Effects of Garden Waste Compost and Bentonite on Muddy Coastal Saline Soil
Abstract
1. Introduction
2. Materials and Methods
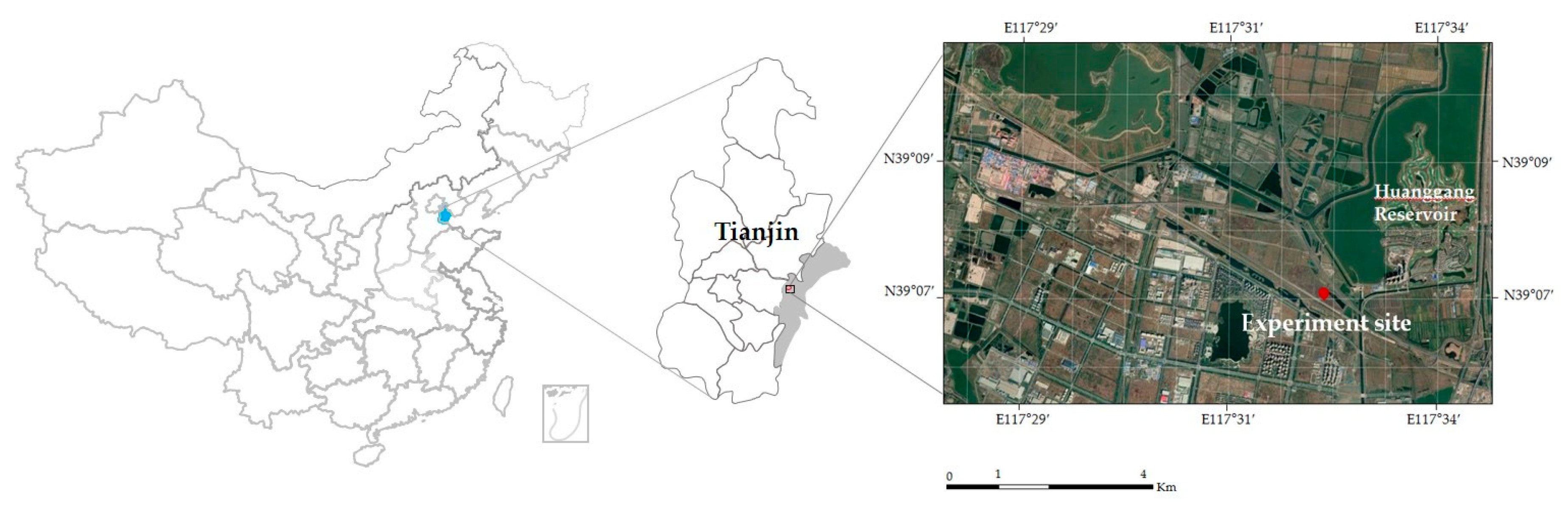
2.1. Experimental Site and Material
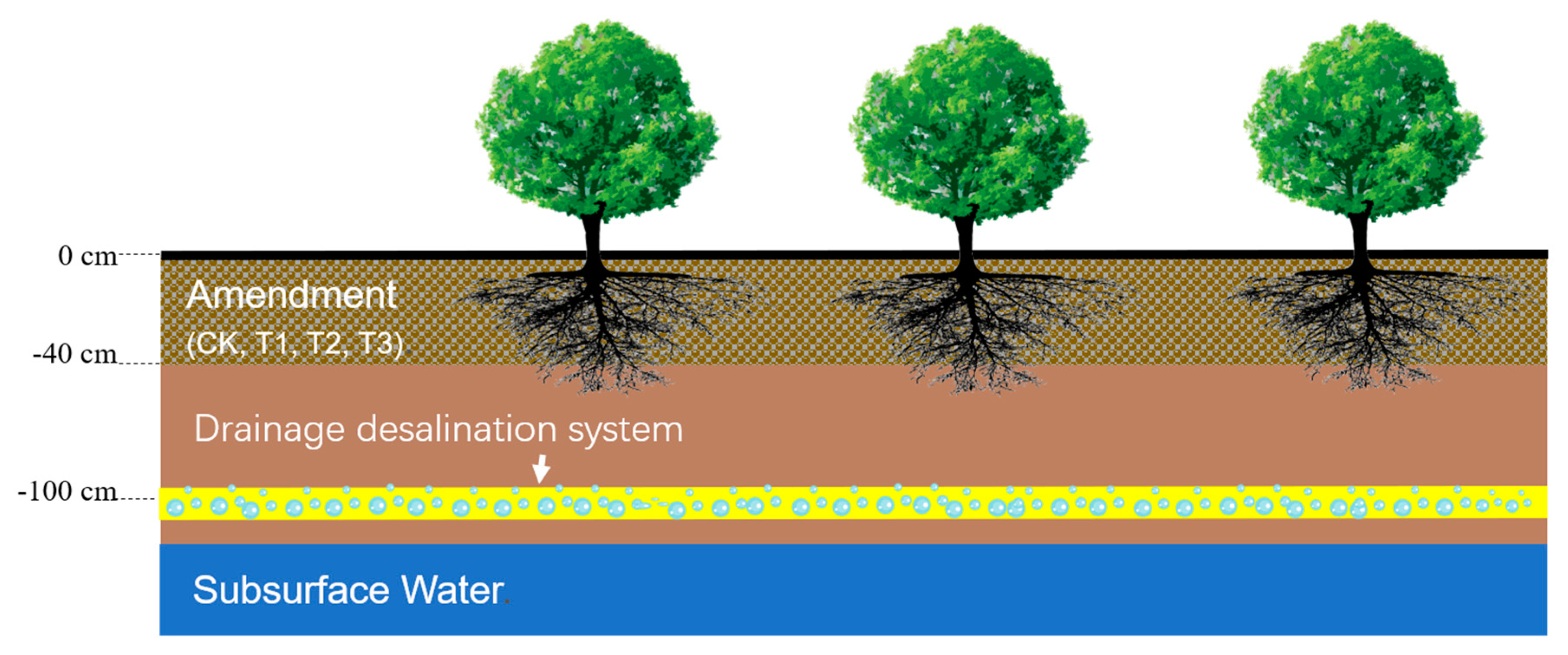
2.2. Experiment
2.3. Measurement and Analysis
- xi: The value of the i-th indicator
- fvi: The eigenvector value of the i-th indicator
- Fj: The jth principal component score
- Q: The soil improvement effect comprehensive evaluation score
- vcj: The variance contribution rate of the j-th principal component
- ac: The cumulative contribution rate of all principal components
3. Results
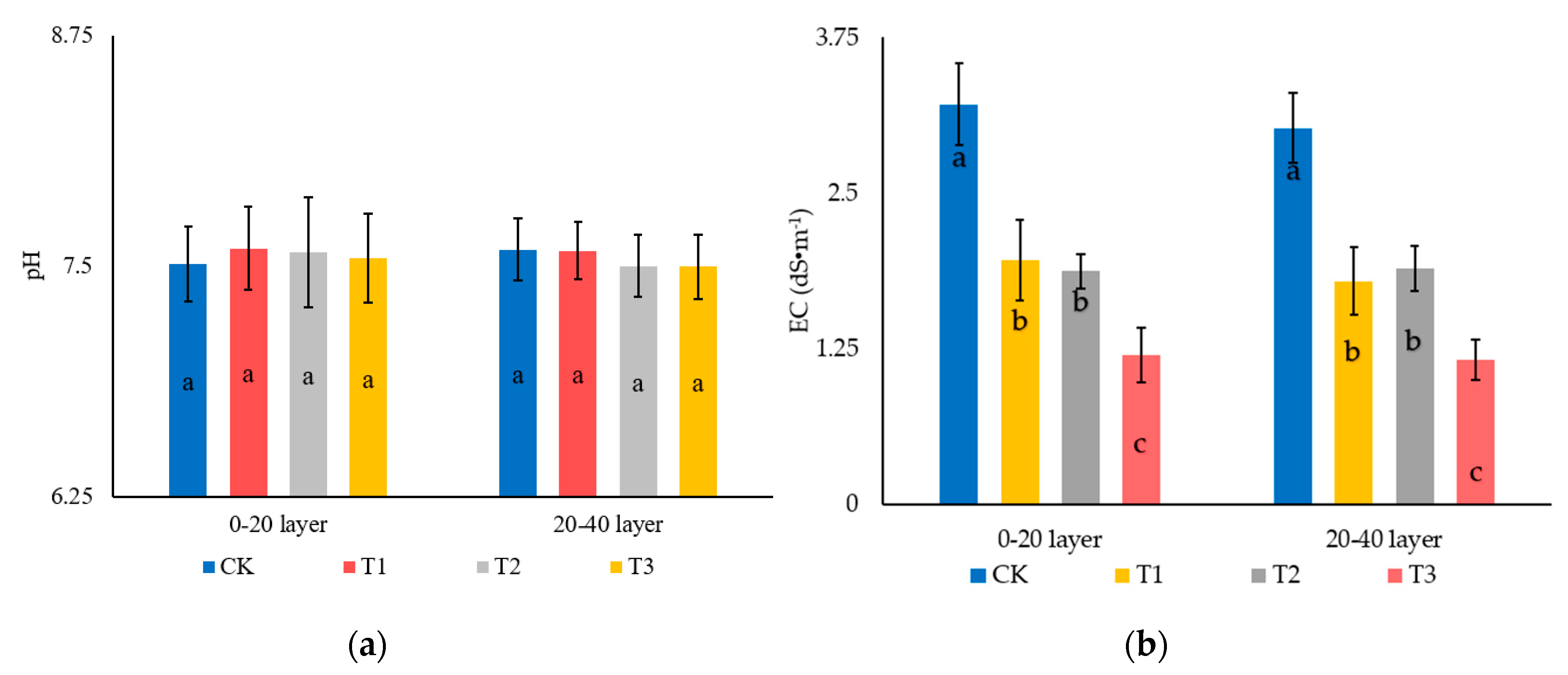
3.1. Effect of Garden Waste and Bentonite on the Soil pH and Electric Conductivity (EC)
3.2. Effect of Garden Waste and Bentonite on Soil Permeability
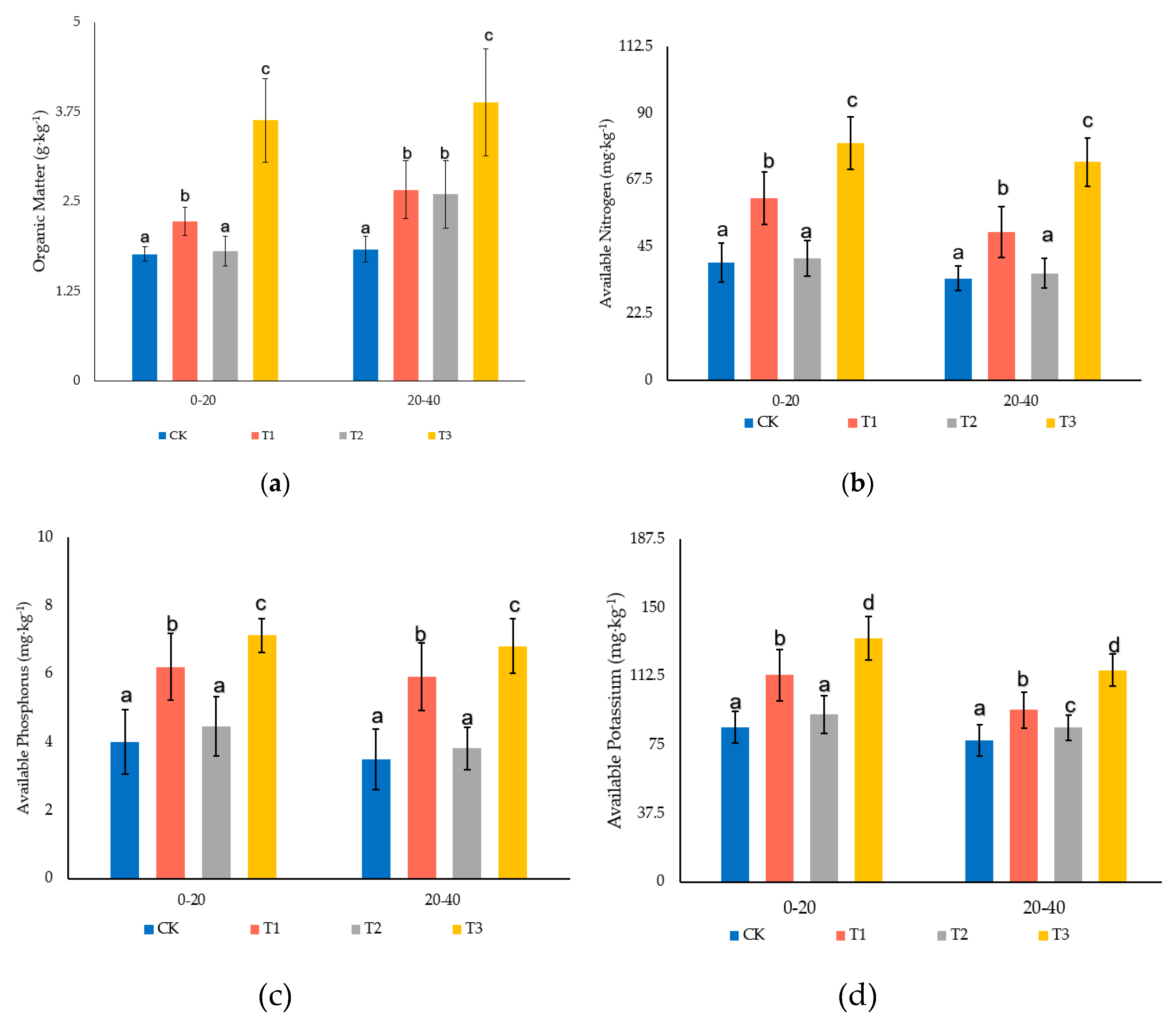
3.3. Effect of Garden Waste and Bentonite on Soil Nutrients
3.4. Effect of Garden Waste and Bentonite on Tree Growth
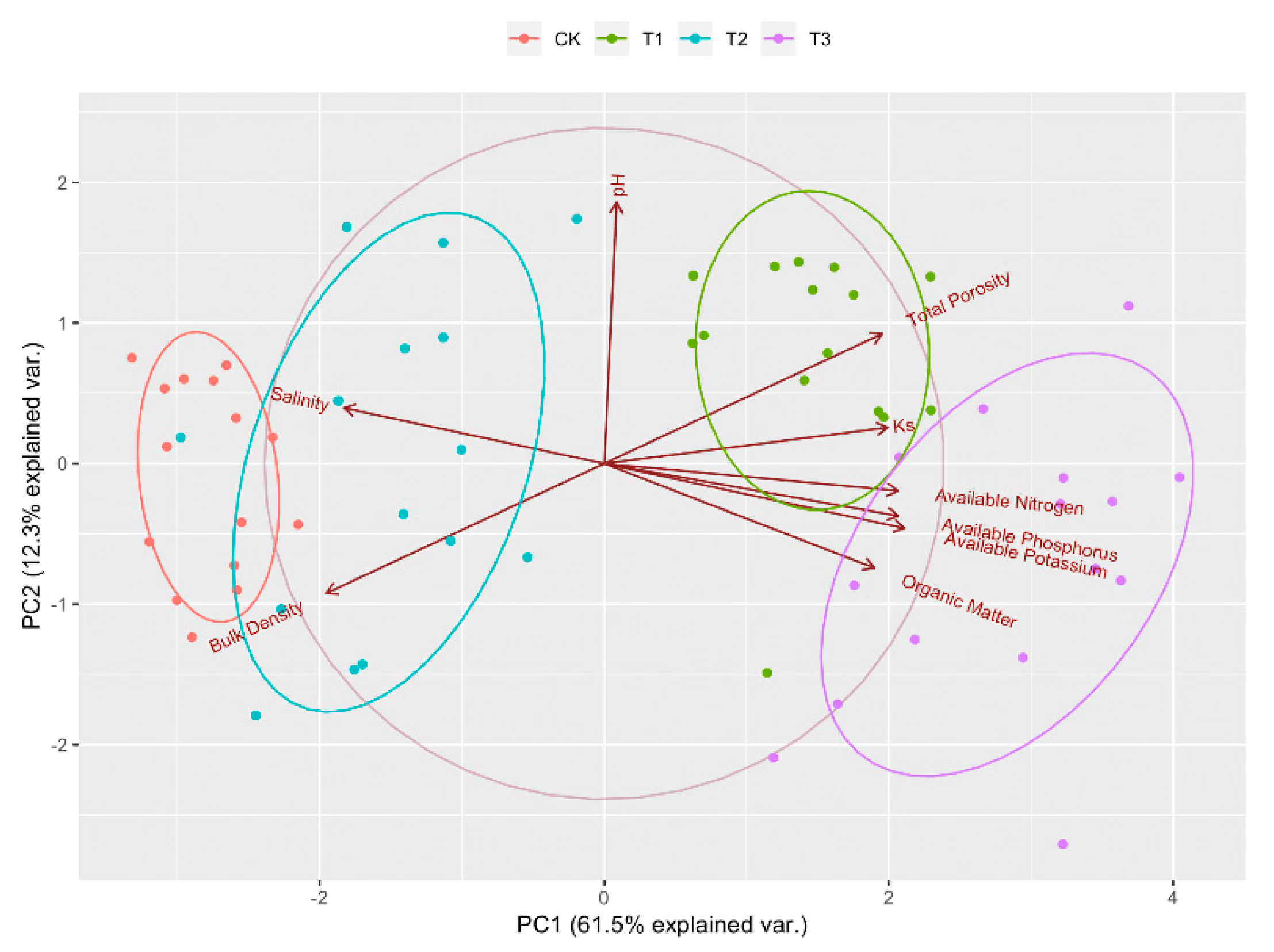
3.5. Comprehensive Evaluation of Soil Improvement Effects under Different Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehta, A.J. Mudshore dynamics and controls. In Muddy Coasts of the World: Processes, Deposits and Function, 1st ed.; Healy, T., Wang, Y., Healy, J., Eds.; Elsevier Science B. V.: Amsterdam, The Netherlands, 2002; pp. 58–60. [Google Scholar]
- Liu, W.L.; Luo, W.; Jia, Z.H.; Pan, Y.X.; Yang, Y.Z.; Pu, F.M. Comprehensive assessment of subsurface drainage construction in the Yellow River Delta. Agric. Res. Arid Areas 2013, 31, 122–126. [Google Scholar]
- Cui, L.L.; Li, G.S.; Ouyang, N.L.; Mu, F.Y.; Yan, F.; Zhang, Y.T.; Huang, X.Y. Analyzing Coastal Wetland Degradation and its Key Restoration Technologies in the Coastal Area of Jiangsu, China. Wetlands 2018, 38, 525–537. [Google Scholar] [CrossRef]
