Abstract
Fresh-cut vegetables, namely those that undergo processes such as washing, sorting, or chopping while keeping their fresh state, constitute an important market element nowadays. Among those operations, the washing step becomes really important due both to the extensive use of water resources and to the utilization of controversial water sanitizing agents, such as chlorine. To ideally eliminate those chlorinated compounds while decreasing water consumption, four novel filtrating technologies (pulsed corona discharge combined with nanofiltration, NF-PCD; classical ultrafiltration, UF; nanofiltration membranes integrating silver nanoparticles, NF-AgNP; and microfiltration with cellulose acetate membranes containing chitin nanocrystals, ChCA) have been proposed to eliminate any contaminating agent in recirculated water. Here, we performed a life cycle assessment (LCA) to assess the environmental effects of introducing these new solutions and to compare those impacts with the burden derived from the current strategy. The novel technologies showed a decreased environmental burden, mainly due to the enhanced water recirculation and the subsequent decrease in energy consumption for pumping and cooling the water stream. The environmental gain would be maintained even if a certain amount of chlorine was still needed. This analysis could serve as an aid to decision-making while evaluating the introduction of new sanitizing techniques.
1. Introduction
In order to benefit from the well-known properties of fresh fruits and vegetables, many customers tend to favor the consumption of “fresh-cut” (FC) products, defined as “those fruits and vegetables that may have undergone procedures such as washing, sorting, trimming, peeling, slicing or chopping that do not affect their fresh life quality” [1]. These products play an important role in the present days, when the time allocated to cooking processes is in a clear decrease [2].
FC vegetables have shown an increased market size when compared with FC fruits, especially due to the sale of salad bags. The value of the European fresh-cut fruit and vegetable market is about 3.4 billion euros, of which salads, vegetables, and fruit account for 62%, 31%, and 7% of the market volume, respectively [3]. Regarding its production, harvested fresh vegetables typically undergo several unit operations to end up with the final FC products. These operations consist of trimming, slicing and shredding, washing, draining, weighing, and packing [4,5].
Washing unit operation is a key step in the production of FC vegetables [6]. Before packaging of shredded produce, it is necessary to remove dirt, pesticide residues, and microorganisms that may lead to quality loss along the shelf life of the final product [7]. This step is performed by immersing produce in tanks of washing water, which is partially recirculated to decrease the total cost of the operation [6].
Of crucial importance is the quality of water that is used for this operation, since water may paradoxically act as a contaminant agent when it is recirculated. The reused water is characterized by a high organic load, which provides nutrients supporting microbial growth [8]. There is, therefore, a need for using a sanitizing agent that can virtually eliminate any possible cross-contamination among water tanks [9]. The most widely used sanitizer is chlorine due to its low cost and effectiveness when eliminating contaminant bacteria through oxidation, although its utilization is controversial since its reaction byproducts have shown a carcinogenic potential [6,10,11]. Indeed, its use has been banned in several countries in the EU [2]. Besides, the organic compounds present in fresh vegetables generate a high chlorine demand, which leads to the rapid consumption of the free chlorine present in the recycled water [11]. Due to this fact, fresh solution of chlorine in water needs to be constantly added, and thus total recirculation of the water flux is not possible, which encourages extensive water usage in the process. This results both in the resource depletion and in the extensive use of energy for pumping and cooling, which is an important issue of the present society as it has been stated by the United Nations. Indeed, this organism claims in its 12th Sustainable Development Goal (SDG) the necessity for ensuring sustainable consumption and production patterns, promoting an efficient use of resources and energy [12].
In order to decrease the water consumption and production costs by increasing the recirculation rates, Fusi et al. proposed the introduction of a water filtering system in the production line of baby lamb leaves [13]. Membrane separation can be used to treat the process water before its recirculation, thus decreasing the organic particles and avoiding cross-contamination [14]. As stated by the authors of [13], the use of this technology would lead to the reduction of electricity needed for pumping water, as well as a general water saving and a further reduction in wastewater production, in line with the 12th SDG. Additionally, the introduction of filtering techniques would replace the controversial use of chlorine.
Membrane devices have become an alternative to traditional water purification processes. A membrane represents a thin physical interface that regulates the pass of certain species through it, depending on their physical and/or chemical properties [15]. Depending on the pore sizes, we can find several types of membranes that can be used for water treatment (Table 1).

Table 1.
Classification of filtering membranes in terms of their pore size [15].
In the present work, we evaluated the environmental effects of introducing different membrane-based tools as water sanitizing agents in the FC industry. This work was carried out as part of the CEREAL project under the 7th Framework Program, which aimed to improve the resource efficiency throughout the postharvest chain of fresh-cut fruits and vegetables. In previous stages of the project, the consortium partners developed and/or evaluated from a technical point of view the suitability of several filtering devices for decontaminating FC produce washing waters. Their work resulted in valuable data, which were later used by the authors to evaluate the environmental impact of a hypothetical large-scale implementation of the developed techs through a life cycle assessment (LCA), with a special focus on the washing operation itself. LCA is a commonly used method to assess the environmental impact of a determined product through its whole life cycle, considering the extraction and processing of the raw materials, the manufacturing and distribution steps, the use and recycling by the consumer, and the final disposal [16]. This technique is broadly applicable to several fields, and food production is among them. It is possible to find publications performing LCA for meat [17], dairy [18,19], crops [20,21], and even edible insect [22] production industries. LCA has also been applied to the FC vegetables and fruit industry on several occasions [23,24,25], but none of them focused on the washing step of the process.
By means of the LCA, we were able to evaluate the potential decrease in the environmental impact of the washing step by the introduction of new sanitizing techniques. Ideally, these new tools would replace the need for chlorine as a sanitizing agent, thus avoiding both the potential issues of this compound related to human health and the costs derived from the infrastructure [26,27].
2. Materials and Methods
LCA was performed according to ISO 14040 and 14044 [28,29], taking into consideration the following stages: (1) goal and scope definition; (2) life cycle inventory analysis (LCI); (3) life cycle impact assessment (LCIA); and finally, life cycle interpretation.
2.1. Goal and Scope Definition
The global aim of the project was the assessment of the environmental profile regarding the washing of fresh-cut vegetables when introducing several membrane-based sanitizing techniques, and comparing them with the reference scenario.
Since cut and packaged lettuce dominate the market of FC vegetables, corresponding to the greatest part of the sales volume [3], the functional unit (FU) was set to 1 ton of cut lettuce to be washed. This FU provides the reference for the normalization of the LCA data and allows for the comparison between the different scenarios.
2.1.1. System Boundaries
Fresh-cut vegetable production is performed according to the steps in Figure 1. A cradle to grave approach would consider all the steps in it. However, in the LCA performed by [13], it was reported that the agricultural and processing phase contributes more than 80% to the total environmental loads in the 12 assessed impact categories, so downstream impacts could be negligible. What is more, the output of washed vegetables needs to meet the requirements for the maximal biological load independently of the sanitizing technique, so the way the washing is performed does not influence the downstream environmental loads. Similarly, we considered that the losses of produce will be equal under the use of the different technologies evaluated here, since they only affect the recirculated water and not the washed produce. Therefore, the impacts of the agricultural phase and the previous processing steps were considered equal among the five scenarios.
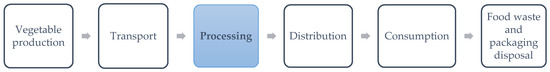
Figure 1.
Cradle to grave production chain of fresh-cut (FC) vegetable production. In blue, the part of the process where this analysis focused.
Thus, we set the system boundaries in the washing phase itself, considering as the input the fresh vegetables and as the output the ready-to-pack vegetables. By limiting the model to one single production step, we avoided any potential errors due to the modeling of further operations, and thus diminished the uncertainty of our results.
2.1.2. Scenarios Definition
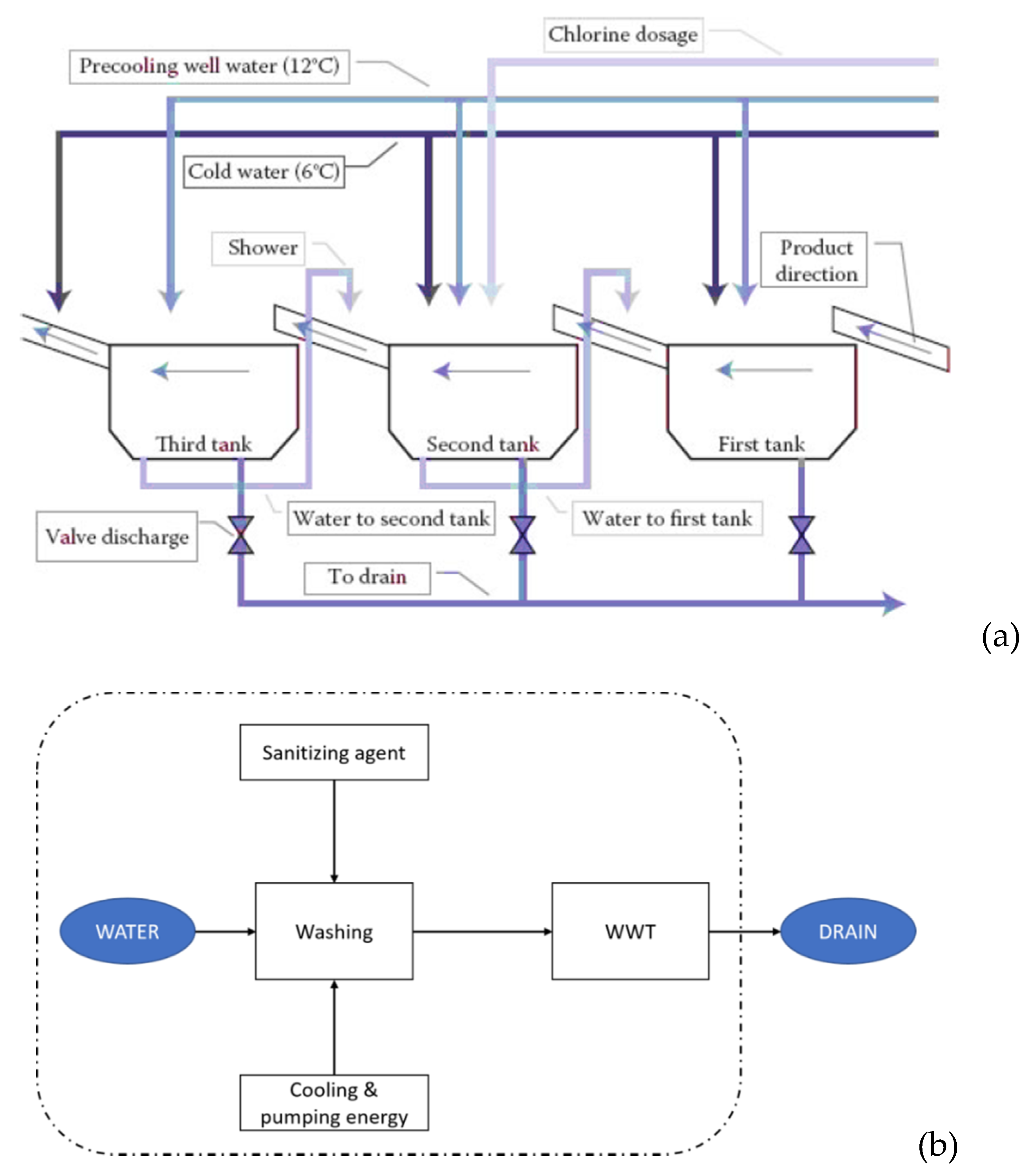
The current operational process for FC lettuce washing in the industry is summarized in Figure 2a [30]. This process is typically performed in three steps corresponding to three washing tanks. In this scenario, water is recirculated from the cleanest tank (the last one) to the previous one. Chlorine is added in the first and/or second tank as a sanitizing agent, while the third tank would perform a rinsing with potable water [30]. The water that is not recirculated to the previous tanks undergoes a wastewater treatment (WWT) before it is drained to the environment. The conceptualized model of this operation can be seen in Figure 2b. This was our reference scenario.
