Ammonium as a Carbon-Free Electron and Proton Source in Microbial Electrosynthesis Processes
Abstract
:1. Introduction
2. Processes
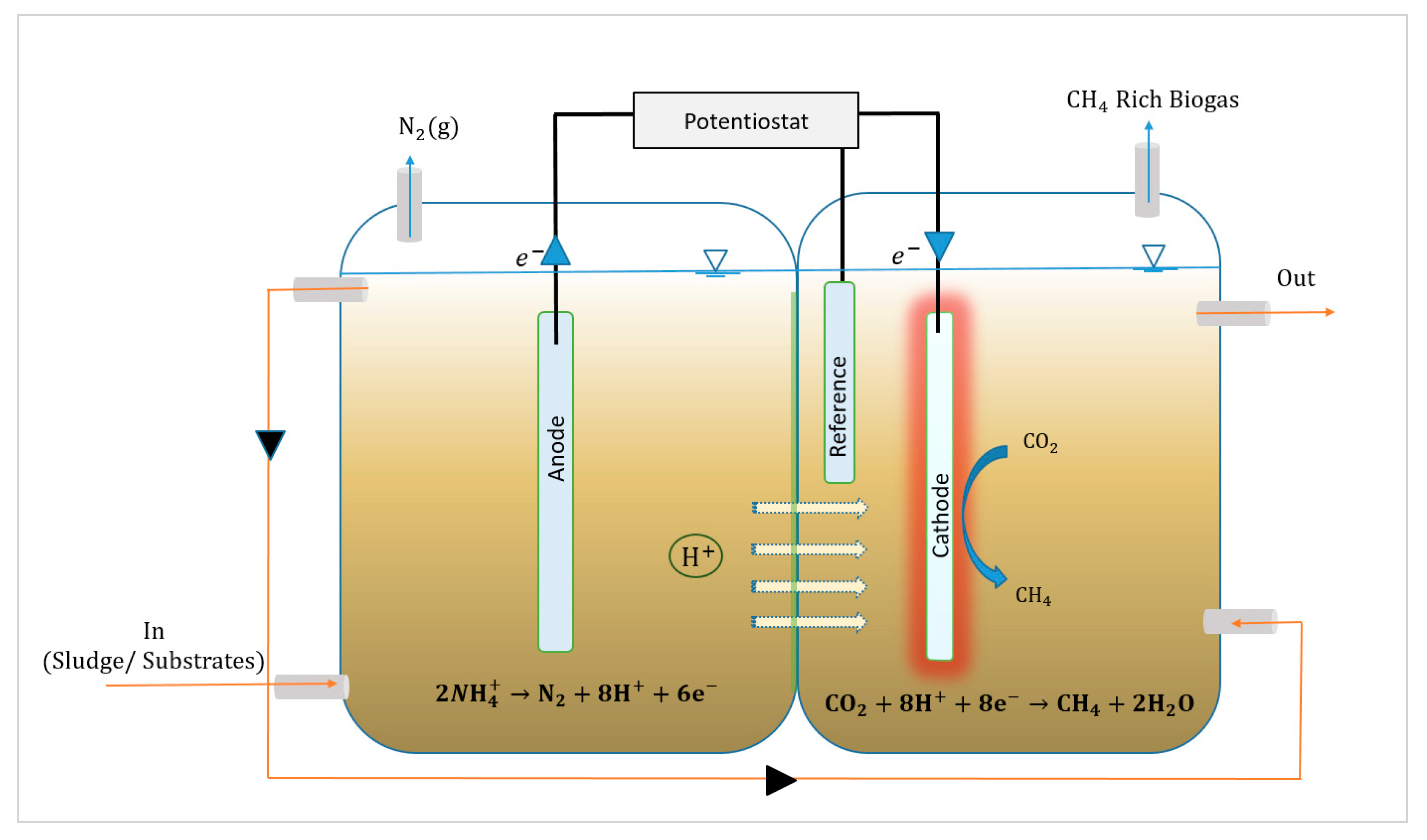
2.1. Anode:Electrochemical Process
2.2. Cathode:Bioelectrochemical Process
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, M.; Song, W.; Buhain, J. Bioenergy and biofuels: History, status, and perspective. Renew. Sustain. Energy Rev. 2015, 42, 712–725. [Google Scholar] [CrossRef]
- Samarakoon, G.; Nelabhotla, A.B.; Dinamarca, C.; Winkler, D.; Bakke, R. Modelling Bio-Electrochemical CO2 Reduction to Methane. In Proceedings of the 10th Trondheim Conference on Carbon Capture, Transport and Storage, Trondheim, Norway, 17–19 June 2019. [Google Scholar]
- Nelabhotla, A.B.T.; Dinamarca, C. Bioelectrochemical CO2 Reduction to Methane: MES Integration in Biogas Production Processes. Appl. Sci. 2019, 9, 1056. [Google Scholar] [CrossRef] [Green Version]
- Schievano, A.; Pant, D.; Puig, S. Editorial: Microbial Synthesis, Gas-Fermentation and Bioelectroconversion of CO2 and Other Gaseous Streams. Front. Energy Res. 2019, 7, 110. [Google Scholar] [CrossRef]
- Nelabhotla, A.B.T.; Dinamarca, C. Electrochemically mediated CO 2 reduction for bio-methane production: A review. Rev. Environ. Sci. Biotechnol. 2018, 17, 531–551. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.-C.; Ahn, Y. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor. Bioresour. Technol. 2018, 259, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bian, B.; Bajracharya, S.; Xu, J.; Pant, D.; Saikaly, P.E. Microbial electrosynthesis from CO2: Challenges, opportunities and perspectives in the context of circular bioeconomy. Bioresour. Technol. 2020, 122863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katuri, K.P.; Kalathil, S.; Ragab, A.; Bian, B.; Alqahtani, M.F.; Pant, D.; Saikaly, P.E. Dual-function electrocatalytic and macroporous hollow-fiber cathode for converting waste streams to valuable resources using microbial electrochemical systems. Adv. Mater. 2018, 30, 1707072. [Google Scholar] [CrossRef] [PubMed]
- Katuri, K.P.; Ali, M.; Saikaly, P.E. The role of microbial electrolysis cell in urban wastewater treatment: Integration options, challenges, and prospects. Curr. Opin. Biotechnol. 2019, 57, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Girguis, P.; Nielsen, L.K. Metabolic and practical considerations on microbial electrosynthesis. Curr. Opin. Biotechnol. 2011, 22, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Service, R. Ammonia—A Renewable Fuel Made from Sun, Air, and Water—Could Power the Globe without Carbon. Science 2018. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M. Removal of ammonium ions by selected natural and synthetic zeolites. Gospod. Surowcami Miner. 2010, 26, 133–148. [Google Scholar]