- Hassan, M.I.; Rahmat, N.H. The Effect of Coastline Changes to Local Community’s Social-Economic. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2016, 42, 25–36. [Google Scholar] [CrossRef]
- Ye, G.F.; Luo, M.J.; Lu, C.Y. Restoration of Degraded Ecosystem and Integrated Management in Coastal Zone. World For. Res. 2006, 19, 5–10. [Google Scholar]
- Peng, S.L.; Zhou, T.; Wang, D.L.; Gao, Y.Z.; Zhong, Z.W.; Xie, D.; Zhou, H.J.; Ji, H.T.; An, S.Q.; Dong, M.; et al. Restoration of Degraded Ecosystem. In Contemporary Ecology Research in China; Li, W.H., Ed.; Springer: Berlin, Germeny, 2015; pp. 235–263. [Google Scholar]
- Sun, J.N.; Yang, R.Y.; Li, W.X.; Pan, Y.H.; Zheng, M.Z.; Zhang, Z.H. Effect of biochar amendment on water infiltration in a coastal saline soil. J. Soil. Sediments. 2018, 18, 3271–3279. [Google Scholar] [CrossRef]
- Zhang, J.F. Coastal Saline Soil Rehabilitation and Utilization Based on Forestry Approaches in China; Springer: Berlin, Germeny, 2014; pp. 154–159. [Google Scholar]
- Zhang, T.; Wang, T.; Liu, K.S.; Wang, L.X.; Wang, K.; Zhou, Y. Effects of different amendments for the reclamation of coastal saline soil on soil nutrient dynamics and electrical conductivity responses. Agric. Water Manag. 2015, 159, 115–122. [Google Scholar] [CrossRef]
- Yin, A.J.; Zhang, M.; Gao, C.; Yang, X.H.; Xu, Y.; Wu, P.B.; Zhang, H. Salinity evolution of coastal soils following reclamation and intensive usage, Eastern China. Environ. Earth Sci. 2016, 75, 1281–1292. [Google Scholar] [CrossRef]
- Wang, L.L.; Li, S.Y.; Sun, X.Y.; Zhang, T.; Fu, Y.; Zhang, H.L. Application of salt-isolation materials to a coastal region: Effects on soil water and salt movement and photosynthetic characteristics of Robinia pseudoacacia. Acta Ecol. Sinica 2015, 35, 1388–1398. [Google Scholar]
- Zhang, F.R.; Zhang, Z. The Reclamation Effects Should Be Considered for Saline Soil Criteria in Soil Classification System. In Developments in Soil Salinity Assessment and Reclamation: Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Shabbir, A.S., Mahmoud, A.A., Faisal, K.T., Eds.; Springer Science+Business Media: Berlin/Heidelberg, Germany, 2013; pp. 357–363. [Google Scholar]
