses and transport mechanisms. According to these results, models belonging to group 1 (Model I and II) show a constant concentration over the time (with the exception of an initial transient phase for Model II); models belonging to group 2 (Model III and IV) show a decreasing exponential trend whereas models belonging to group 3 (Model V and VI) exhibit a temporal trend represented by a bell-shaped curve.
Model I is the most basic, as it neglects fate and transport mechanisms over time. Therefore, the dimensionless concentration C
w/C
w0 is constant. Conversely, Model II solves the transport equation, and it considers a constant source; therefore, at the initial time t = 0 the value of C
w/C
w0 is zero and after a transient phase reaches a constant value. Although both models showed a similar trend over the time, Model II depends on the spatial coordinates
x,
y, and
z. The models belonging to group 1 can be used when the reduction processes of the source, like leaching and volatilization losses or biodegradation, are negligible, so the source can be modelled as constant. Pascuzzi et al. [
71] used the model I for evaluating the risk for human health assuming a steady redeposition on the soil of pollutants in the selected site. The C
W/C
W0 was equal to 3.33 × 10
−2, while according to our results the C
W/C
W0 calculated through the application of the SAM coefficient ranges between 0.167 and 0.444. These different values are related to the differences in the adopted reference schemes, which cause a stronger attenuation process in the scheme presented by Pascuzzi et al. They consider a source thickness L
1 of 0.2 m and a distance between the bottom of the source and the water table L
f equal to 6m, while in our case L
1 ranges between 1 m and 4 m and L
f is 5 m.
Di Gianfilippo et al. [
51] used Model I to reproduce the transport of leaching substances from alkaline waste material assuming a constant source. They carried out a Monte Carlo analysis to evaluate a wide range of scenario conditions, varying the input parameters within selected intervals. The SAM value was estimated using a value of L
1 varying from 0.1 m to 1 m and a value of L
f from 5 to 10 m, thus obtaining a value of C
W/C
W0 ranging between 9.9 × 10
−3 and 0.167. These concentrations are slightly lower than those calculated in this work because of the different geometrical conditions, as in the previous case.
The solutions of the group 2 consist of exponential laws, describing the depletion processes of the source. Model III assumes instantaneous mixing in the unsaturated zone, and it does not consider transport mechanisms. Therefore, Cw/Cw0 is spatially constant along the vertical axis, and it decreases over time. In Model IV the exponential law is multiplied by αleach. In this scenario, the value of αleach is approximately equal to 1, because of the selected type of soil and the contaminant. In consequence, the exponential part is dominant. The depletion coefficients μ1 and μ2 are equal to 3.469 × 10−9 s−1 and 4.800 × 10−9 s−1, respectively. They are indicators of the depletion rate of the contaminant and differ only for the retardation coefficient. Therefore, in these conditions, the results of the two models are very similar. For group 2, the source depletion processes (bio-chemical degradation and leaching losses) are predominant, while the transport mechanisms are less relevant.
Models V and VI can be considered as the more advanced ones as they consider both the source depletion and a transient transport equation. This causes the distinctive bell-shape, yielding a dimensionless concentration peak lower than the other models I-IV. The solutions contain two parts: a time-dependent exponential law and the space- and time-dependent solution of the transport equation. For Model V the first part takes into account only the biochemical degradation, while for Model VI, it considers leaching and volatilization losses. The second part of the solution is different due to the initial and boundary conditions of the transport equation. For these reasons, the peaks of the solutions differ between the Models V and VI. In this scenario, for Model V the maximum of the normalized concentration is 4.338 × 10−2 at 8.17 y, while in Model VI, the maximum of the normalized concentration corresponds to 4.920 × 10−2 at 8.08 y. These models allow to describe the fate and transport mechanisms more thoroughly than the other models. In this case, the predicted concentrations are approximately an order of magnitude lower than those obtained by using the other models.
