Mass Transfer Performance Study for CO2 Absorption into Non-Precipitated Potassium Carbonate Promoted with Glycine Using Packed Absorption Column
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
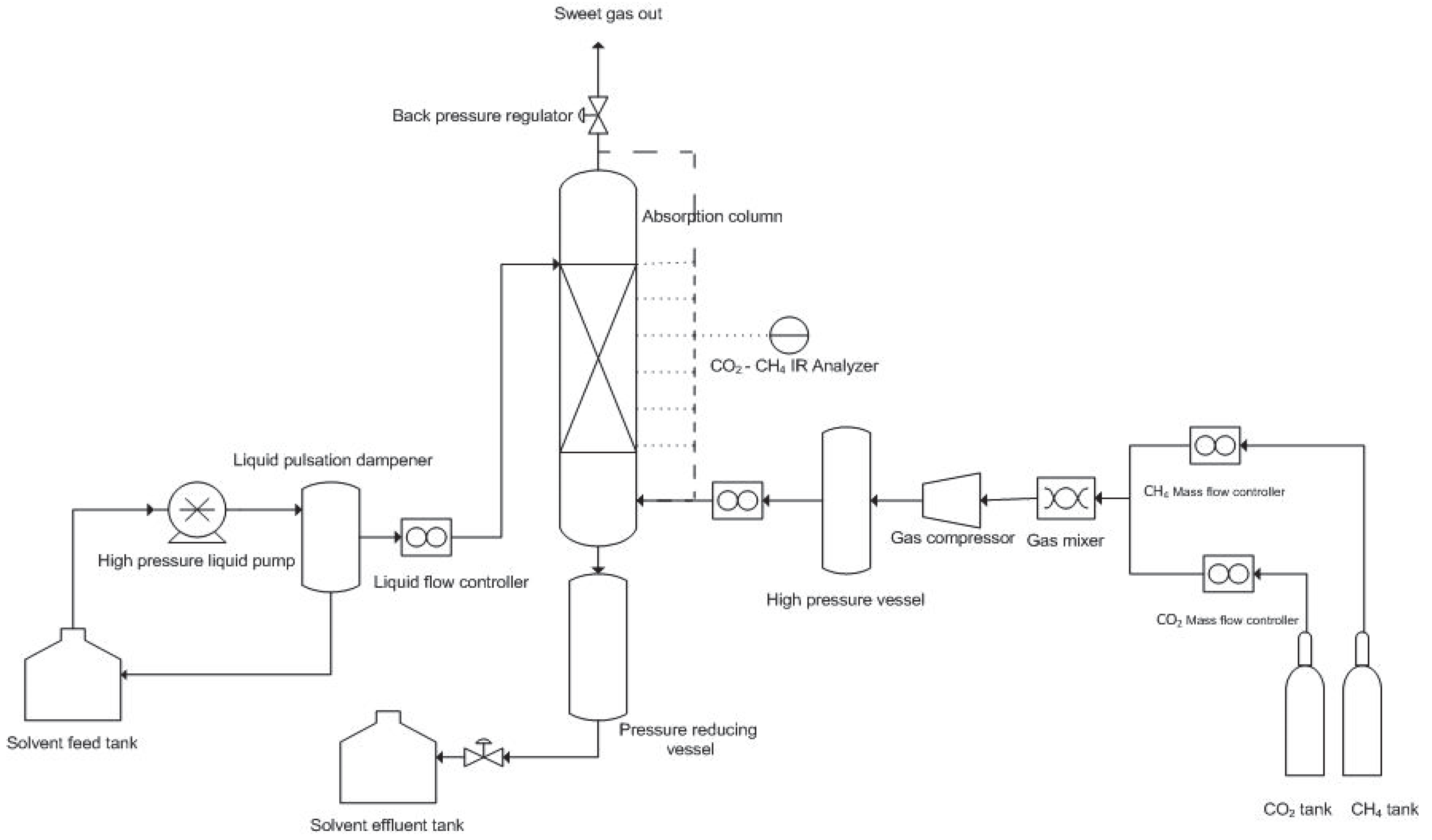
2.2. Process Description
2.3. Reactive Absorption Model for and Determination
2.3.1. Reaction and Kinetic Mechanism of CO2 Absorption into PCGLY Blended Solvent
2.3.2. Two-Film Theory
2.3.3. Reaction Model
2.3.4. Mass Balance
- The system is at steady-state operation.
