First Report of the Dinoflagellate Genus Effrenium in the East Sea of Korea: Morphological, Genetic, and Fatty Acid Characteristics
Abstract
1. Introduction
2. Materials and Methods
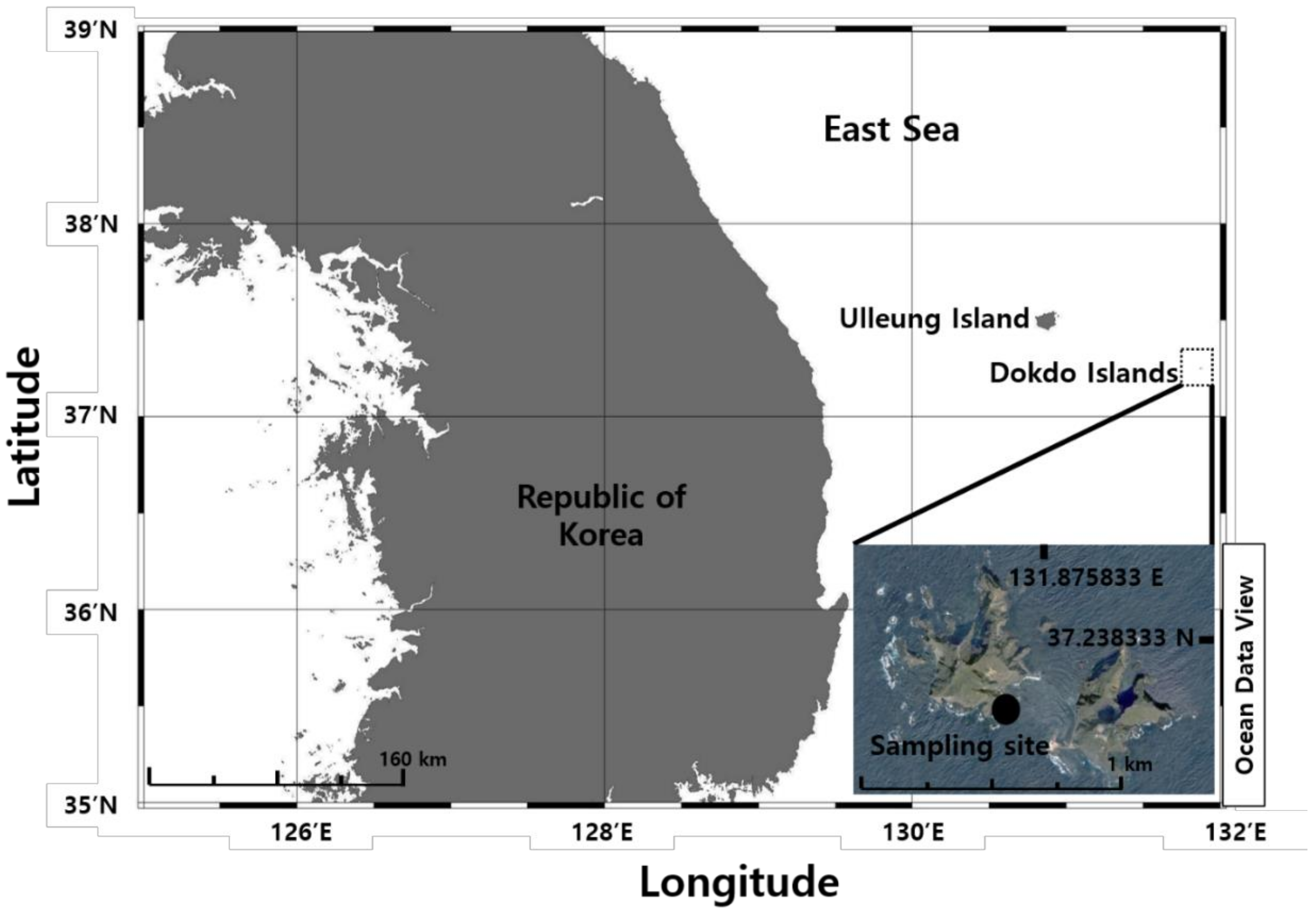
2.1. Sample Collection and Isolation
2.2. Morphological Identification
2.3. Molecular Identification
2.4. Fatty acid Composition Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jeong, H.J. The Interactions between Microzooplanktonic Grazers and Dinoflagellates Causing Red Tides in the Open Coastal Waters off Southern California. Ph.D. Thesis, University of California, San Diego, CA, USA, 1995; p. 139. [Google Scholar]
- Park, T.G.; Lim, W.A.; Park, Y.T.; Lee, C.K.; Jeong, H.J. Economic impact, management and mitigation of red tides in Korea. Harmful Algae 2013, 30, S131–S143. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.D.; Kim, J.S.; Seong, K.A.; Kang, N.S.; Kim, T.H. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci. J. 2010, 45, 65–91. [Google Scholar] [CrossRef]
- Hwang, B.S.; Yoon, E.Y.; Kim, H.S.; Yih, W.; Park, J.Y.; Jeong, H.J.; Rho, J. Ostreol A: A new cytotoxic compound isolated from the epiphytic dinoflagellate Ostreopsis cf. ovata from the coastal waters of Jeju Island, Korea. Bioorg. Med. Chem. Lett. 2013, 23, 3023–3027. [Google Scholar] [PubMed]
- Jang, S.H.; Jeong, H.J.; Kwon, J.E. High contents of eicosapentaenoic acid and docosahexaenoic acid in the mixotrophic dinoflagellate Paragymnodinium shiwhaense and identification of putative omega-3 biosynthetic genes. Algal Res. 2017, 25, 525–537. [Google Scholar] [CrossRef]
- Yoon, E.Y.; Park, J.Y.; Jeong, H.J.; Rho, J. Fatty acid composition and docosahexaenoic acid (DHA) content of the heterotrophic dinoflagellate Oxyrrhis marina fed on dried yeast: Compared with algal prey. Algae 2017, 32, 67–74. [Google Scholar] [CrossRef][Green Version]
- Peltomaa, E.; Hällfors, H.; Taipale, S.J. Comparison of diatoms and dinoflagellates from different habitats as sources of PUFAs. Mar. Drugs 2019, 17, 233. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef]
- Galloway, A.W.; Winder, M. Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS ONE 2015, 10, e0130053. [Google Scholar] [CrossRef]
- Taipale, S.J.; Vuorio, K.; Strandberg, U.; Kahilainen, K.K.; Järvinen, M.; Hiltunen, M.; Peltomaa, E.; Kankaala, P. Lake eutrophication and brownification downgrade availability and transfer of essential fatty acids for human consumption. Environ. Int. 2016, 96, 156–166. [Google Scholar] [CrossRef]
- Brett, M.T.; Müller-Navarra, D.C.; Ballantyne, A.P.; Ravet, J.L.; Goldman, C.R. Daphnia fatty acid composition reflects that of their diet. Limnol. Oceanogr. 2006, 51, 2428–2437. [Google Scholar] [CrossRef]
- LaJeunesse, T.C. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 2002, 141, 387–400. [Google Scholar]