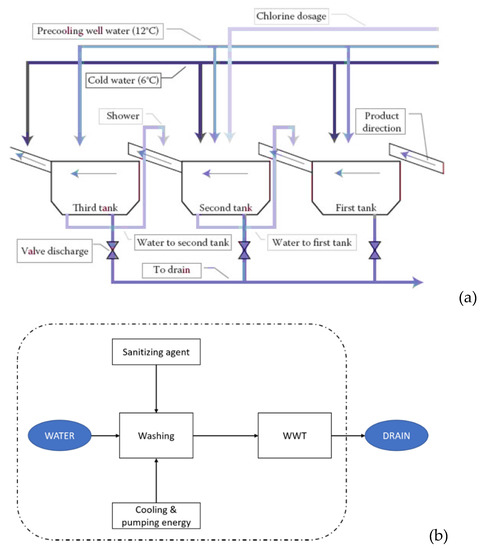
Figure 2.
Description of how the washing step of the FC lettuce production is performed within the reference scenario. (a) Washing tanks structure, where FC produce enters by the right side of the picture and passes through the different washing tanks [31]. Water flow is schematically represented in (b), where the discontinuous line sets the boundaries of our scenario. The inputs of the system are freshwater, cooling and pumping energy, and the sanitizing agent, chlorine. The main output is wastewater, which is processed in the wastewater treatment (WWT) plant before it is returned to the technosphere. Another output of the process (not shown in the picture) is the unreacted chlorine.
In order to enhance water recirculation decreasing its consumption by 50% and to eliminate the chlorine as a sanitizing agent, the project consortium partners proposed and analyzed four membrane-based methods that can be used to remove part of the organic load within the reused water:
- A hybrid depuration system, based upon the utilization of ozone gas combined with inorganic filtering membranes. In this scenario, the oxidizing role of chlorine is replaced by ozone. The main drawback when using ozone as an oxidizing agent is its high cost, which can be diminished by ozonation using pulsed corona discharge (PCD), though it shows enhanced energy efficiency when compared with other methods [31,32]. As an active species, ozone oxidizes the organic compound present in water, but to a lesser extent than chlorine [33]. That is why ozonation has been combined with nanofiltration (NF) membranes in several studies [31,34,35]—to prevent membrane fouling by degrading the organic matter. The combination of these technologies as a means of water purification in the washing step of FC lettuce production has been proposed in [36] by a member of the CEREAL project consortium.
- Standard ultrafiltration (UF) membranes. When it comes to alternative water treatment processes, UF is one of the most widely used [37,38]. This technology has the ability to remove colloids, particles, bacteria, and viruses from water [39]. However, the major drawback of UF systems in a large-scale application is membrane fouling [40], which is treated through backwashes—pumping water backwards through the filters media [41]. The use of filtering membranes alone for the treatment of FC washing water has also been previously reported [42].
- Microfiltration (MF) membranes made of cellulose acetate (CA) and chitin nanocrystals. CA-based membranes are extensively used in industrial-scale applications since they are derived from an abundant natural polymer such as cellulose. However, they show poor mechanical strength and chemical and thermal stability [43]. Thus, this material needs to be reinforced in order to meet the requirements for its actual utilization. Chitin nanocrystals (ChNC) can be used for this aim. They are macromolecules that act as structural polymers in the exoskeleton of arthropods, in the cell walls of fungi and yeast, and in other microorganisms [44]. Besides their good mechanical properties, ChNC also possesses antifungal and antibacterial properties [45]. This behavior prevents the biofilm formation and the subsequent fouling of the membrane, providing a successful means of water filtering [45,46].
- Nanofiltration using ceramic membranes coated with biocide silver nanoparticles (AgNP). The use of fine-pore membranes is combined with silver, which has long been known to exhibit good antibacterial ability for a considerable range of microorganisms, and thus AgNP are commercialized as antimicrobial agents [47,48]. This combination is able to successfully treat water under an acceptable flux rate with excellent bacterial losses [49].
From this point to the end of the document, the four technologies will be denoted as NF-PCD (nanofiltration-pulsed corona display), UF (ultrafiltration), ChCA (chitin-cellulose acetate) and NF-AgNP (nanofiltration-silver nanoparticles), respectively.
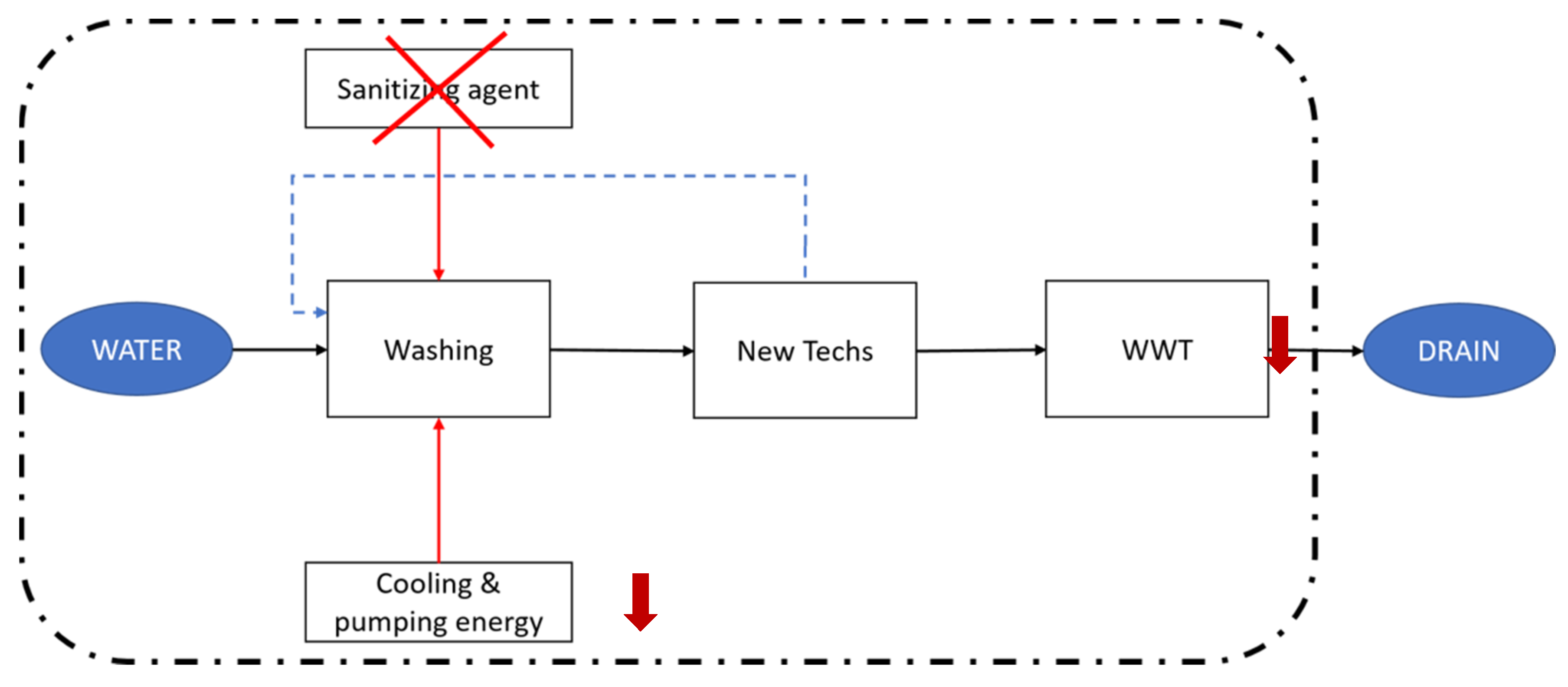
By using these technologies, the conceptualized scenario changes, as described in Figure 3. The elimination of the sanitizing agent would also imply the displacement of the associated infrastructure. What is more, an enhanced water recirculation would decrease the energy consumption for cooling and pumping the freshwater input, while it would also decrease the stream to be treated by the WWT plant.
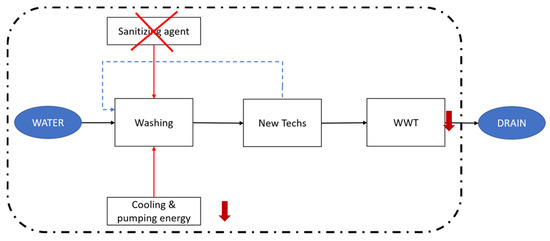
Figure 3.