- Fast reaction mechanism occurs in the liquid film of the gas-liquid interface.
- The gas and liquid flow rates are constant throughout the column.
2.4. CO2 Removal Efficiency
2.5. Mean Absolute Error (MAE) Was Determined as Given in Equation (26)
3. Results
3.1. CO2 Absorption Performance Behaviour
3.1.1. Effect of Liquid Flow Rate on CO2 Absorption and Mass Transfer Performances
3.1.2. Effect of Gas Flow Rate on CO2 Absorption Performances
3.1.3. Effect of Operational Pressure on CO2 Absorption Performances
3.1.4. Effect of Inlet Liquid Temperature on CO2 Absorption Performance
3.2. Comparative Study of Absorption Performance with MDEA
PCGLY as Potential Green Solvent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Roser, M. CO2 and Other Greenhouse Gas Emissions; Our World in Data: Oxford, UK, 2019. [Google Scholar]
- Ahmad, S.; Ab Kadir MZ, A.; Shafie, S. Current perspective of the renewable energy development in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 897–904. [Google Scholar] [CrossRef]
- Tan, L.S.; Shariff, A.M.; Lau, K.K.; Bustam, M.A. Factors affecting CO2 absorption efficiency in packed column: A review. J. Ind. Eng. Chem. 2012, 18, 1874–1883. [Google Scholar] [CrossRef]
- Global Natural Gas. Insight; IGU: Barcelona, Spain, 2017.
- The Outlook for Energy: A View to 2040; Exxonmobil: Irving, TX, USA, 2017.
- Darman, N.H.; Harun, A.R. Technical Challenges and Solutiond on Natural Gas Development in Malaysia. In Proceedings of the 4th Workshop of the China-Sichuan Basin Case Study, Beijing, China, 30 May–3 June 2006. [Google Scholar]
- Halim, H.; Shariff, A.; Bustam, M. High pressure CO2 absorption from natural gas using piperazine promoted 2-amino-2-methyl-1-propanol in a packed absorption column. Sep. Purif. Technol. 2015, 152, 87–93. [Google Scholar] [CrossRef]
- Hairul, N.A.H.; Shariff, A.M.; Bustam, M.A. Mass transfer performance of 2-amino-2-methyl-1-propanol and piperazine promoted 2-amino-2-methyl-1-propanol blended solvent in high pressure CO22 absorption. Int. J. Greenh. Gas Control 2016, 49, 121–127. [Google Scholar] [CrossRef]
- Tan, L.S.; Lau, K.K.; Bustam, M.A.; Shariff, A.M. Removal of high concentration CO2 from natural gas at elevated pressure via absorption process in packed column. J. Nat. Gas Chem. 2012, 21, 7–10. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Adewole, J.K.; Leo, C.P.; Ismail, S.; Sultan, A.S.; Olatunji, S.O. Prediction of plasticization pressure of polymeric membranes for CO2 removal from natural gas. J. Membr. Sci. 2015, 480, 39–46. [Google Scholar] [CrossRef]
- Wu, S.Y.; Liu, Y.F.; Chu, C.Y.; Li, Y.C.; Liu, C.M. Optimal Absorbent Evaluation for the CO2 Separating Process by Absorption Loading, Desorption Efficiency, Cost, and Environmental Tolerance. Int. J. Green Energy 2014, 12, 1025–1030. [Google Scholar] [CrossRef]
- Ma’mum, S.; Svendsen, H.F.; Hoff, K.A.; Juliussen, O. Selection of new absorbents for carbon dioxide capture. Energy Convers. Manag. 2007, 48, 251–258. [Google Scholar] [CrossRef]
- Thee, H.; Nicholas, N.J.; Smith, K.H.; da Silva, G.; Kentish, S.E.; Stevens, G.W. A kinetic study of CO2 capture with potassium carbonate solutions promoted with various amino acids: Glycine, sarcosine and proline. Int. J. Greenh. Gas Control 2014, 20, 212–222. [Google Scholar] [CrossRef]
- Shuaib, S.M.; Shariff, A.M.; Bustam, M.A.; Murshid, G. Physical properties of aqueous solutions of potassium carbonate + glycine as a solvent for carbon dioxide removal. J. Serb. Chem. Soc. 2014, 79, 719–727. [Google Scholar] [CrossRef]