- Lee, S.Y.; Jeong, H.J.; Kang, N.S.; Jang, T.Y.; Jang, S.H.; LaJeunesse, T.C. Symbiodinium tridacnidorum sp. nov., a dinoflagellate common to Indo-Pacific giant clams, and a revised morphological description of Symbiodinium microadriaticum Freudenthal, emended Trench & Blank. Eur. J. Phycol. 2015, 50, 155–172. [Google Scholar]
- Lee, S.Y.; Jeong, H.J.; Kang, N.S.; Jang, T.Y.; Jang, S.H.; Lim, A.S. Morphological characterization of Symbiodinium minutum and S. psygmophilum belonging to clade B. Algae 2014, 29, 299–310. [Google Scholar]
- Lobban, C.S.; Schefter, M.; Simpson, A.G.B.; Pochon, X.; Pawlowski, J.; Foissner, W. Maristentor dinoferus n. gen., n. sp. a giant heterotrich ciliate (Spirotrichea: Heterotrichida) with zooxanthellae, from coral reefs on Guam, Mariana Islands. Mar. Biol. 2002, 40, 411–423. [Google Scholar]
- Pochon, X.; Garcia-Cuestos, L.; Baker, A.C.; Castella, E.; Pawlowski, J. One-year survey of a single Micronesian reef reveals extraordinarily rich diversity of Symbiodinium types in soritid foraminifera. Coral Reefs 2007, 26, 867–882. [Google Scholar] [CrossRef]
- Trench, R.K. Microalgal-invertebrate symbioses: A review. Endocytobiosis Cell Res. 1993, 9, 135–175. [Google Scholar]
- Jeong, H.J.; Lee, S.Y.; Kang, N.S.; Yoo, Y.D.; Lim, A.S.; Lee, M.J.; Kim, H.S.; Yih, W.; Yamashita, H.; LaJeunesse, T.C. Genetics and morphology characterize the dinoflagellate Symbiodinium voratum, n. sp., (Dinophyceae) as the sole representative of Symbiodinium clade E. J. Eukaryot. Microbiol. 2014, 61, 75–94. [Google Scholar] [CrossRef]
- Lee, S.B. Isolation and Structure Determination of Bioactive Secondary Metabolites from Benthic Marine Dinoflagellates and Sponges. Master’s Thesis, Kunsan National University, Gunsan, Korea, 2016. [Google Scholar]
- Nakamura, H.; Asari, T.; Ohizumi, Y.; Kobayashi, J.; Yamasu, T.; Murai, A. Isolation of zooxanthellatoxins, novel vasoconstrictive substances from the zooxanthella Symbiodinium sp. Toxicon 1993, 31, 371–376. [Google Scholar] [CrossRef]
- LaJeunesse, T.C.; Parkinson, E.J.; Gabrielson, P.W.; Jeong, H.J.; Reimer, J.D.; Voolstra, C.R.; Santos, S.R. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018, 28, 2570–2580. [Google Scholar] [CrossRef]
- Chang, F.H. Winter phytoplankton and microzooplankton populations off the coast of Westland, New Zealand, 1979. N. Z. J. Mar. Freshw. Res. 1983, 17, 279–304. [Google Scholar] [CrossRef]
- Polne-Fuller, M. A novel technique for preparation of axenic cultures of Symbiodinium (Pyrrophyta) through selective digestion by amoebae. J. Phycol. 1991, 27, 552–554. [Google Scholar] [CrossRef]