- Haj-Amor, Z.; Bouri, S. Subsurface Drainage System Performance, Soil Salinization Risk, and Shallow Groundwater Dynamic Under Irrigation Practice in an Arid Land. Arab. J. Sci. Eng. 2019, 44, 467–477. [Google Scholar] [CrossRef]
- Nemr, A.M.E.; Sikaily, A.E.; Khaled, A. Total and Leachable Heavy Metals in Muddy and Sandy Sediments of Egyptian Coast Along Mediterranean Sea. Environ. Monit. Assess. 2007, 129, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Awn, S.H.A. Stabilization of Saline Soils by Different Active Techniques. Diyala J. Eng. Sci. 2015, 8, 46–60. [Google Scholar]
- Anandhanarayanan, G.; Murugaiyan, V. Effects Of Salt Solutions and Sea Water on the Geotechnical Properties of Soil—A Review. Int. J. Engi. Res. Tech. 2014, 3, 1819–1824. [Google Scholar]
- Gong, X.Q.; Li, S.Y.; Michael, A.C.; Scott, X.C.; Wu, Q.; Wang, L.; An, Z.F.; Sun, X.Y. Spent mushroom substrate and cattle manure amendments enhance the transformation of garden waste into vermicomposts using the earthworm Eisenia fetida. J. Environ. Manag. 2019, 248, 109263. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Sun, X.Y.; Li, S.Y.; Qu, B.P.; Wan, L. Reutilization of Green Waste as Compost for Soil Improvement in the Afforested Land of the Beijing Plain. Sustainability 2018, 10, 2376. [Google Scholar] [CrossRef]
- Lohri, C.R.; Diener, S.; Zabaleta, I.; Mertenzt, A.; Zurbrügg, C. Treatment technologies for urban solid biowaste to create value products: A review with focus on low- and middle-income settings. Rev. Environ. Sci. BioTech. 2017, 16, 81–130. [Google Scholar] [CrossRef]
- Mohan, S.M. Biodegradation of Garden Waste, Market Waste Using Eisenia fetida and Eudrilus eugenia and Assessment of Manure Quality on Tomato. J. Instit. Eng. (India) Ser. A. 2014, 95, 75–82. [Google Scholar] [CrossRef]