Schematic representation of the water flow in the new proposed scenarios. Water that exits the washing tanks is treated by means of the new technologies and recirculated to the tanks. Therefore, the flux to be processed by the WWT plant is decreased, as well as the cooling and pumping energy needs. Properly designed filtering techniques would dismiss the need for using a chlorine sanitizing agent. The discontinuous lines set the system boundaries, as in Figure 2b.
2.2. Life Cycle Inventory
Briefly, the data concerning the different washing elements across the five scenarios were obtained from three different sources:
- Primary data, which were kindly supplied by the different partners in the CEREAL project consortium. As it was stated in the introductory part, these data resulted from previous stages of this same project, where the consortium developed and/or evaluated at lab-scale the technologies here assessed, reaching conclusions such as the expected water saving and electricity usage.
- Secondary data retrieved from background databases. In this work, we used the Ecoinvent v3.2 database to gather the remaining missing data and to model the lacking processes [50]. This is a widely used database in the framework of LCA due to its three main strengths: the data’s reliability, transparency, and the independence of the host institutions [51].
- Secondary data collected from a profound literature search. Fortunately, data concerning the manufacturing of the filtering devices had been previously reported and were here used for elaborating the inventory.
Ecoinvent unit processes were preferentially used for systems modeling. When any unit process was missing from that database, bespoke ones were compiled from scientific references. Processes were designed including the same factors and assumptions as of the equivalent Ecoinvent ones in order to ensure consistency across the whole LCI. Within the reference system, the inventory takes into account three fundamental items: (1) water intake from the general supply network; (2) chlorination infrastructure, considering both the sanitizing tanks and the purchase of the chemical compound; (3) energy supplies needed for water pumping and cooling, considering the Spanish electricity mixture. As it was previously stated, the output elements of the process are, on the one hand, the wastewaters that need to be treated and, on the other hand, the unreacted chlorine.
For the remaining evaluated scenarios, the application of the different filtering techniques eliminates the need for sanitizing chlorine. Thus, all the derived infrastructure is removed from the following inventories.
With regards to the NF-PCD membrane, the materials and energy intakes needed for the pulsed corona display (PCD) device construction were extracted from [36], and its disposal was based upon the recycling of the steel utilized for this purpose. This technology was combined with a standard nanofiltration membrane. Due to the lack of primary data and/or literature information, we modeled the production of the membrane using data from a reverse osmosis device recorded on Ecoinvent database (FILMTECTM SW30HR-380). The aforementioned process documented in Ecoinvent did not consider the device disposal, so we assumed that the ceramic NF membrane was disposed of in an inorganic residue landfill.
For the inventory regarding the NF-AgNP scenario, we lacked once again primary information in terms of membrane manufacturing. Thus, the filtering membrane serving as the basis of the device was the same Ecoinvent reverse osmosis standard as in the NF-PCD scenario, as well as its disposal. This membrane was coated with silver nanoparticles (AgNP), whose production was modeled based on the report in [52].
As for the ultrafiltration membrane system, the inventory relied on an already modeled system in Ecoinvent, which considered the production, utilization, and disposal of the membrane. Thus, the only output that needed to be taken into account was the wastewater that was subsequently treated.
Finally, the cellulose acetate (CA) membrane production was modeled basing on [53]. The process started with the pretreatment of the Kraft cellulose paste from corn starch, which subsequently underwent an acetylation or esterification step with acetic anhydride. This resulted in cellulose tri- or diacetate in the form of fine powder or flakes. The manufacturing of the final membrane from the cellulose acetate was also modeled thanks to the data in [53]. The membrane was finally coated with chitin bactericide nanocrystals, corresponding to 5% of the total weight [45]. The chitin was obtained from crab shell residues. The inventory regarding this step was modeled based on the data in [54]. As a byproduct, it generated a protein paste, which was employed as animal feeding, and therefore here allocated as avoided impact.
The summarized LCI can be found in Table 2. The full quantitative information of the inventories concerning each scenario can be viewed in Supplementary Materials.

Table 2.