- Nordgård, A.S.R.; Bergland, W.H.; Vadstein, O.; Mironov, V.; Bakke, R.; Østgaard, K.; Bakke, I. Anaerobic digestion of pig manure supernatant at high ammonia concentrations characterized by high abundances of Methanosaeta and non-euryarchaeotal archaea. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D.; Tsuchihashi, R.; Metcalf & Eddy; Eecom, I.A. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGRAW-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- He, Z.; Kan, J.; Wang, Y.; Huang, Y.; Mansfeld, F.; Nealson, K.H. Electricity production coupled to ammonium in a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 3391–3397. [Google Scholar] [PubMed]
- Jadhav, D.A.; Ghangrekar, M.M. Effective ammonium removal by anaerobic oxidation in microbial fuel cells. Environ. Technol. 2015, 36, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Candido, L.; Gomes, J.A.C.P. Evaluation of anode materials for the electro-oxidation of ammonia and ammonium ions. Mater. Chem. Phys. 2011, 129, 1146–1151. [Google Scholar] [CrossRef]
- Ray, S.G.; Ghangrekar, M.M. Mechanisms of Charge Transfer during Bio-Cathodic Electro-Synthesis of CO2 neutral Methane. Adv. Biotech. Micro. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.-H.; Lacroix, R.; Quéméner, E.D.-L.; Bureau, C.; Midoux, C.; Bouchez, T. Upscaling of Microbial Electrolysis Cell Integrating Microbial Electrosynthesis: Insights, Challenges and Perspectives. bioRxiv 2019, 609909. [Google Scholar] [CrossRef]
- Nelabhotla, A.B.T. Electrochemical Unit Integration with Biogas Production Processes; University of South-Eastern Norway: Nortoden, Norway, 2020; ISBN 978-82-7206-547-7. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivalingam, V.; Dinamarca, C.; Samarakoon, G.; Winkler, D.; Bakke, R. Ammonium as a Carbon-Free Electron and Proton Source in Microbial Electrosynthesis Processes. Sustainability 2020, 12, 3081. https://doi.org/10.3390/su12083081
Sivalingam V, Dinamarca C, Samarakoon G, Winkler D, Bakke R. Ammonium as a Carbon-Free Electron and Proton Source in Microbial Electrosynthesis Processes. Sustainability. 2020; 12(8):3081. https://doi.org/10.3390/su12083081
Chicago/Turabian StyleSivalingam, Vasan, Carlos Dinamarca, Gamunu Samarakoon, Dietmar Winkler, and Rune Bakke. 2020. "Ammonium as a Carbon-Free Electron and Proton Source in Microbial Electrosynthesis Processes" Sustainability 12, no. 8: 3081. https://doi.org/10.3390/su12083081