- Lakhdar, A.; Rabhi, M.; Ghnaya, T.; Montemurro, F.; Jedidi, N.; Abdelly, C. Effectiveness of compost use in salt-affected soil. J. Hazard. Mater. 2009, 171, 29–37. [Google Scholar] [CrossRef]
- Rusterholz, H.P.; Wirz, D.; Baur, B. Garden waste deposits as a source for non-native plants in mixed deciduous forests. Appl. Veg. Sci. 2012, 15, 329–337. [Google Scholar] [CrossRef]
- Tang, J.; Xu, Y. Agricultural and Forestry Waste Composts as Substitutes for Peat in Potting Media: Effects on Root Growth and Fractal Features of New Guinea impatiens (Impatiens hawkeri). Agric. Res. 2016, 5, 269–276. [Google Scholar] [CrossRef]
- Tits, M.; Elsen, A.; Bries, J.; Vandendriessche, H. Short-term and long-term effects of vegetable, fruit and garden waste compost applications in an arable crop rotation in Flanders. Plant Soil 2014, 376, 43–59. [Google Scholar] [CrossRef]
- Andersson, S.; Nilsson, S.I.; Saetre, P. Leaching of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) in mor humus as affected by temperature and pH. Soil Biol. Biochem. 2000, 32, 1–10. [Google Scholar] [CrossRef]
- Chinsamy, M. Garden-waste-vermicompost leachate alleviates salinity stress in tomato seedlings by mobilizing salt tolerance mechanisms. Plant Growth Regul. 2013, 71, 41. [Google Scholar] [CrossRef]
- Wang, L.L.; Sun, X.Y.; Li, S.Y.; Zhang, T.; Zhang, W.; Zhai, P. Application of organic amendments to a coastal saline soil in north China: Effects on soil physical and chemical properties and tree growth. PLoS ONE 2014, 9, 89185. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Garcia, C.; Gonzalez, J.L.; Hernandez, M.T. Use of organic amendment as a strategy for saline soil remediation: Influence on the physical, chemical and biological properties of soil. Soil Biol. Biochem. 2006, 38, 1413–1421. [Google Scholar] [CrossRef]