- Yamashita, H.; Koike, K. The genetic identity of free-living Symbiodinium obtained over a broad latitudinal range in the Japanese coast. Phycol. Res. 2013, 61, 68–80. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Moestrup, Ø.; Park, T.G. Gyrodiniellum shiwhaense n. gen., n. sp., a new planktonic heterotrophic dinoflagellate from the coastal waters of western Korea: Morphology and ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2011, 58, 284–309. [Google Scholar] [CrossRef] [PubMed]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group and strain specific genetic makers for globally distributed Alexandrium (Dinophyceae) II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Kibler, S.R.; Reece, K.S.; Stokes, N.A.; Steidinger, K.A.; Millie, D.F.; Bendis, B.J.; Pigg, R.J.; Tester, P.A. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J. Phycol. 2003, 39, 754–761. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software v.4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MrBayes: Bayesian inference of phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Moestrup, Ø.; Shin, W.G.; Nam, S.W.; Park, J.Y.; de Salas, M.F.; Kim, K.W.; Noh, J.H. Description of a new planktonic mixotrophic dinoflagellate Paragymnodinium shiwhaense n. gen., n. sp. from the coastal waters off western Korea: Morphology, pigments, and ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2010, 57, 121–144. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Breuer, G.; Evers, W.A.C.; de Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of fatty acid content and composition in microalgae. J. Vis. Exp. 2013, 80, e50628. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.S.; Lee, J.A.; Jang, H.S.; Kim, K.M.; Lim, E.S.; Yoon, M.; Hong, J.W. First record of a marine microalgal species, Chlorella gloriosa (Trebouxiophyceae) isolated from the Dokdo Islands, Korea. Korean J. Environ. Biol. 2019, 37, 526–534. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Lindberg, K.; Daugbjerg, N. Studies on woloszynskioid dinoflagellates IV: The genus Biecheleria gen. Nov. Phycol. Res. 2009, 57, 203–220. [Google Scholar] [CrossRef]
- Fensome, R.A.; Taylor, F.J.R.; Norris, G.; Sargeant, W.A.S.; Wharton, D.I.; William, G.L. A Classification of Living and Fossil Dinoflagellates; Sheridan Press: Hanover, Germany, 1993; p. 351. [Google Scholar]
- Gou, W.L.; Sun, J.; Li, X.Q.; Zhen, Y.; Xin, Z.; Yu, Z.G.; Li, R.X. Phylogenetic analysis of a free-living strain of Symbiodinium isolated from Jiaozhou Bay, P.R. China. J. Exp. Mar. Biol. Ecol. 2003, 296, 135–144. [Google Scholar] [CrossRef]
- Jeong, H.J.; Yoo, Y.D.; Kang, N.S.; Lim, A.S.; Seong, K.A.; Lee, S.Y.; Lee, M.J.; Lee, K.H.; Kim, H.S.; Shin, W.G.; et al. Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium. Proc. Natl. Acad. Sci. USA 2012, 109, 12604–12609. [Google Scholar] [CrossRef]
- Shao, P.; Chen, Y.Q.; Zhou, H.; Yuan, J.; Qu, L.H.; Zhao, D.; Lin, Y.S. Genetic variability in the Gymnodiniaceae ITS regions: Implications for species identification and phylogenetic analysis. Mar. Biol. 2004, 144, 215–224. [Google Scholar] [CrossRef]