Elements acting as inputs and outputs of the different considered scenarios, namely nanofiltration-pulsed corona display scenario (NF-PCD), nanofiltration-silver nanoparticle filtration scenario (NF-AgNP), ultrafiltration scenario (UF), and chitin-cellulose acetate membrane scenario (ChCA). The items represent the components of the LCA inventories.
2.3. Life Cycle Impact Assessment
For modeling the life cycles within the different scenarios, we used SimaPro v8. In order to evaluate the environmental impacts, we used the ReCiPe method, whose primary objective is to transform the list of life cycle inventory results into a limited number of impact indicator scores, categorized in 18 midpoint indicators and 3 endpoint indicators [57]. Here, we assessed 12 ReCiPe midpoint indicators (same as [13]): climate change, ozone depletion, terrestrial acidification, freshwater eutrophication and ecotoxicity, marine eutrophication and ecotoxicity, human toxicity, photochemical oxidant formation, terrestrial ecotoxicity, and water and fossil depletion. We also considered the three endpoint indicators: damage to human health, damage to ecosystems, and damage to resource availability. The main strength of this methodology is that it ensures that the different impacts are not assessed more than once in different indicators, and thus ReCiPe scores are extensively used in the life cycle impact assessments [13,58,59,60,61].
2.4. Sensitivity Analysis
The introduction of these new techniques in industrial-scale applications is expected to create a water recirculation rate increased by 50% with respect to our reference scenario. However, this value might not be reached, since the conditions under which the washing operation is performed in the factory substantially differ from their application in laboratory conditions. In order to be able to properly assess if these membranes could decrease the environmental loads on a larger scale, we performed a sensitivity analysis. We evaluated whether the four new technologies would maintain a significant environmental gain in the case that the maximum water savings were 20%, 30%, or 40% of the consumption within the reference scenario.
On the other hand, we considered that in larger scales the use of a determined concentration of chlorine might still be needed in order to limit membrane fouling and maintain the standards of produce quality. To explore this possibility, another sensitivity analysis was performed concerning the chlorine addition in the proposed scenarios. We evaluated the environmental gain when the concentration of chlorine in the washing water was 100%, 50%, and 20% of the concentration used in our reference scenario. It should be noted that although in the first case the concentration of chlorine was the same as in our reference, the enhanced recirculation rate was maintained, so that a lesser freshwater input flux needed to be treated with the sanitizing agent. As a result, the net consumption of chlorine decreased.
Both analyses were performed focusing on the single score endpoint indicator, defined as the sum of the three ReCiPe endpoint impact scores. Besides, in the two analyses, we considered as significant a decrease in the single score indicator of 20%, compared to the reference scenario.
3. Results
3.1. Impacts Evaluation
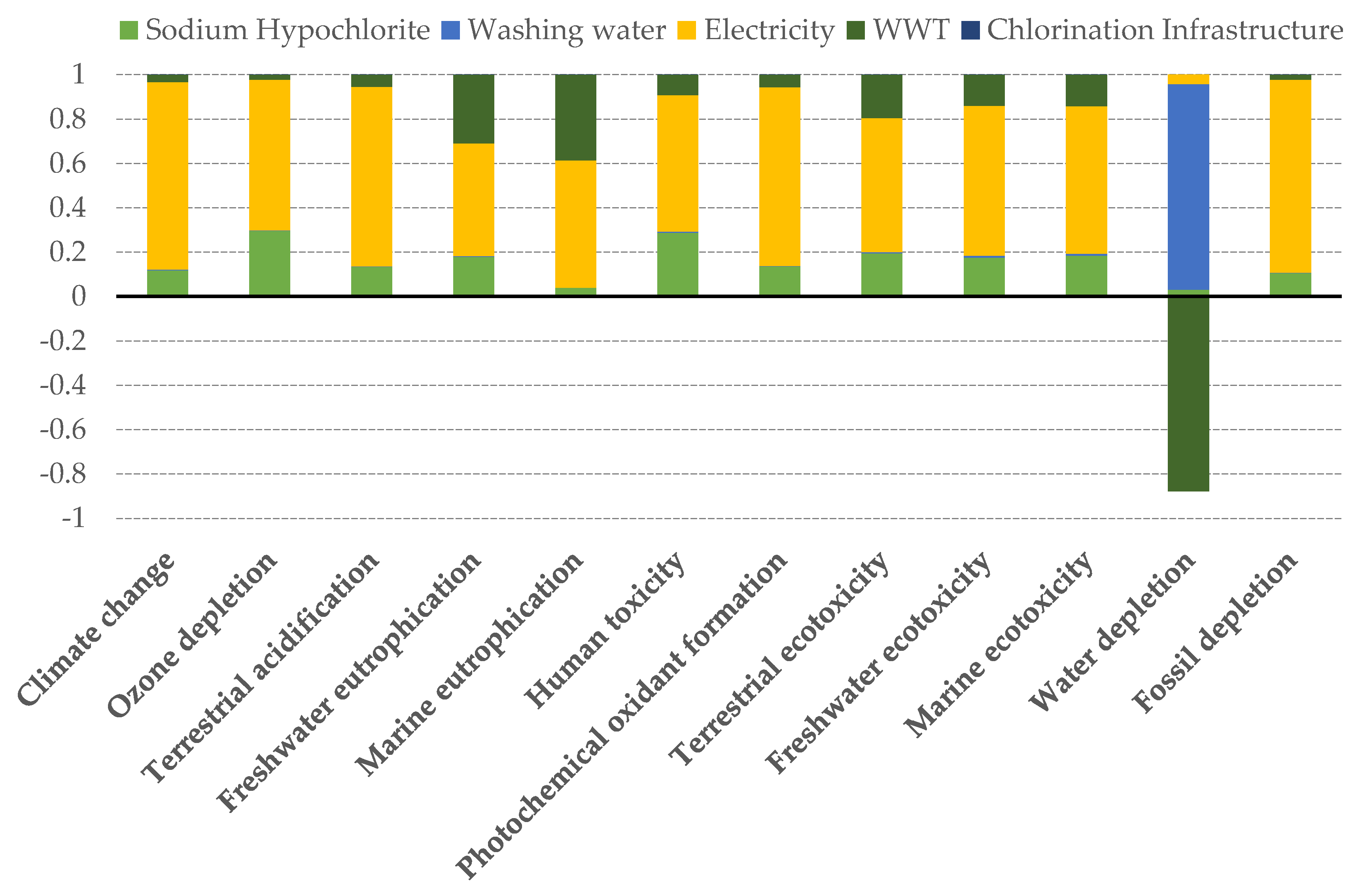
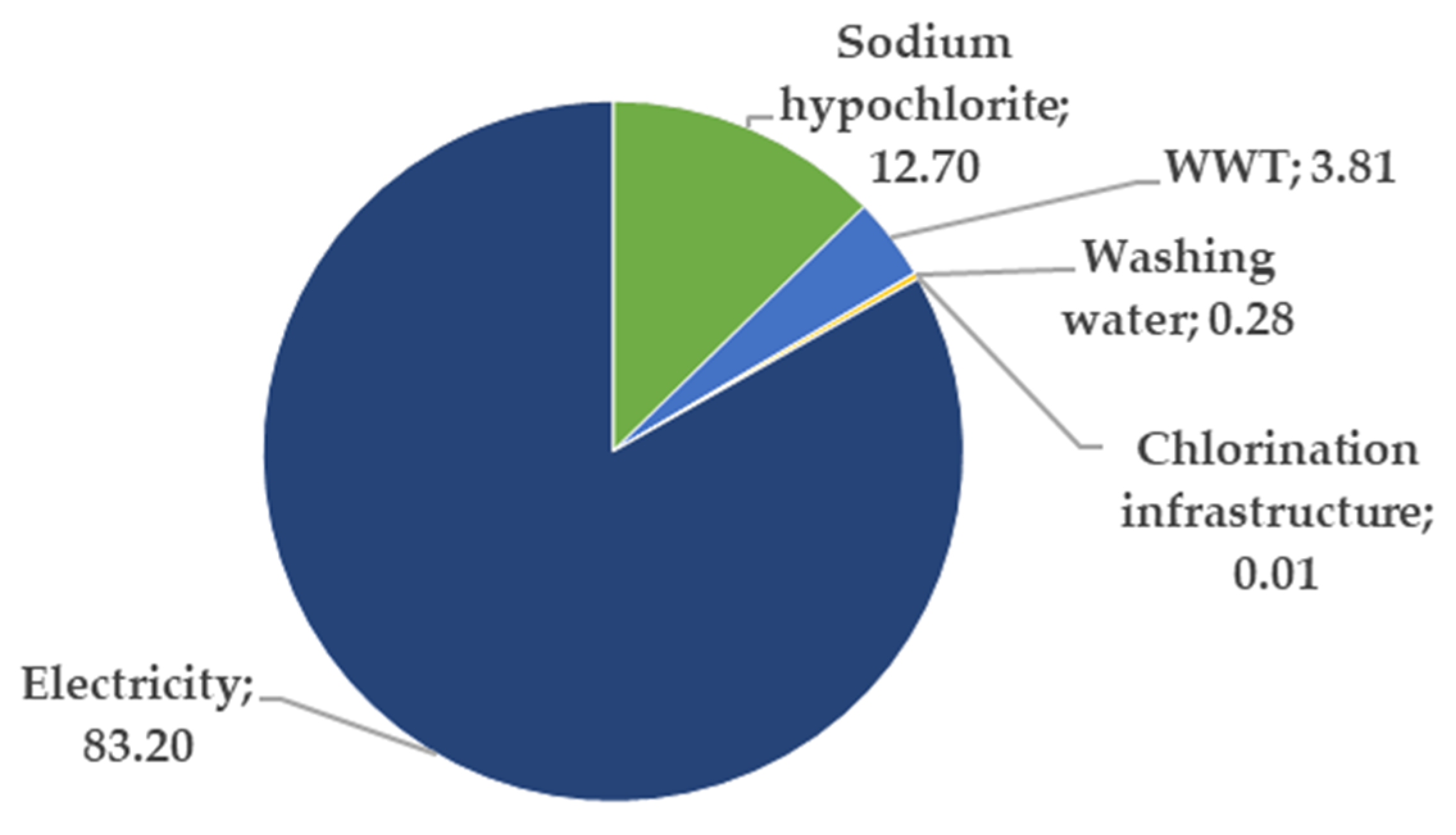
The impact scores for each of the 12 ReCiPe midpoint categories calculated according to our reference scenario are presented in Figure 4. In almost all the considered categories, major impacts were due to the energy consumption, followed by the chlorination process and wastewater treatment as the principal environmentally damaging components.
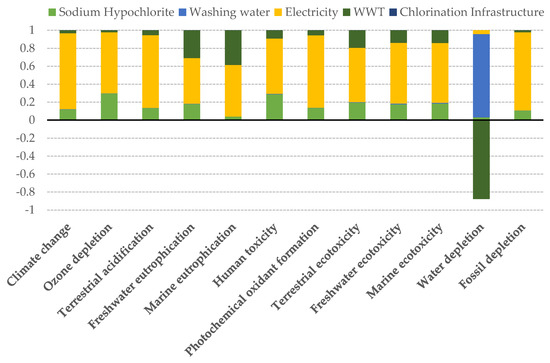
Figure 4.
Relative contribution of the different components of the reference scenario to each midpoint impact indicator.
It is worth noting the negative contribution of WWT to the water depletion impact score. This is due to the fact that once the water is treated in the WWT plant it is returned to the technosphere, and thus the net water consumption is diminished.
The overall impact of the different elements in the reference scenario were evaluated according to the endpoint indicators, and the final result is presented in Figure 5. The electricity consumption is responsible for more than 80% of the total environmental burden.
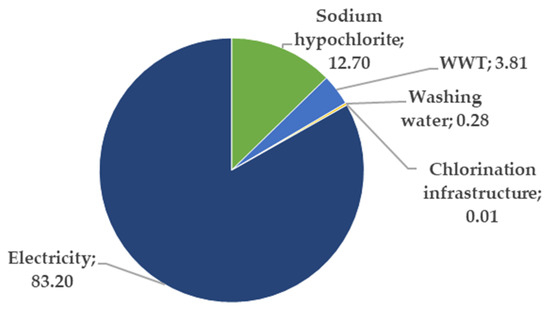
Figure 5.
Contribution (in %) of each of the components of our reference scenario to the single score endpoint indicator. This single score is conceptualized as the sum of the three endpoint indicators stated in Section 2.3.
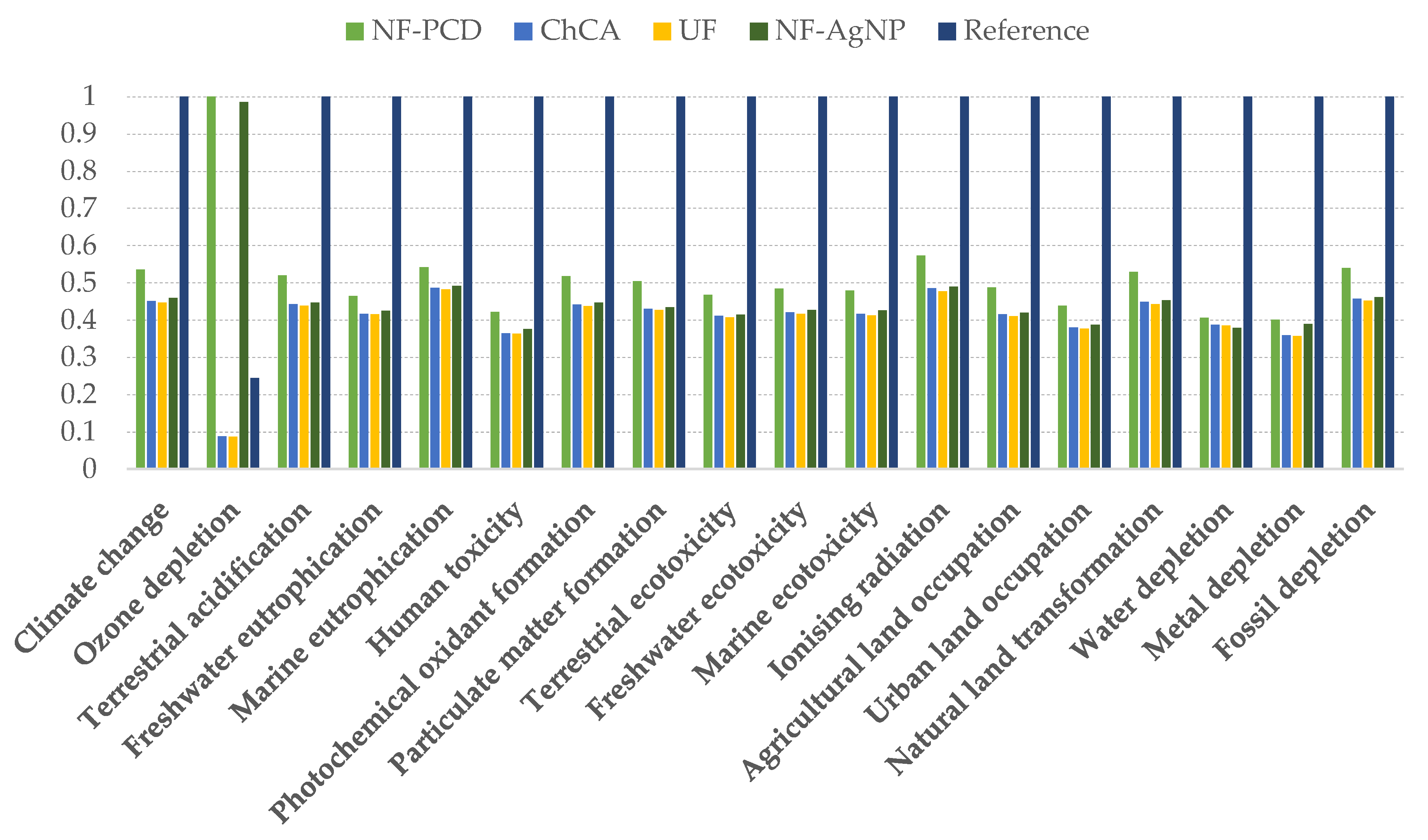
When introducing the different filtering techniques in the system, the impacts of 11 out of 12 midpoint categories decreased compared to our reference, independently of the considered scenario (Figure 6). Only NF scenarios showed an increased impact in the category of ozone depletion, due to the modeled membrane production. The data related to the membrane manufacturing were extracted from Ecoinvent, as stated in the inventory, and included the formation of chlorofluorocarbons, namely CFC-113, which is a major contributor to the ozone layer depletion.