- Bernal, M.P.; Sommer, S.G.; Chadwick, D.; Chen, Q.; Li, G.X.; Michel, F.C. Chapter Three. Current Approaches and Future Trends in Compost Quality Criteria for Agronomic Environmental, and Human Health Benefits. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2017; Volume 144, pp. 143–233. [Google Scholar]
- Zhang, Y.Z. Review on research progress of bentonite. Adv. Environ. Prot. 2019, 9, 496–501. [Google Scholar] [CrossRef]
- Yssaad, H.; Amine, B.B. Effect of Salinity and Bentonite on the Characteristics of Mineral Soil and Behavior of Leguminous Plants (Vicia faba L.). In Developments in Soil Salinity Assessment and Reclamation: Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Shahid, S.A., Abdelfattah, M.A., Taha, F.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 783–794. [Google Scholar]
- Zhou, L.; Monreal, C.M.; Xu, S.T.; McLaughlin, N.B.; Zhang, H.Y.; Hao, G.C.; Liu, J.H. Effect of bentonite-humic acid application on the improvement of soil structure and maize yield in a sandy soil of a semi-arid region. Geoderma 2019, 338, 269–280. [Google Scholar] [CrossRef]
- Xiu, Y.; Iqbal, A.; Zhu, C.; Wu, G.; Chang, Y.P.; Li, N.; Cao, Y.; Zhang, W.B.; Zeng, H.M.; Chen, S.Y.; et al. Improvement and transcriptome analysis of root architecture by overexpression of Fraxinus pennsylvanica DREB2A transcription factor in Robinia pseudoacacial L. ‘Idaho’. Plant Biotech. J. 2016, 14, 1456–1469. [Google Scholar] [CrossRef]
- Shahzad, H.; Imran, M.; Iqbal, M.; Javed, A.; Bilal, H.M.; Hussain, S.; Hussain, Z.; Huma, B.; Iqbal, S.; Safdar, A. Soil Physical Health Indices, Wue and Wheat Yield Under Different Mulching Amendment. Sylwan 2016, 160, 367–387. [Google Scholar]
- Farren, N.J.; Dunmore, E.R.; Mead, M.I.; Nadzir, M.S.M.; Samah, A.A.; Phang, S.M.; Bandy, B.J.; Sturges, W.T. Chemical Characterisation of Water-soluble Ions in Atmospheric Particulate Matter on the East Coast of Peninsular Malaysia. Atmos. Chem. Phys. 2018, 3, 1–33. [Google Scholar] [CrossRef]