For these reasons, in some applications, the models of these groups are preferable. Di Sante et al. [
72] used Model VI to assess the sanitary and environmental risk of a disused industrial plant contaminated with polycyclic aromatic hydrocarbons, heavy hydrocarbons and polychlorinated biphenyls. Although the Italian guidelines [
73] consider adequate a steady-state model to reproduce leaching phenomena, the authors suggest the use of a transient model for contaminants with high experimental leaching value, because they are more suitable to reproduce real conditions. They evaluated concentrations of aromatic fraction of heavy hydrocarbons and benzo(a)pyrene after 20 years roughly equal to 0.40 mg/L e 1 × 10
−6 mg/L, respectively. Their results consider not only the leaching process but also the uniform dilution in groundwater, which is not considered by this paper. Furthermore, their results cannot be expressed as a dimensionless quantity. For these reasons, a direct comparison with the results of this work is not possible.
The different output concentrations of the models lead to different amounts of contaminant reaching the water table. In order to determine and compare the mass value reaching the groundwater for the different models, the mass flux (
J) from unsaturated zone to groundwater is integrated over the time.
J is governed only by water phase advection, and it is evaluated as suggested in [
43]:
where
Auz-gw is the exchange area between the two zones.
For all the models but Model II,
Auz-gw is equal to
A, and the dissolved concentration
Cw is constant over the area. In the paper describing Model II [
43],
Auz-gw is bigger than
A, and the concentration is variable in space; hence, it is necessary to integrate
C(
x,y,t) over space and time.
For the considered contaminant, soil type and source thickness, the mass reaching the water table, considering a period of twenty years and forty years, has been calculated starting from an initial source concentration
Cw0 (100 μg/dm
3); see
Table 6. As can be noted, the third group of models returns a lower contaminant mass than the others. After 20 years, the exponential models belonging to group 2 exhibit the highest values, while extending the period of time to 40 years Model I exhibits the maximum value of
J.
4.2. Predicted Concentration of Different Contaminants over Time
The physico-chemical characteristics of the contaminant, e.g., the volatility and the degradation rate [
52,
74], strongly affect its mobility through the unsaturated zone as well as its fate in time. The selected models are solved using the contaminants properties listed in
Table 5, fixing the thickness of the source
L1 = 2 m and a sand soil.
The transport time ranges between some orders of magnitude according to the contaminant mobility. Hence, in order to overcome this discrepancy and compare the results consistently, the dimensionless concentration has been plotted versus a dimensionless time τ, calculated as the ratio between the physical time and a reference time tref. This criterion has not been used for Model I because the results are not depended by time and contaminant properties. In this case, tref has not been considered, and the dimensionless concentration is plotted versus physical time. The value of the tref cannot be evaluated the same way for all the models. For models belonging to the groups 2 and 3, the dimensionless concentration goes to zero after a certain period of time, depending on contaminants and medium properties. Therefore, the value of tref has been set equal to the time when Cw/Cw0 reaches the residual value of 0.1%. For model II, the value of tref has been calculated with a different criterion, as in this case Cw/Cw0 tends to a horizontal asymptote: It is set equal to the time when the variation of the concentration is negligible (<0.01 μg/dm3). As one can perceive, in this case the value of tref depends on the temporal discretization, which has been set equal to 1 month for Benzene, Vinyl chloride and Trichloroethylene and 1 year for Benzo(a)pyrene and Aldrin.
The values of
tref are listed in
Table 7. It can be observed that Model III has the highest
tref for all the contaminants. More generally, the models belonging to group 2 exhibit a higher t
ref than the other models for the contaminants characterized by a greater mobility (Benzene, Vinyl Chloride, and Trichloroethylene). For Benzo(a)pyrene and Aldrin, the reference time
tref of Model V is not reported because C
w is negligible.
For the Model II (
Figure 3b), it is possible to observe a marked distinction in the trend concentration between contaminants characterized by a less mobility (Benzo(a)pyrene and Aldrin) and the others (Benzene, Vinyl chloride, Trichloroethylene). The first group of contaminants reaches a steady-state concentration higher than the others: the C
w/C
w0 ratio is equal to 0.443 for Benzo(a)pyrene and 0.358 for Aldrin. The second group reaches lower values, the C
w/C
w0 ratio is about equal to 0.090 for the three contaminants. These results are in accordance with the application of the model presented by Locatelli et al. [
75], considering the different hydrogeological setting of the simulated site. Locatelli et al. [
75] used the model II to simulate leaching of benzene and toluene in a simulated contamination scenario; at 5 meters, they obtain a C
w/C
w0 ratio corresponding to 0.01 for benzene and 0.04 for toluene. The lower value compared to the obtained value in this paper can be attributed to the different hydrogeological setting and the different assessment of some parameters, like the degradation constant.