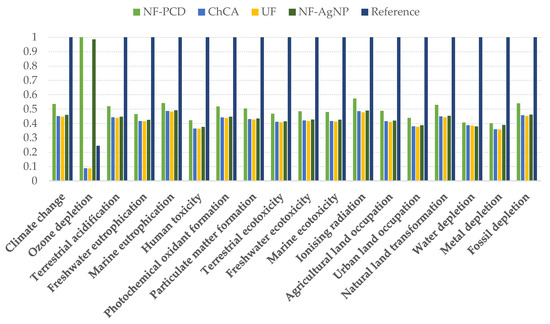
Figure 6.
Midpoint impact indicators of the different scenarios within the 12 considered categories. The results are shown relative to the scenario where the indicator had its maximum value. NF-PCD: nanofiltration-pulsed corona display scenario; ChCA: chitin-cellulose acetate membrane scenario; UF: ultrafiltration scenario; NF-AgNP: nanofiltration-silver nanoparticles scenario.
In spite of it, the use of water purification membranes entailed an overall endpoint impact reduction of 55% in the case of NF-AgNP and UF scenario, whereas NF-PCD and ChCA filtration showed an impact reduction of 47% and 56%, respectively, when compared to the reference scenario.
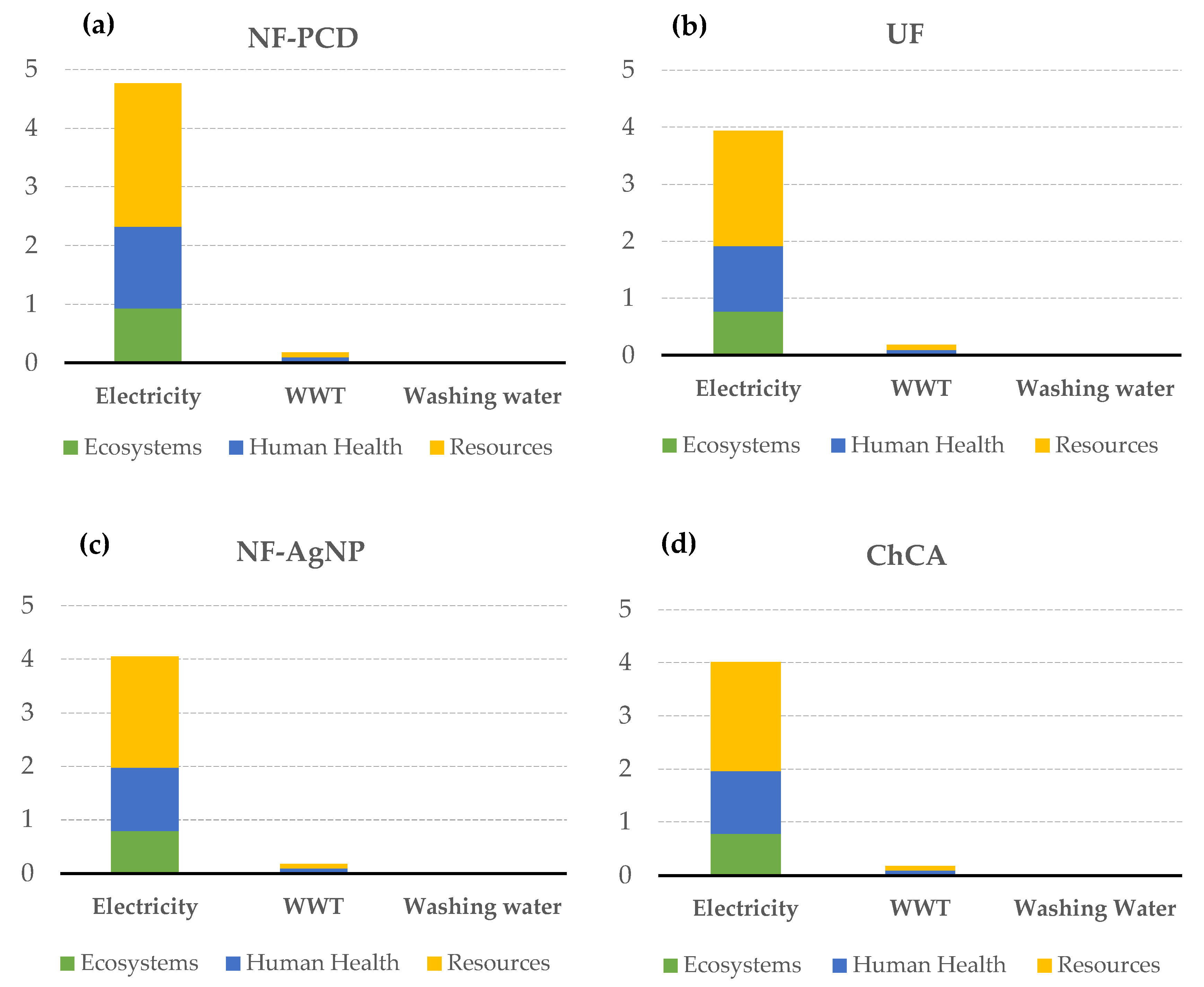
When the endpoint impacts were allocated to the different elements of each of the scenarios, it was noticed that once again the major impacts were due to the energy consumption, followed by the wastewater treatment. The remaining elements entailed negligible impacts (Figure 7).
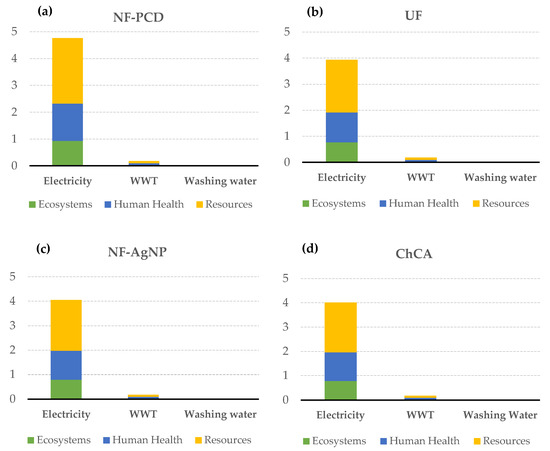
Figure 7.
Absolute contribution to the different endpoint indicators of electricity consumption, WWT, and washing water usage in the case of pulsed corona display scenario (NF-PCD) (a), ultrafiltration scenario (UF) (b), silver nanoparticles scenario (NF-AgNP) (c) and chitin-cellulose acetate membrane scenario (ChCA) (d). The rest of the processes concerning membrane manufacturing, maintenance, and disposal showed negligible contributions and thus were not here represented.
3.2. Sensitivity Analysis
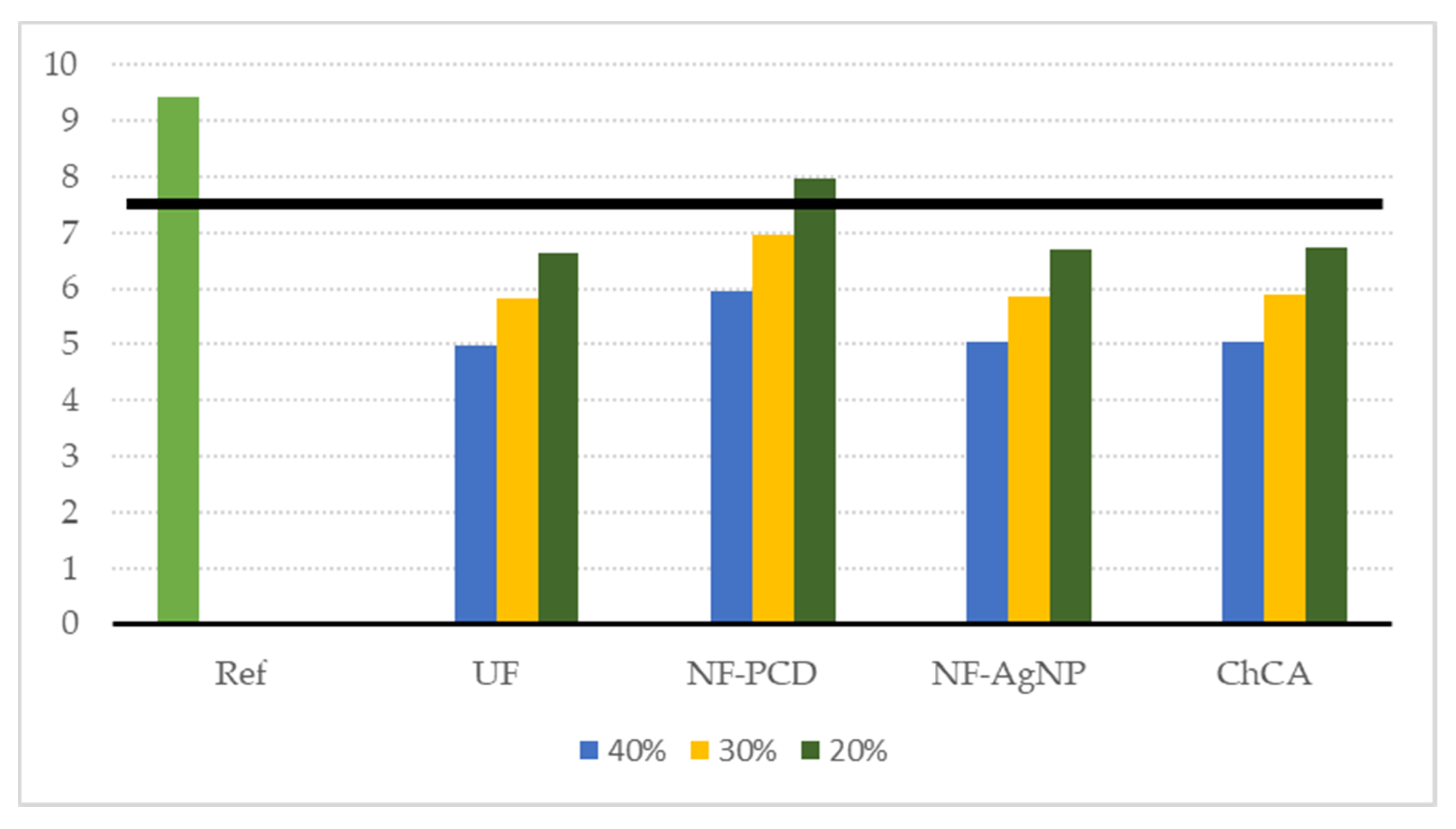
The sensitivity analysis on water consumption confirmed that the latter overall impact reduction, in terms of the ReCiPe single score endpoint indicator, was generally maintained when water recirculation rates were reduced (Figure 8). In all proposed scenarios but NF-PCD, the environmental gain was significant when compared to the reference for every considered recirculation rate, with reductions of the overall impact scores greater than 20%.