- McBride, B.B.; Muller-Parker, G.; Jakobsen, H.H. Low thermal limit of growth rate of Symbiodinium californium (Dinophyta) in culture may restrict the symbiont to southern populations of its host anemones (Anthopleura spp.; Anthozoa, Cnidaria). J. Phycol. 2009, 45, 855–863. [Google Scholar] [CrossRef]
- Awai, K.; Matsuoka, R.; Shioi, Y. Lipid and fatty acid compositions of Symbiodinium strains. In Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9–13 July 2012. [Google Scholar]
- Tsirigoti, A.; Tzovenis, I.; Koutsaviti, A.; Economou-Amilli, A.; Ioannou, E.; Melkonian, M. Biofilm cultivation of marine dinoflagellates under different temperatures and nitrogen regimes enhances DHA productivity. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Mansour, M.P.; Volkman, J.K.; Jackson, A.E.; Blackburn, S.I. The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 1999, 35, 710–720. [Google Scholar] [CrossRef]
- Nichols, P.D.; Jones, G.J.; De Leeuw, J.W.; Johns, R.B. The fatty acid and sterol composition of two marine dinoflagellates. Phytochemistry 1984, 23, 1043–1047. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Aizdaicher, N.A. Fatty acid composition of 15 species of marine microalgae. Phytochemistry 1995, 39, 351–356. [Google Scholar] [CrossRef]
- Volkman, J.K. Fatty acids of microalgae used as feedstocks in aquaculture. In Fats for the Future; Cambie, R.C., Ed.; Ellis Horwood: Chichester, UK, 1989; pp. 263–283. [Google Scholar]
- Barreira, L.; Pereira, H.; Gangadhar, K.N.; Custódio, L.; Varela, J. Medicinal effects of microalgae-derived fatty acids. In Handbook of Marine Microalgae; Kim, S.K., Ed.; Academic Press: Cambridge, UK, 2015; pp. 209–231. [Google Scholar]
- Mehta, L.R.; Dworkin, R.H.; Schwid, S.R. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat. Clin. Pr. Neurol. 2009, 5, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [PubMed]
- Marszalek, J.R.; Lodish, H.F. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: Breastmilk and fish are good for you. Annu. Rev. Cell Dev. Biol. 2005, 21, 633–657. [Google Scholar] [CrossRef] [PubMed]
- Jeromson, S.; Gallagher, I.J.; Galloway, S.D.; Hamilton, D.L. Omega-3 fatty acids and skeletal muscle health. Mar. Drugs 2015, 13, 6977–7004. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X. Omega-3: A link between global climate change and human health. Biotechnol. Adv. 2011, 29, 388–390. [Google Scholar] [CrossRef]
- Jang, H.S.; Kang, N.S.; Kim, K.M.; Jeon, B.H.; Park, J.S.; Hong, J.W. Description and application of a marine microalga Auxenochlorella protothecoides isolated from Ulleung-do. J. Life Sci. 2017, 27, 1152–1160. [Google Scholar]
- Kim, K.M.; Kang, N.S.; Jang, H.S.; Park, J.S.; Jeon, B.H.; Hong, J.W. Characterization of Heterochlorella luteoviridis (Trebouxiaceae, Trebouxiophyceae) isolated from the Port of Jeongja in Ulsan, Korea. J. Mar. Biosci. Biotechnol. 2017, 9, 22–29. [Google Scholar]