- Shen, S.; Yang, Y.; Wang, Y.; Ren, S.; Han, J.; Chen, A. CO2 absorption into aqueous potassium salts of lysine and proline: Density, viscosity and solubility of CO2. Fluid Phase Equilibria 2015, 399, 40–49. [Google Scholar] [CrossRef]
- Hamzehie, M.E.; Najibi, H. Experimental and theoretical study of carbon dioxide solubility in aqueous solution of potassium glycinate blended with piperazine as new absorbents. J. CO2 Util. 2016, 16, 64–77. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Grant, T.; Anderson, C.; Hooper, B. Comparative life cycle assessment of potassium carbonate and monoethanolamine solvents for CO2 capture from post combustion flue gases. Int. J. Greenh. Gas Control 2014, 28, 35–44. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Akbari, V.; Hamid, M.K.A.; Manan, Z.A. Rate-based simulation and comparison of various promoters for CO2 capture in industrial DEA-promoted potassium carbonate absorption unit. J. Ind. Eng. Chem. 2015, 22, 306–316. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Azmi, M.S.; Bustam, M.A. Study of CO2 solubility in Aqueous Blend of Potassium Carbonate Promoted with Glycine. Appl. Mech. Mater. 2014, 625, 19–23. [Google Scholar] [CrossRef]
- Hu, G.; Nicholas, N.J.; Smith, K.H.; Mumford, K.A.; Kentish, S.E.; Stevens, G.W. Carbon dioxide absorption into promoted potassium carbonate solutions: A review. Int. J. Greenh. Gas Control 2016, 53, 28–40. [Google Scholar] [CrossRef]
- Kim, Y.E.; Choi, J.H.; Nam, S.C.; Yoon, Y.I. CO2 absorption capacity using aqueous potassium carbonate with 2-methylpiperazine and piperazine. J. Ind. Eng. Chem. 2012, 18, 105–110. [Google Scholar] [CrossRef]
- Ramazani, R.; Mazinani, S.; Jahanmiri, A.; Bruggen, B.V.D. Experimental investigation of the effect of addition of different activators to aqueous solution of potassium carbonate: Absorption rate and solubility. Int. J. Greenh. Gas Control 2016, 45, 27–33. [Google Scholar] [CrossRef]
- Raksajati, A.; Ho, M.T.; Wiley, D.E. Reducing the cost of CO2 capture from flue gases using aqueous chemical absorption. Ind. Eng. Chem. Res. 2013, 52, 16887–16901. [Google Scholar] [CrossRef]
- Guo, D.; Thee, H.; Tan, C.Y.; Chen, J.; Fei, W.; Kentish, S.; Stevens, G.W. Amino acids as carbon capture solvents: Chemical kinetics and mechanism of the glycine+ CO22 reaction. Energy Fuels 2013, 27, 3898–3904. [Google Scholar] [CrossRef]
- Smith, K.H.; Harkin, T.; Mumford, K.; Kentish, S.; Qader, A.; Anderson, C.; Hooper, B.; Stevens, G.W. Outcomes from pilot plant trials of precipitating potassium carbonate solvent absorption for CO2 capture from a brown coal fired power station in Australia. Fuel Process. Technol. 2017, 155, 252–260. [Google Scholar] [CrossRef]
- Smith, K.; Andrew, L.; Mumford, K.; Li, S.; Indrawan; Thanumurthy, N.; Anderson, C.; Hooper, B.; Kentish, S.; Stevens, G. Pilot plant results for a precipitating potassium carbonate solvent absorption process promoted with glycine for enhanced CO2 capture. Fuel Process. Technol. 2015, 135, 60–65. [Google Scholar] [CrossRef]
- Echt, W.; Meister, P. Design, fabrication and startup of an offshore membrane CO2 removal system. In Proceedings of the 88th Annual Convention, Gas Processors Association, P-28, San Antonio, TX, USA, 8–10 March 2009. [Google Scholar]