The Cw/Cw0 ratios for Benzo(a)pyrene and Aldrin in the Model II are higher than in Model I (Cw/Cw0 = 0.286). This proves that in some circumstances, the Model I is not the most conservative approach, as it is typically considered (ASTM, 2000). This result can be related to the fact that in Model I the SAM is assumed simplistically constant for all the contaminants.
In Model III, as can be seen from
Figure 3c, the results collapse on the same curve as consequence of the use of the dimensionless time τ. This occurs in Model IV to some extent. In fact, as previously explained, Model IV consists of two terms: α
dep and α
leach. The former is characterized by scale invariance while the latter is approximately equal to one for all the contaminants. The minimum value of α
leach corresponding to 0.930 has been obtained for Benzo(a)pyrene.
In Model V the concentrations of contaminants with low mobility (Benzo(a)pyrene and Aldrin) is closed to zero during the whole considered range of time (10
4–10
5 years) because of the exponential part of the solution. Therefore, the results of this model (
Figure 3e) are shown only for the three contaminants Benzene, Vinyl Chloride and Trichloroethylene. In
Figure 3f. sketching the results of Model VI, it is possible to identify two groups of contaminants, characterized by low and high mobility, respectively. The concentration of contaminants belonging to the first group reach a maximum value earlier than the second group.
Comparison between Different Degradation Constants
The contaminant degradation into the environment, λ, simulated through a first order kinetics, takes into account both chemical mechanisms (hydrolysis, redox reductions and photodegradations) and biodegradation. Several mechanisms are involved, and each of them can be affected by many environmental variables, making the assessments of these coefficients highly uncertain [
76,
77]. The λ values can vary by more than one order of magnitude for each contaminant in a natural porous media [
15,
78]. For these reasons, a sensitivity analysis based on the variation of λ has been carried out. The comparison is developed with respect to the Trichloroethylene compound, in the same conditions of the previous sections: depth equal to z = 5 m, source thickness
L1 equal to 2 m and the unsaturated zone composed by sand. In Howard [
66], the values of λ for Trichloroethylene in groundwater range from 4.852 × 10
−9 s
−1 to 2.499 × 10
−8 s
−1. Three values have then been chosen for the comparison: the first λ
1 is the minimum value (4.852 × 10
−9 s
−1), the second λ
2 is the mean of logarithms of the two extremes (1.101 × 10
−9 s
−1), and the third λ
3 is the maximum value (2.499 × 10
−8 s
−1). The results of the comparison are shown in
Figure 4.
As already highlighted, Model I is independent of contaminant properties; therefore, the ratio C
w/C
w0 is constant, equal to 0.286 for all the values of λ. In Model II (
Figure 4b), little differences related to the decay coefficients can be noted. The obtained C
w/C
w0, equal to 0.0901, 0.0884 and 0.0848 for λ
1, λ
2 and λ
3, respectively, present similar values, reaching the steady state conditions in a small period of time.
In the Models III and IV (
Figure 4c,d), the λ coefficients play the same role, affecting the depletion coefficients μ
1 and μ
2, see
Table 1.
The variation of λ affects Model V more than Model VI (
Figure 4e,f). In particular, it influences the C
w/C
w0 peak (maximum concentration) as well as the time when it occurs. In both models, the higher is the value of λ, the earlier and lower is the peak. In Model V, C
w/C
w0 peaks correspond to 0.0438 at 8 y, 0.0094 at 7.83 y, 0.00033 at 7.42 y for the three values of λ considered, respectively, whereas in Model VI the C
w/C
w0 are equal to 0.0492 at 8.08 y, 0.0247 at 7.92 y and 0.0053 at 7.83 y.
4.3. Comparison between Different Soils
The physical properties of soil represent a further element that influences the fate and transport mechanisms of the contaminants. Simulations with three different soils (sand, loam, clay) have been carried out, in order to evaluate the corresponding differences in the models. Again, the comparison has been developed with respect to the Trichloroethylene compound in the same conditions depicted in the previous section. The three types of soils are characterized by the same organic carbon fraction, whereas they differ in grain size, water content, hydraulic conductivity and infiltration rate. The soil parameters values are listed in
Table 4.