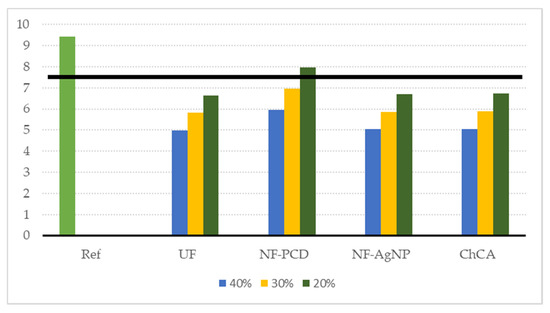
Figure 8.
Sensitivity analysis on the water consumption decrease. We considered, on each of the proposed scenarios, a water consumption decrease of 40%, 30%, and 20% with respect to the reference scenario (where water saving is null). The black solid line denotes an environmental gain of 20% compared to the reference.
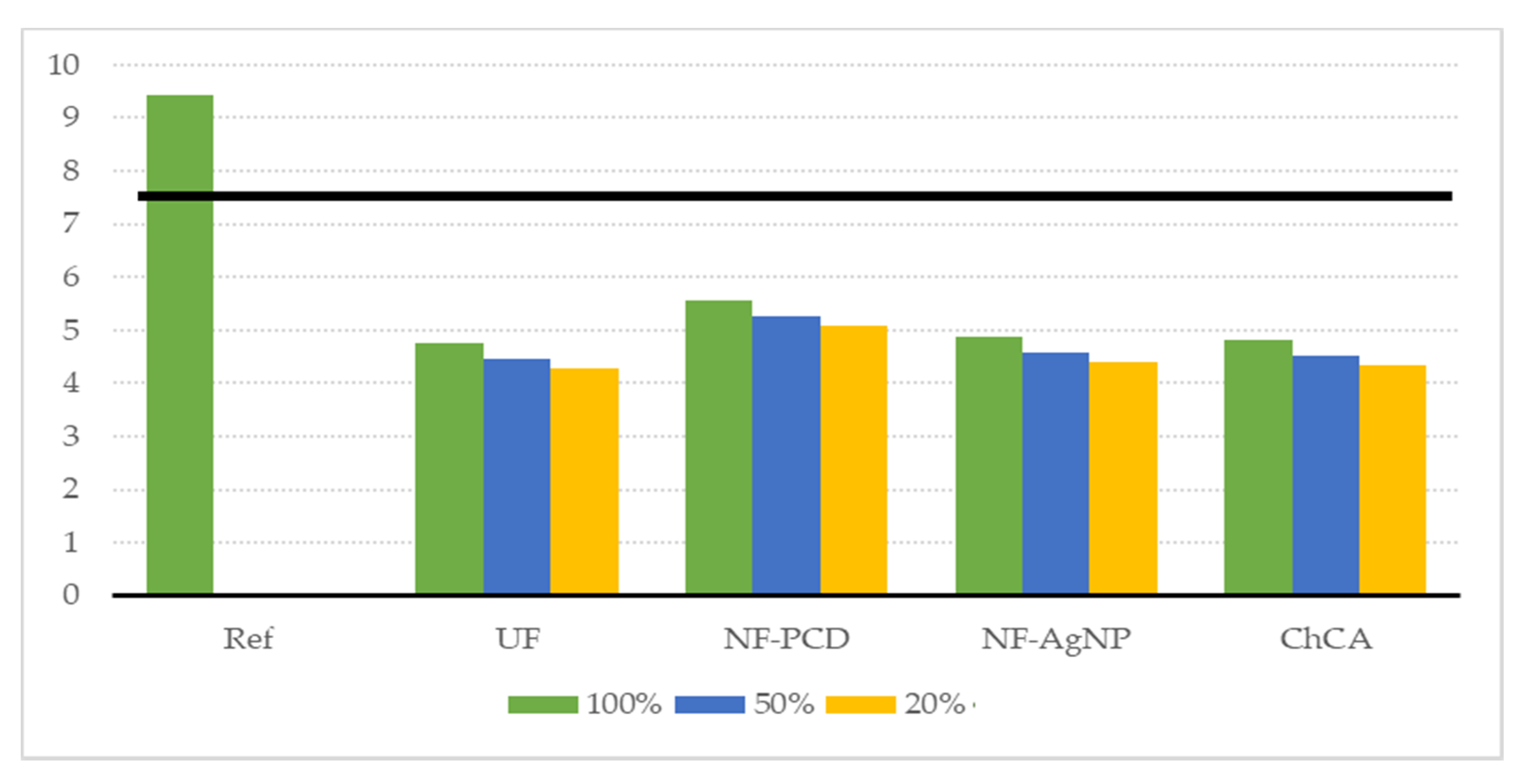
As for the sensitivity analysis concerning the chlorine addition, the results are shown in Figure 9. It can be noticed that the environmental gain is maintained even if the same concentration of chlorine as in the reference scenario is used to sanitize the recirculated washing water. This is due to the fact that the main contributor to the decrease in the environmental burden is once again the water saving. Even if we maintain the usage of chlorine, keeping a reduction of 50% in freshwater consumption is still nearly as beneficial as not using chlorine at all according to the ReCiPe indicators.
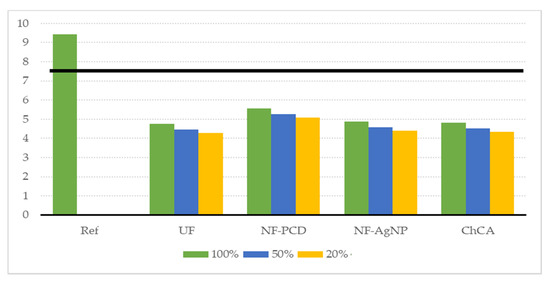
Figure 9.
Result of the sensitivity analysis performed regarding the chlorine addition. It was considered here that an addition of chlorine concentrations of 100%, 50%, and 20%, referred to the added chlorine concentration on the reference scenario. The solid black line denotes our sensitivity limit of 20% reduction on the endpoint single score.
4. Discussion
The inclusion of filtering membranes as washing water sanitizing techniques appears to be clearly convenient from an environmental point of view. The major impact reduction was due to the energy savings derived from a decreased water stream to be cooled and pumped. This is consistent with the results reported by Fusi et al. [13], who reported a decrease in the environmental burden when introducing filtering techniques in the process. However, they did not evaluate the impact of the manufacturing of the filtering device. Considering the inventory here developed, we clarified that the impact due to this manufacturing and usage was negligible in the endpoint impact assessment.
This fact also validates the use of secondary data, both from articles and from the Ecoinvent database. The processes stored on databases and the data collected from articles entailed different aims, and consequently adapting those to our purposes may have led to imprecisions on the calculations of midpoint and endpoint impact indicators. However, the filtering infrastructure represented less than 0.0001% of the single-score endpoint indicator, so incorrectness in our procedures would not affect the main conclusions of the study.
The only issue related to modelization using Ecoinvent data was related to the ozone depletion indicator, which appeared to be a major issue in the nanofiltration scenarios. This could be due to the fact that Figure 6 plots the indicators relative to the scenario where their value is greatest, and the absolute value might not be enough high to entail actual harm. However, the modeled scenarios considered just an approximation of the real filtering device, since no NF system inventory was found in the secondary data retrieval. This component was replaced by a reverse osmosis device, present in the Ecoinvent database. The manufacturing of this membrane includes the use of polyester resin, a viscous liquid resin that is usually combined with fiberglass to serve as a supporting element [62], the use of which leads to CFC formation. Actual NF device manufacturing does not require the use of this compound, as it seems when analyzing the other membranes inventories, and thus the contribution to ozone depletion could be tackled. Thus, Ecoinvent databases are extremely useful but the LCA results reached with their data must be carefully analyzed.
The main reason for the reduction of the environmental burden in the four scenarios is the enhanced water recirculation, which results in decreases in the energy from water cooling and pumping. For the water being rinsed, it has been reported that 1–2 °C is the optimal water temperature for most FC products, in order to successfully remove traces of chlorine [4]. Here, we considered a cool water temperature of 6 °C, as suggested in [30]. Part of the savings on energy could be allocated to decrease even more the water temperature and enhance the final FC produce quality. It is worth pointing out that in our analysis the input water temperature was set to the average temperature in the Spanish general supply network, i.e., 20 °C. The input temperature will depend on the season of the year, on the country where the production takes place, or even on the temperature of alternative water sources, such as wells or rivers. As a result, even further energy saving could be achieved and this, together with a disminished water use, would contribute to the accomplishment of the 12th SDG proposed by the United Nations.