- Abdul Halim, H.N.; Shariff, A.M.; Tan, L.S.; Bustam, M.A. Mass transfer performance of CO2 absorption from natural gas using monoethanolamine (MEA) in high pressure operations. Ind. Eng. Chem. Res. 2015, 54, 1675–1680. [Google Scholar] [CrossRef]
- Hairul, N.; Shariff, A.; Bustam, M. Process behaviour in a packed absorption column for high pressure CO2 absorption from natural gas using PZ+ AMP blended solution. Fuel Process. Technol. 2017, 157, 20–28. [Google Scholar] [CrossRef]
- Thee, H.; Smith, K.H.; Silva, G.D.; Kentish, S.E.; Stevens, G.W. Carbon dioxide absorption into unpromoted and borate-catalyzed potassium carbonate solutions. Chem. Eng. J. 2012, 181, 694–701. [Google Scholar] [CrossRef]
- McCabe, W.; Smith, J.C.; Hariot, P. Unit Operation of Chemical Engineering, 7th ed.; McGraw-Hill Companies, Inc.: Singapore, 2005. [Google Scholar]
- Zeng, Q.; Guo, Y.; Niu, Z.; Lin, W. Mass transfer coefficients for CO2 absorption into aqueous ammonia solution using a packed column. Ind. Eng. Chem. Res. 2011, 50, 10168–10175. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Tontiwachwuthikul, P. High-efficiency structured packing for CO2 separation using 2-amino-2-methyl-1-propanol (AMP). Sep. Purif. Technol. 1997, 12, 67–79. [Google Scholar] [CrossRef]
- Wellek, R.; Brunson, R.; Law, F. Enhancement factors for gas-absorption with second-order irreversible chemical reaction. Can. J. Chem. Eng. 1978, 56, 181–186. [Google Scholar] [CrossRef]
- Hairul, N.; Shariff, A.M.; Tay, W.H.; Mortel, A.M.A.; Lau, K.K.; Tan, L.S. Modelling of high pressure CO2 absorption using PZ+ AMP blended solution in a packed absorption column. Sep. Purif. Technol. 2016, 165, 179–189. [Google Scholar] [CrossRef]
- Cullinane, J.T.; Rochelle, G.T. Carbon dioxide absorption with aqueous potassium carbonate promoted by piperazine. Chem. Eng. Sci. 2004, 59, 3619–3630. [Google Scholar] [CrossRef]
- Kumar, N.; Rao, D.P. Design of a packed column for absorption of carbon dioxide in hot K2CO3 solution promoted by arsenious acid. Gas. Sep. Purif. 1989, 3, 152–155. [Google Scholar] [CrossRef]
- Shen, S.; Feng, X.; Zhao, R.; Ghosh, U.K.; Chen, A. Kinetic study of carbon dioxide absorption with aqueous potassium carbonate promoted by arginine. Chem. Eng. J. 2013, 222, 478–487. [Google Scholar] [CrossRef]
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High performance promoter-free CO2 absorption using potassium carbonate solution in an ultrasonic irradiation system. J. CO2 Util. 2017, 21, 383–394. [Google Scholar] [CrossRef]
- Mustafa, N.F.A.; Shariff, A.M.; Halim, H.N.A.; Tay, W.H.; Yusof, S.M.M. CO2 removal efficiency from natural gas at elevated pressure of packed absorption CO2 lumn using potassium carbonate promoted with glycine. Sci. Proc. Ser. 2019, 1, 55–57. [Google Scholar]
- Lau, R.; Peng, W.; Valesquez-Vargas, L.G.; Yang, G.Q.; Fan, L.S. Gas− liquid mass transfer in high-pressure bubble columns. Ind. Eng. Chem. Res. 2004, 43, 1302–1311. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, X.; Yu, K. Review of mass-transfer correlations for packed columns. Ind. Eng. Chem. Res. 2005, 44, 8715–8729. [Google Scholar] [CrossRef]
- Naami, A.; Edali, M.; Sema, T.; Idem, R.; Tontiwachwuthikul, P. Mass transfer performance of CO2 absorption into aqueous solutions of 4-diethylamino-2-butanol, monoethanolamine, and N-methyldiethanolamine. Ind. Eng. Chem. Res. 2012, 51, 6470–6479. [Google Scholar] [CrossRef]