- Manikan, V.; Nazir, M.Y.M.; Kalil, M.S.; Isa, M.H.M.; Kader, A.J.A.; Yusoff, W.M.W.; Hamid, A.A. A new strain of docosahexaenoic acid producing microalga from Malaysian coastal waters. Algal Res. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Thompson, P.A.; Guo, M.X.; Harrison, P.J.; Whyte, J.N. Effects of variation in temperature. II. On the fatty acid composition of eight species of marine phytoplankton. J. Phycol. 1992, 28, 488–497. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary ω-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ω-6/ω-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. (Maywood) 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- Turchini, G.M.; Ng, W.K.; Tocher, D.R. Fish oil replacement and alternative lipid sources in aquaculture feeds; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2010; p. 533. [Google Scholar]
- De Roos, B.; Sneddon, A.A.; Sprague, M.; Horgan, G.W.; Brouwer, I.A. The potential impact of compositional changes in farmed fish on its health-giving properties: Is it time to reconsider current dietary recommendations? Public Health Nutr. 2017, 20, 2042–2049. [Google Scholar] [CrossRef]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Chen, B. Effects of dietary essential fatty acids on reproduction rates of subtropical calanoid copepod, Acartia erythraea. Mar. Ecol. Prog. Ser. 2012, 455, 95–110. [Google Scholar] [CrossRef]
- Jonasdottir, S.H. Effects of food quality on the reproductive success of Acartia tonsa and Acartia hudsonica—Laboratory observations. Mar. Biol. 1994, 121, 67–81. [Google Scholar] [CrossRef]
- Taipale, S.J.; Kahilainen, K.K.; Holtgrieve, G.W.; Peltomaa, E.T. Simulated eutrophication and browning alters zooplankton nutritional quality and determines juvenile fish growth and survival. Ecol. Evol. 2018, 8, 2671–2687. [Google Scholar] [CrossRef]






| Species | Strain | LC | Date | T | S | GBAN |
|---|---|---|---|---|---|---|
| E. voratum | MABIKLP88 | Dokdo Islands | September 2016 | 24 | 35 | MN904916 |
| Primer Name | Amplifies | Sequence (5′-3′) | Reference |
|---|---|---|---|
| Forward primer D1R | LSU rDNA | ACC CGC TGA ATT TAA GCA TA | [26] |
| Reverse primer LSUB | LSU rDNA | ACG AAC GAT TTG CAC GTC AG | [27] |
| Mastigote Character Traits | MABIKLP88 | SvFL 1 | CCMP421 |
|---|---|---|---|
| Strain locality | Dokdo Islands, Korea | Jeju Island, Korea | Cook Strait, New Zealand |
| Shape in ventral view | Mushroom | Mushroom | Mushroom |
| AP length (μm; living cells) | 9.42–15.6 (12) | 10.8–16.2 (13.1) | 10.1–17.1 (12.8) |
| Cell width (μm; living cells) | 7.08–11.9 (9.7) | 7.8–11.5 (9.5) | 8.1–14.4 (10.3) |
| Ratio of length to width (living cells) | 1.12–1.4 (1.2) | 1.1–1.8 (1.4) | 1.2–1.3 (1.2) |
| AP length (μm; SEM) | 7.3–12.7 (9.6) | 8.5–12.4 (10.5) | 7.0–13.0 (11.7) |
| Cell width (μm; SEM) | 5.52–11.4 (7.7) | 6.4–9.8 (8.2) | 5.8–10.9 (9.2) |