The possibility of saving water in the FC production chain had been previously explored in the literature. To date, water decontamination has been addressed using different physical methods (UV light [63], pulsed light [64], power ultrasound [65]), biological methods [66], or chemical methods (such as hydrogen peroxide [67] or citric acid [68]) As a drawback, most of these technologies have been reported to need long treatment times, which increases the turnover rate [69]. Furthermore, there are no studies so far considering these techniques from an environmental point of view, as we did here by means of the LCA. To decrease the residence times, most of these technologies have been combined together or with chlorine sanitizing agents, although this entails a high cost [70].
The membrane-based techniques here considered have the potential ability to totally replace the need for adding chlorine as a sanitizing agent, representing at the same time an environmentally friendly alternative to the current strategy. The wide use of chlorine in the process has been reported to entail some public health issues, including the formation of carcinogenic by-products such as chloroform or trihalomethanes, chloramines, and haloacetic acids [26,27]. Due to this fact, the use of these compounds in the production chain has already been prohibited in some European countries, namely Belgium, Denmark, Germany and The Netherlands [2]. The evaluated systems may offer an appropriate alternative for the FC vegetable washing at these locations.
However, laboratory conditions are indeed just an approximation of the real factory scenario. The efficiency of the system will clearly depend on the quality and dirtiness of the freshly harvested produce that enters the process. Ideally, the biocide or oxidant compounds that are combined with membranes within the techs will be sufficient to avoid membrane fouling, as it was reported in previous stages of the project. Lab-scale experiments showed that applying backwashes periodically would be sufficient to prevent the clogging of the system, but further experiments on a larger scale are needed to evaluate this issue.
Nevertheless, our sensitivity analysis showed that even if small amounts of chlorine were needed to keep the biological load standards for the washed produce, a significant environmental gain would still be achieved. Indeed, the differences between reducing up to 20% the chlorine concentration and keeping it as in our reference scenario are negligible for all the evaluated techniques when compared to the impacts derived from the current strategy. Furthermore, chlorine is a relatively cheap compound with an important oxidizing ability, so its combination with the new sanitizing techniques would probably increase the life span of their components and decrease the associated maintenance costs.
Indeed, this study has mainly focused on the environmental traits of the proposed filtering devices. The results are thus preliminary, and they just show the environmental viability of the devices. Further studies should be carried out in order to assess whether these technologies would be applicable on a larger scale. Special attention should be paid to the potential issues related to membrane fouling. Larger streams of water to be treated might carry some vegetable residues, which were not considered when testing the decontaminating ability of the membranes. The use of backwashes should be tested in larger scales and not only when washing leafy vegetables, but also some others whose residue may be smaller with higher clogging potential, such as broccoli.
Larger scale studies would also lead to new primary data that could be used to complement the LCA performed here. The use of an inventory mainly composed of direct primary data will lead to more robust conclusions, and will eliminate any possible imprecision due to estimations.
Moreover, this study has been limited to the environmental point of view. If these new techs are to be introduced in a real manufacturing process, also an economic analysis should be performed to confirm that the advantages of the membrane-based devices do not entail a disproportionate cost in the total operation.
5. Conclusions
In this work, we have presented four new membrane techniques that aim to address two major issues regarding FC vegetable washing operations: the current low water recirculation rate and the controversial use of chlorinated compounds as sanitizing agents. Introducing these devices on the FC production chain has been proven to be beneficial from an environmental point of view, since they are able to reduce the total impacts derived from the washing process. The environmental load reduction is mainly due to the decrease in water consumption, which subsequently implies decreased electricity consumption for water cooling and pumping. This environmental gain is maintained even if the increases in water recirculation rates are limited and/or the addition of small quantities of chlorine are still needed to ensure produce quality. On the whole, membrane-based sanitizing techniques appear to give a sustainable option for the sanitizing of FC washing water. Larger scale experiments should be performed to ensure technical viability. This environmental analysis would also complement an economic study of the decision-making process when assessing the alternatives to the current FC production chain strategy.
Supplementary Materials
The Supplementary Materials are available online at https://www.mdpi.com/2071-1050/12/9/3674/s1.
Author Contributions
Investigation, M.V., M.P.L., H.M.-P., and J.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Plan of Science, Technology, and Innovation of the Principality of Asturias, grant number FC-GRUPIN-IDI/2018/000225.
Acknowledgments
The authors wish to thank the European Union’s Seventh Programme for research and technological development FP7-ERANET-SUSFOOD “CEREAL Project” for the financial support given, along with the national Institute for Agricultural and Food Research and Technology Ref. ERA35-CEREAL-INIA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kader, A.A.; Gil, M.I. Fresh-cut fruit and vegetables. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2008; pp. 475–504. ISBN 978-1-84569-184-4. [Google Scholar]
- Baselice, A.; Colantuoni, F.; Lass, D.A.; Nardone, G.; Stasi, A. Trends in EU consumers’ attitude towards fresh-cut fruit and vegetables. Food Qual. Prefer. 2017, 59, 87–96. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Elena Russo, M.; Marzocchella, A. Pre-treatment and enzymatic hydrolysis of lettuce residues as feedstock for bio-butanol production. Biomass Bioenergy 2017, 96, 172–179. [Google Scholar] [CrossRef]
- Turatti, A. Process Design, Facility and Equipment Requirements. In Advances in Fresh-Cut Fruits and Vegetables Processing; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 339–360. ISBN 9781420071238. [Google Scholar]
- Patrick, V.; Mazollier, J. Overview of the European Fresh-cut Produce Industry. In Fresh-Cut Fruits and Vegetables: Science, Technology and Market; Lamikanra, O., Ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9781498729949. [Google Scholar]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Allende, A.; Selma, M.V. Treatments to Ensure Safety of Fresh-cur Fruits and Vegetables. In Advances in Fresh-Cut Fruits and Vegetables Processing; Martin-Belloso, O., Soliva-Fortuny, R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 221–230. [Google Scholar]
- Teng, Z.; van Haute, S.; Zhou, B.; Hapeman, C.J.; Millner, P.D.; Wang, Q.; Luo, Y. Impacts and interactions of organic compounds with chlorine sanitizer in recirculated and reused produce processing water. PLoS ONE 2018, 13, e0208945. [Google Scholar] [CrossRef] [PubMed]
- Van Haute, S.; Tryland, I.; Veys, A.; Sampers, I. Wash water disinfection of a full-scale leafy vegetables washing process with hydrogen peroxide and the use of a commercial metal ion mixture to improve disinfection efficiency. Food Control 2015, 50, 173–183. [Google Scholar] [CrossRef]
- Weng, S.; Luo, Y.; Li, J.; Zhou, B.; Jacangelo, J.G.; Schwab, K.J. Assessment and speciation of chlorine demand in fresh-cut produce wash water. Food Control 2016, 60, 543–551. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, B.; Van Haute, S.; Nou, X.; Zhang, B.; Teng, Z.; Turner, E.R.; Wang, Q.; Millner, P.D. Association between bacterial survival and free chlorine concentration during commercial fresh-cut produce wash operation. Food Microbiol. 2018, 70, 120–128. [Google Scholar] [CrossRef]
- Goal 12 Sustainable Development Knowledge Platform. Available online: https://sustainabledevelopment.un.org/sdg12 (accessed on 6 April 2020).
- Fusi, A.; Castellani, V.; Bacenetti, J.; Cocetta, G.; Fiala, M.; Guidetti, R. The environmental impact of the production of fresh cut salad: A case study in Italy. Int. J. Life Cycle Assess. 2016, 21, 162–175. [Google Scholar] [CrossRef]
- Allende, A.; Selma, M.V.; López-Gálvez, F.; Villaescusa, R.; Gil, M.I. Impact of wash water quality on sensory and microbial quality, including Escherichia coli cross-contamination, of fresh-cut escarole. J. Food Prot. 2008, 71, 2514–2518. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Ilgin, M.A.; Gupta, S.M. Environmentally conscious manufacturing and product recovery (ECMPRO): A review of the state of the art. J. Environ. Manag. 2010, 91, 563–591. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gudiño, J.; Monteiro, A.N.T.R.; Espagnol, S.; Blanco-penedo, I.; Garcia-launay, F. Life Cycle Assessment of Iberian Traditional Pig Production System in Spain. Sustainability 2020, 12, 627. [Google Scholar] [CrossRef]
- Pierucci, S.; Klemeš, J.J.; Piazza, L.; Bakalis, S.; Falcone, G.; De Luca, A.I.; Stillitano, T.; Iofrida, N.; Strano, A.; Piscopo, A.; et al. Shelf Life Extension to Reduce Food Losses: The Case of Mozzarella Cheese. In Proceedings of the Chemical Engeneering Transactions, Milano, Italy, 28–31 May 2017. [Google Scholar]
- Partearroyo, T.; de Samaniego-Vaesken, M.L.; Ruiz, E.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Current food consumption amongst the spanish anibes study population. Nutrients 2019, 11, 2663. [Google Scholar] [CrossRef] [PubMed]