It is worth noting that the analyzed models consider the sorption process only through the soil-water partition coefficient, which is a synthetic parameter considering the characteristics of the soil, the contaminant and their interaction. This parameter should be assessed experimentally with reference to the specific soil-contaminant complex. In this analysis, it has been chosen to evaluate the partition coefficient referring only to the contaminant characteristics. For this reason, the obtained results for the different types of soils are affected only by the difference between hydrological characteristics of the soils, while the differences related to the sorption capacity are neglected. This assumption has resulted in a lower partition coefficient value of the clays than the experimental values in these soils and, consequently, a higher value of the simulated contaminant concentration. Clay soils have, in fact, a higher sorption behavior than the other soils because of the high surface area and negative charged sites of the soils particles.
As previously seen, Model I is independent by the soil parameters (
Figure 5a). Model II (
Figure 5b) shows a similar behavior for sand and loam, reaching the steady state C
w/C
w0 ratios corresponding to 0.0902 and 0.0836, at 1.67 y and 2.42 y respectively. A lower mobility could be observed for clay. In this case the steady concentration, C
w/C
w0 equal to 0.0538, is reached at t = 7.67 y. Hence, the leaching velocity has a greater impact compared to the sorption capacity.
Model III and IV give similar trends as shown in
Figure 5c,d, respectively. In Model III, the time needed to reach the 0.1% of the initial concentration, is equal to 63.17 y, 72.67 y, 69.83 y whereas, in Model IV the time needed to reach the 0.1% of the initial concentration is equal to 45.67 y, 63.00 y and 68.58 y for sand, clay and loam, respectively.
Models V and VI are most influenced by soil parameters, which affect both the time at which the maximum concentration occurs at z = 5 m and the related values. The transport mechanism lasts very long when Model V is applied for clay. Hence the concentration is so extremely low and therefore undetectable. The Cw/Cw0 peaks are equal to 4.380 × 10−2 at 8 y and 8.651 × 10−3 at 18.67 y in sand and loam, respectively. In Model VI the peaks are of the same order of magnitude for sand and loam, whereas it is negligible for clay. In this case, the Cw/Cw0 ratios are equal to 4.920 × 10−2 at 8.08 y and 1.509 × 10−2 at 19.75 y for sand and loam, respectively.
4.4. Comparison between Different Source Thickness
As can be seen in
Table 1, the thickness of the source L
1 determines the soil attenuation of the contaminant in the Model I, and the time needed to deplete the source in the models composed of an exponential part (Models III to VI). Model II is not influenced by the change of source thickness because it does not take into account the source depletion and soil attenuation. The simulations have been carried out taking into account the scheme depicted in
Figure 1, with three different source depths: L
1 = 1 m, 2 m and 4 m, using sand as type of soil and Trichloroethylene as contaminant. Again, the dimensionless concentration C
w/C
w0 has been calculated at the depth z = 5 m. The concentrations calculated with Model I (
Figure 6a) shows a significant variation in relation to the source thickness, being equal to 0.167, 0.286 and 0.444, for L
1 equal to 1 m, 2 m, 4 m respectively.
In the exponential Models III and IV (
Figure 6c,d), the source thickness has a different effect. The depletion coefficients μ
1 and μ
2 (
Table 1) may be used as indicators of the contaminant depletion velocity. The time needed to reach the 0.1% of the initial concentration increases with thickness, reaching the values of 58, 64 and 73 years in the Model III. In Model IV the time needed to reach the 0.1% of the initial concentration is equal to 27, 46 and 70 years for the three values of L
1.
In the Model V, an increased thickness of the source determines delayed and higher peaks. The Cw/Cw0 ratios are 2.25 × 10−2 (t = 7.92 y), 4.38 × 10−2 (t = 8.00 y) and 8.24 × 10−2 (t = 8.25 y) for L1 equal to 1 m, 2 m, 4 m respectively.
In the Model VI, the increasing of the source thickness lead to higher but earlier peaks. The Cw/Cw0 ratios are equal to 3.19 × 10−2 (t = 8.08 y), 4.92 × 10−2 (t = 8.08 y) and 7.93 × 10−2 (t = 8.00 y), for L1 equal to 1 m, 2 m, 4 m respectively.