- Regalado, C.M.; Muñoz-Carpena, R. Estimating the saturated hydraulic conductivity in a spatially variable soil with different permeameters: A stochastic Kozeny–Carman relation. Soil Tillage Res. 2004, 77, 189–202. [Google Scholar] [CrossRef]
- Wu, S.W.; Zhang, Y.; Tan, Q.L.; Sun, X.C.; Wei, W.H.; Hu, C.X. Biochar is superior to lime in improving acidic soil properties and fruit quality of Satsuma mandarin. Sci. Total Environ. 2020, 714, 136722. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.R.; Cole, C.V.; Watanabe, S.; Dean, L.A. Estimation of Available Phosphorous in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954; pp. 1–8.
- Su, H.; Fang, Z.; Tsang, P.E.; Zhang, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Pang, Y.X.; Zhang, Q.; Jiao, Z.; Chen, Q. Comprehensive evaluation of regional clean energy development levels based on principal component analysis and rough set theory. Renew. Energy 2018, 122, 643–653. [Google Scholar] [CrossRef]
- Lin, W.S.; Mu, D.; Wang, L.P. Correlation between the Growth of Dominant Trees and Surface Soil Physiochemical Properties of Conifer and Broad-Leaved Mixed Forest at Different Succession Stages. Scientia Silvae Sinicae 2016, 52, 17–25. [Google Scholar]
- XU, X.L.; Liu, Y.; Feng, J.L. Attribute Coordinate Comprehensive Evaluation Model Combining Principal Component Analysis. Intell. Sci. II 2018, 539, 60–69. [Google Scholar]
- Rayhani, M.H.T.; Yanful, E.K.; Fakher, A. Desiccation-induced cracking and its effect on the hydraulic conductivity of clayey soils from Iran. Can. Geotech. J. 2007, 44, 276–283. [Google Scholar] [CrossRef]
- Guo, Y.; Su, J.G.; Dong, Y.; Wolch, J. Application of land use regression techniques for urban greening: An analysis of Tianjin, China. Urban Forest. Urban. Green. 2019, 38, 11–21. [Google Scholar] [CrossRef]