- Xu, B.; Gao, H.; Luo, X.; Liao, H.; Liang, Z. Mass transfer performance of CO2 absorption into aqueous DEEA in packed columns. Int. J. Greenh. Gas Control 2016, 51, 11–17. [Google Scholar] [CrossRef]
- Fu, K.; Rongwong, W.; Liang, Z.; Na, Y.; Idem, R.; Tontiwachwuthikul, P. Experimental analyses of mass transfer and heat transfer of post-combustion CO2 absorption using hybrid solvent MEA–MeOH in an absorber. Chem. Eng. J. 2015, 260, 11–19. [Google Scholar] [CrossRef]
- Ling, H.; Gao, H.; Liang, Z. Comprehensive solubility of N2O and mass transfer studies on an effective reactive N, N-dimethylethanolamine (DMEA) solvent for post-combustion CO2 capture. Chem. Eng. J. 2019, 355, 369–379. [Google Scholar] [CrossRef]
- Pinsent, B.; Pearson, L.; Roughton, F. The kinetics of combination of carbon dioxide with hydroxide ions. Trans. Faraday Soc. 1956, 52, 1512–1520. [Google Scholar] [CrossRef]
- Yi, F.; Zou, H.K.; Chu, G.W.; Shao, L.; Chen, J.F. Modeling and experimental studies on absorption of CO2 by Benfield solution in rotating packed bed. Chem. Eng. J. 2009, 145, 377–384. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Idem, R.; Tontiwachwuthikul, P.P.T.; Liang, Z. Analysis of solubility, absorption heat and kinetics of CO2 absorption into 1-(2-hydroxyethyl) pyrrolidine solvent. Chem. Eng. Sci. 2017, 162, 120–130. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Alwi, S.R.W.; Manan, Z.A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. Int. J. Greenh. Gas Control 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Smith, K.H.; Anderson, C.J.; Tao, W.; Endo, K.; Mumford, K.A.; Kentish, S.E.; Qader, A.; Hooper, B.; Stevens, G.W. Pre-combustion capture of CO2—Results from solvent absorption pilot plant trials using 30 wt% potassium carbonate and boric acid promoted potassium carbonate solvent. Int. J. Greenh. Gas Control 2012, 10, 64–73. [Google Scholar] [CrossRef]









| Operating Parameters | Value (Unit) |
|---|---|
| MDEA concentration | 1.3 (kmol/m3) |
| PCGLY concentration | 1.2 (kmol/m3) |
| Liquid flow rate | 7.22 (m3/m2.h) |
| Gas flow rate | 41.72 (kmol/m2.h) |
| Operating pressure | 4.04 (MPa) |
| CO2 concentration | 20 (%) |
| Liquid inlet temperature | 333 (K) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, N.F.A.; Mohd Shariff, A.; Tay, W.H.; Abdul Halim, H.N.; Mhd Yusof, S.M. Mass Transfer Performance Study for CO2 Absorption into Non-Precipitated Potassium Carbonate Promoted with Glycine Using Packed Absorption Column. Sustainability 2020, 12, 3873. https://doi.org/10.3390/su12093873
Mustafa NFA, Mohd Shariff A, Tay WH, Abdul Halim HN, Mhd Yusof SM. Mass Transfer Performance Study for CO2 Absorption into Non-Precipitated Potassium Carbonate Promoted with Glycine Using Packed Absorption Column. Sustainability. 2020; 12(9):3873. https://doi.org/10.3390/su12093873
Chicago/Turabian StyleMustafa, Nur Farhana Ajua, Azmi Mohd Shariff, Wee Horng Tay, Hairul Nazirah Abdul Halim, and Siti Munirah Mhd Yusof. 2020. "Mass Transfer Performance Study for CO2 Absorption into Non-Precipitated Potassium Carbonate Promoted with Glycine Using Packed Absorption Column" Sustainability 12, no. 9: 3873. https://doi.org/10.3390/su12093873
APA StyleMustafa, N. F. A., Mohd Shariff, A., Tay, W. H., Abdul Halim, H. N., & Mhd Yusof, S. M. (2020). Mass Transfer Performance Study for CO2 Absorption into Non-Precipitated Potassium Carbonate Promoted with Glycine Using Packed Absorption Column. Sustainability, 12(9), 3873. https://doi.org/10.3390/su12093873