- Clement, O.; Olatayo Jekayinfa, S.; Jekayinfa, S.O.; Pecenka, R.; Jaiyeoba, F.; Ogunlade, C.A.; Oni, O. Life Cycle Assessment of Local Rice Production and Processsing in Nigeria. In Proceedings of the Internarional Commission of Agricultural and Biosystems Engineering, Ibadan, Nigeria, 22–25 October 2018. [Google Scholar]
- Ghasemi-Mobtaker, H.; Kaab, A.; Rafiee, S. Application of life cycle analysis to assess environmental sustainability of wheat cultivation in the west of Iran. Energy 2020, 193, 116768. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life cycle assessment of edible insects for food protein: A review. Agron. Sustain. Dev. 2016, 36. [Google Scholar] [CrossRef]
- Moreno, J.; Pablos, C.; Marugán, J. Quantitative Methods for Life Cycle Assessment (LCA) Applied to the Vegetable Industry. In Quantitative Methods for Food Safety and Quality in the Vegetable Industry; Springer: New York, NY, USA, 2018; pp. 255–293. [Google Scholar]
- Tasca, A.L.; Nessi, S.; Rigamonti, L. Environmental sustainability of agri-food supply chains: An LCA comparison between two alternative forms of production and distribution of endive in northern Italy. J. Clean. Prod. 2017, 140, 725–741. [Google Scholar] [CrossRef]
- Ilari, A.; Duca, D. Energy and environmental sustainability of nursery step finalized to “fresh cut” salad production by means of LCA. Int. J. Life Cycle Assess. 2018, 23, 800–810. [Google Scholar] [CrossRef]
- Bull, R.J.; Crook, J.; Whittaker, M.; Cotruvo, J.A. Therapeutic dose as the point of departure in assessing potential health hazards from drugs in drinking water and recycled municipal wastewater. Regul. Toxicol. Pharmacol. 2011, 60, 1–19. [Google Scholar] [CrossRef]
- Legay, C.; Rodriguez, M.J.; Sérodes, J.B.; Levallois, P. Estimation of chlorination by-products presence in drinking water in epidemiological studies on adverse reproductive outcomes: A review. Sci. Total Environ. 2010, 408, 456–472. [Google Scholar] [CrossRef]
- ISO/IEC ISO 14040:2006. Environmental Management—Life Cycle Assessment—Principles and Framework; International Organization for Standardization (ISO): Genève, Switzerland, 2006. [Google Scholar]
- ISO/IEC ISO 14044:2006. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; International Organization for Standardization (ISO): Genève, Switzerland, 2006. [Google Scholar]
- Colelli, G.; Amodio, M.L. Factor Affecting Quality and Safety of Fresh-cut Fruits and Vegetables. In Fresh-Cut Fruits and Vegetables: Technology, Physiology and Safety; Pareek, S., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 194–222. [Google Scholar]
- Ajo, P.; Preis, S.; Vornamo, T.; Mänttäri, M.; Kallioinen, M.; Louhi-Kultanen, M. Hospital wastewater treatment with pilot-scale pulsed corona discharge for removal of pharmaceutical residues. J. Environ. Chem. Eng. 2018, 6, 1569–1577. [Google Scholar] [CrossRef]
- Ajo, P.; Kornev, I.; Preis, S. Pulsed Corona Discharge in Water Treatment: The Effect of Hydrodynamic Conditions on Oxidation Energy Efficiency; ACS Publications: Washington, DC, USA, 2015. [Google Scholar]
- Williams, R.C.; Sumner, S.S.; Golden, D.A. Inactivation of Escherichia coli O157:H7 and Salmonella in Apple Cider and Orange Juice Treated with Combinations of Ozone, Dimethyl Dicarbonate, and Hydrogen Peroxide. J. Food Sci. 2006, 70, M197–M201. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Arola, K.; Kallioinen, M.; Reinikainen, S.P.; Hatakka, H.; Mänttäri, M. Advanced treatment of membrane concentrate with pulsed corona discharge. Sep. Purif. Technol. 2018, 198, 121–127. [Google Scholar] [CrossRef]
- Johansson, T.; Manttari, M.; Kallioinen, M. Pulsed Corona Discharge and Membrane Filtration for Purification of Lettuce Washing Waters; LUT University: Lappenranta, Finland, 2016. [Google Scholar]
- Mierzwa, J.C.; da Silva, M.C.C.; Veras, L.R.V.; Subtil, E.L.; Rodrigues, R.; Li, T.; Landenberger, K.R. Enhancing spiral-wound ultrafiltration performance for direct drinking water treatment through operational procedures improvement: A feasible option for the Sao Paulo Metropolitan Region. Desalination 2012, 307, 68–75. [Google Scholar] [CrossRef]
- Hernando, M.D.; Petrovic, M.; Radjenovic, J.; Fernández-Alba, A.R.; Barceló, D. Chapter 4.2 Removal of pharmaceuticals by advanced treatment technologies. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 50, pp. 451–474. ISBN 9780444530523. [Google Scholar]
- Tian, J.Y.; Ernst, M.; Cui, F.; Jekel, M. Correlations of relevant membrane foulants with UF membrane fouling in different waters. Water Res. 2013, 47, 1218–1228. [Google Scholar] [CrossRef]
- Chew, C.M.; Aroua, M.K.; Hussain, M.A.; Ismail, W.M.Z.W. Evaluation of ultrafiltration and conventional water treatment systems for sustainable development: An industrial scale case study. J. Clean. Prod. 2016, 112, 3152–3163. [Google Scholar] [CrossRef]
- Basu, O.D. Backwashing. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–3. [Google Scholar]
- Nelson, H.; Singh, R.; Toledo, R.; Singh, N. The use of a submerged microfiltration system for regeneration and reuse of wastewater in a fresh-cut vegetable operation. Sep. Sci. Technol. 2007, 42, 2473–2481. [Google Scholar] [CrossRef]
- Chung, H.Y.; Hall, J.R.B.; Gogins, M.A.; Crofoot, D.G.; Weik, T.M. Polymer, Polymer Microfiber, Polymer Nanofiber and Applications Including Filter Structures. U.S. Patent 7090715B2, 15 August 2006. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. Oxf. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Goetz, L.A.; Jalvo, B.; Rosal, R.; Mathew, A.P. Superhydrophilic anti-fouling electrospun cellulose acetate membranes coated with chitin nanocrystals for water filtration. J. Membr. Sci. 2016, 510, 238–248. [Google Scholar] [CrossRef]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Marques, P.A.A.P.; Neto, C.P.; Trindade, T.; Daina, S.; Sadocco, P. Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 2009, 5, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, X. Silver nanoparticle decorated cellulose nanofibrous membrane with good antibacterial ability and high water permeability. Appl. Mater. Today 2017, 9, 130–135. [Google Scholar] [CrossRef]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Ecoinvent—The World’s Leading LCA Database Launches Version 3.0. Available online: https://www.psi.ch/en/media/our-research/ecoinvent-the-worlds-leading-lca-database-launches-version-30 (accessed on 10 February 2020).
- Zhang, H.; Hortal, M.; Dobon, A.; Jorda-Beneyto, M.; Bermudez, J.M. Selection of Nanomaterial-Based Active Agents for Packaging Application: Using Life Cycle Assessment (LCA) as a Tool. Packag. Technol. Sci. 2017, 30, 575–586. [Google Scholar] [CrossRef]
- Manda, B.M.K.; Worrell, E.; Patel, M.K. Innovative membrane filtration system for micropollutant removal from drinking water - Prospective environmental LCA and its integration in business decisions. J. Clean. Prod. 2014, 72, 153–166. [Google Scholar] [CrossRef]
- Muñoz, I.; Rodríguez, C.; Gillet, D.M.; Moerschbacher, B. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef]
- Dong, S.; Li, J.; Kim, M.H.; Park, S.J.; Eden, J.G.; Guest, J.S.; Nguyen, T.H. Human health trade-offs in the disinfection of wastewater for landscape irrigation: Microplasma ozonation: Vs. chlorination. Environ. Sci. Water Res. Technol. 2017, 3, 106–118. [Google Scholar] [CrossRef]
- Rodrigo, A.; Wesche, M.; Llorca, I.; Scholl, S. Environmental assessment of new decontamination and sanitation techniques for fresh-cut products. In Proceedings of the Fouling and Cleaning in Food Processing, Cambridge, UK, 31 March–2 April 2014. [Google Scholar]
- ReCiPe|PRé Sustainability. Available online: https://www.pre-sustainability.com/recipe (accessed on 28 January 2020).
- Ernstoff, A.; Tu, Q.; Faist, M.; Del Duce, A.; Mandlebaum, S.; Dettling, J. Comparing the Environmental Impacts of Meatless and Meat-Containing Meals in the United States. Sustainability 2019, 11, 6235. [Google Scholar] [CrossRef]
- Yelboga, M. LCA Analysis of Grafted Tomato Seedling Production in Turkey. Sustainability 2019, 12, 25. [Google Scholar] [CrossRef]
- Vigil, M.; Marey-Pérez, M.F.; Martinez Huerta, G.; Álvarez Cabal, V. Is phytoremediation without biomass valorization sustainable?—Comparative LCA of landfilling vs. anaerobic co-digestion. Sci. Total Environ. 2015, 505, 844–850. [Google Scholar] [CrossRef] [PubMed]
- García, S.G.; Montequín, V.R.; Fernández, R.L.; Fernández, F.O. Evaluation of the synergies in cogeneration with steel waste gases based on Life Cycle Assessment: A combined coke oven and steelmaking gas case study. J. Clean. Prod. 2019, 217, 576–583. [Google Scholar] [CrossRef]
- Ouarhim, W.; Zari, N.; Bouhfid, R.; Qaiss, A. El kacem Mechanical performance of natural fibers–based thermosetting composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Cambridge, UK, 2019; pp. 43–60. [Google Scholar]
- Selma, M.V.; Allende, A.; López-Gálvez, F.; Conesa, M.A.; Gil, M.I. Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry. Food Microbiol. 2008, 25, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Manzocco, L.; Ignat, A.; Bartolomeoli, I.; Maifreni, M.; Nicoli, M.C. Water saving in fresh-cut salad washing by pulsed light. Innov. Food Sci. Emerg. Technol. 2015, 28, 47–51. [Google Scholar] [CrossRef]
- Elizaquível, P.; Sánchez, G.; Selma, M.V.; Aznar, R. Application of propidium monoazide-qPCR to evaluate the ultrasonic inactivation of Escherichia coli O157:H7 in fresh-cut vegetable wash water. Food Microbiol. 2012, 30, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Spricigo, D.A.; Bardina, C.; Cortés, P.; Llagostera, M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013, 165, 169–174. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, M.; Chen, H. Efficacy of washing with hydrogen peroxide followed by aerosolized antimicrobials as a novel sanitizing process to inactivate Escherichia coli O157:H7 on baby spinach. Int. J. Food Microbiol. 2012, 153, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Choi, M.-R.; Park, J.-W.; Park, K.-H.; Chung, M.-S.; Ryu, S.; Kang, D.-H. Use of Organic Acids to Inactivate Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes on Organic Fresh Apples and Lettuce. J. Food Sci. 2011, 76, M293–M298. [Google Scholar] [CrossRef]
- Manzocco, L.; Ignat, A.; Bot, F.; Calligaris, S.; Valoppi, F. Efficient management of the water resource in the fresh-cut industry: Current status and perspectives. Trends Food Sci. Technol. 2015, 46, 286–294. [Google Scholar] [CrossRef]
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).