- Zhang, T.; Li, S.; Sun, X.; Zhang, Y.; Gong, X.; Fu, Y.; Jia, L. The Earthworm Eisenia fetida Can Help Desalinate a Coastal Saline Soil in Tianjin, North China. PLoS ONE 2015, 10, e0144709. [Google Scholar] [CrossRef]
- Eilenbrod, K.D. Self-healing in fractured fine-grained soils. Can. Geotech. J. 2003, 40, 435–449. [Google Scholar] [CrossRef]
- Liu, Z.; Rong, Q.; Zhou, W.; Liang, G. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 2017, 12, e0172767. [Google Scholar]
- Zhang, C.; Feng, X. Natural and human-induced effects on grain size of surface sediments along the Lianyungang muddy coast, China. Chin. J. Oceanol. Limnol. 2011, 2, 387–397. [Google Scholar] [CrossRef]

| Index | Value a |
|---|---|
| pH | 7.69 ± 0.42 |
| EC (mS·cm−1) | 4.89 ± 0.35 |
| Bulk density (g·cm−3) | 1.53 ± 0.08 |
| Total porosity (%) | 39.37 ± 2.41 |
| Hydraulic conductivity (cm·s−1) | 0.63 × 10−5 ± 0.10−5 |
| Organic matter (g·kg−1) | 1.73 ± 0.01 |
| Available nitrogen (mg·kg−1) | 41.21 ± 1.22 |
| Available phosphorus (mg·kg−1) | 4.53 ± 0.39 |
| Available potassium (mg·kg−1) | 58.2 ± 2.41 |
| CO32− (g·kg−1) b | 0.03 ± 0.01 |
| HCO3− (g·kg−1) b | 0.17 ± 0.01 |
| Cl− (g·kg−1) b | 4.75 ± 0.02 |
| Ca2+ (g·kg−1) b | 0.83 ± 0.01 |
| Mg2+ (g·kg−1) b | 0.68 ± 0.01 |
| K+ (g·kg−1) b | 0.37 ± 0.01 |
| Na+ (g·kg−1) b | 6.48 ± 0.15 |
| SO42− (g·kg−1) b | 2.86 ± 0.18 |
| Index | Value a |
|---|---|
| pH | 7.17 ± 0.31 |
| EC (mS·cm−1) | 1.21 ± 0.05 |
| Bulk density (g·cm−3) | 0.15 ± 0.01 |
| Total carbon (%) | 42.93 ± 3.72 |
| Total nitrogen (%) | 0.82 ± 0.04 |
| C:N ratio | 52.34 ± 3.21 |
| Water content | 40.17 ± 2.55 |
| Index | Value a |
|---|---|
| pH | 6.65 ± 0.22 |
| EC (mS·cm−1) | 1.62 ± 0.14 |
| Bulk density (g·cm−3) | 0.34 ± 0.02 |
| Total carbon (%) | 68 ± 2.71 |
| Total nitrogen (%) | 2.57 ± 0.18 |
| Available phosphorus (mg·kg−1) | 498 ± 9.46 |
| Available potassium (mg·kg−1) | 1079 ± 8.08 |
| Germination index (%) | 99 ± 1.31 |
| Humic acid carbon: Fulvic acid carbon ratio (CHA/CFA) | 1.71 ± 0.1 |
| Treatment | Soil Bulk Density (g·cm−3) | Total Porosity (%) | Saturated Hydraulic Conductivity (cm·s−1) |
|---|---|---|---|
| CK | 1.53 ± 0.02a | 43.35 ± 0.91a | 0.74 × 10−5 ± 0.30 × 10−5a |
| T1 | 1.31 ± 0.04bc | 51.13 ± 1.14c | 8.67 × 10−4 ± 0.29 × 10−4b |
| T2 | 1.49 ± 0.03ab | 44.38 ± 1.65ab | 1.18 × 10−5 ± 0.10 × 10−5a |
| T3 | 1.37 ± 0.01c | 49.3 ± 1.06c | 6.63 × 10−4 ± 0.13 × 10−4c |
| Variable | PC1 | PC2 | PC3 |
|---|---|---|---|
| Available nitrogen (AN) | 0.885 | −0.202 | 0.069 |
| Available phosphorus (AP) | 0.876 | −0.101 | 0.287 |
| Available potassium (AK) | 0.86 | −0.173 | −0.067 |
| Saturated hydraulic conductivity (Ks) | 0.83 | 0.105 | −0.146 |
| Bulk density (BD) | −0.819 | −0.403 | 0.359 |
| Total porosity (TP) | 0.819 | 0.403 | −0.359 |
| Organic matter (OM) | 0.789 | −0.332 | 0.307 |
| Salt | −0.78 | 0.186 | −0.196 |
| pH | 0.096 | 0.778 | 0.61 |
| Treatment | F1 | F2 | F3 | Q |
|---|---|---|---|---|
| CK | 106.27 | −15.00 | 20.48 | 77.78 |
| T1 | 156.08 | −24.10 | 29.36 | 113.84 |
| T2 | 120.55 | −18.17 | 22.37 | 87.96 |
| T3 | 194.00 | −31.24 | 36.91 | 141.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Sun, X.; Li, S. Effects of Garden Waste Compost and Bentonite on Muddy Coastal Saline Soil. Sustainability 2020, 12, 3602. https://doi.org/10.3390/su12093602
Li J, Sun X, Li S. Effects of Garden Waste Compost and Bentonite on Muddy Coastal Saline Soil. Sustainability. 2020; 12(9):3602. https://doi.org/10.3390/su12093602
Chicago/Turabian StyleLi, Jingnan, Xiangyang Sun, and Suyan Li. 2020. "Effects of Garden Waste Compost and Bentonite on Muddy Coastal Saline Soil" Sustainability 12, no. 9: 3602. https://doi.org/10.3390/su12093602
APA StyleLi, J., Sun, X., & Li, S. (2020). Effects of Garden Waste Compost and Bentonite on Muddy Coastal Saline Soil. Sustainability, 12(9), 3602. https://doi.org/10.3390/su12093602