| Ratio of length to width (SEM) | 0.83–1.4 (1.2) | 1.2–1.4 (1.3) | 1.2–1.4 (1.3) |
| EAV length (μm) | 1.71–2.72 (2.25) | 1.75–3.09 (2.45) | 1.98–3.19 (2.64) |
| EAV width (μm) | 0.15–0.26 (0.21) | 0.15–0.27 (0.2) | 0.18–0.29 (0.22) |
| Numbers of aligned knobs on EAV | 11–12 | 9–13 | 9–13 |
| Cingulum displaced by cell length | 0.11–0.26 (0.18) | 0.13–0.21 (0.15) | 0.10–0.19 (0.15) |
| Cingulum displaced by cell width | 0.49–0.80 (0.62) | 0.48–0.85 (0.65) | 0.55–1.10 (0.78) |
| Numbers of cingular plates | 17–20 | 17–20 | 17–20 |
| Numbers of sulcal plates | 9 | 9 | 9 |
| Numbers of apical plates | 5 | 5 | 5 |
| Numbers of intercalary plates | 5 | 5 | 5 |
| Numbers of precingular plates | 8 | 8 | 8 |
| Numbers of postcingular plates | 6 | 6 | 6 |
| Numbers of antapical plates | 2 | 2 | 2 |
| Existence of eyespot type E | Yes | Yes | Yes |
| Plate formula | x, EAV, 5′, 5a, 8′′, 9s, 17–20c, 6′′′, 2′′′′, PE | x, EAV, 5′, 5a, 8′′, 9s, 17–20c, 6′′′, 2′′′′, PE | x, EAV, 5′, 5a, 8′′, 9s, 17–20c, 6′′′, 2′′′′, PE |
| Reference | This study | [18] | [18] |
| Collection Location | Strain | GenBank Accession No. | E. voratum MABIKLP88 |
|---|---|---|---|
| Jeju Island, Korea | SVIC1 | HE653239 | 0 (0) |
| SVFL1 | HF568830 | 0 (0) | |
| SVFL2 | HF568831 | 0 (0) | |
| SVFL3 | HF568832 | 0 (0) | |
| SVFL4 | HF568833 | 0 (0) | |
| SVFL5 | HF568834 | 0 (0) | |
| SVFL6 | HF568835 | 0 (0) | |
| Tsushima Island, Japan | TSP-C2-Sy | KF364604 | 0 (0) |
| Cook Strait, New Zealand | CCMP421 | KF364603 | 0 (0) |
| Blanes, Spain | RCC1521 | KF364606 | 1 (0.2) |
| Santa Barbara, CA, USA | rt-383 | KF364605 | 1 (0.2) |
| Component | Content (%) | Note |
|---|---|---|
| Lauric acid (C12:0) | 0.6 | |
| Myristic acid (C14:0) | 3.5 | |
| Pentadecanoic acid (C15:0) | 0.3 | |
| Palmitic acid (C16:0) | 22.1 | SFA (major) |
| Palmitoleic acid (C16:1 n-7) | 9.3 | |
| Hexadecadienoic acid (C16:2 n-4) | 0.4 | |
| Stearic acid (C18:0) | 0.7 | |
| Oleic acid (C18:1 n-9) | 3.3 | |
| Linoleic acid (C18:2 n-6) | 0.6 | |
| g-linolenic acid (C18:3 n-6) | 0.9 | |
| α-linolenic acid (C18:3 n-3) | 0.3 | |
| Stearidonic acid (C18:4 n-3) | 15.2 | Omega-3 PFUA (major) |
| Eicosapentaenoic acid (C20:5 n-3) | 10.9 | Omega-3 PFUA (major) |
| Docosahexaenoic acid (C22:6 n-3) | 25.4 | Omega-3 PFUA (major) |
| Unidentified | 6.5 |
| Culture | Collection Region | LC | Latitude | Longitude | Reference |
|---|---|---|---|---|---|
| MABIKLP88 | Western North Pacific | Dokdo Islands, South Korea | 37.240486 N | 131.870853 E | This study |
| SvIC 1 | Western North Pacific | Jeju Island, South Korea | 33.276667 N | 126.170556 E | [39] |
| SvFL 1 | Western North Pacific | Jeju Island, South Korea | 33.468611 N | 126.324444 E | [39] |
| SvFL 2–5 | Western North Pacific | Jeju Island, South Korea | 33.277778 N | 126.719067 E | [39] |
| SvFL 6 | Western North Pacific | Jeju Island, South Korea | 33.276667 N | 126.170556 E | [18] |
| MJa-B6-Sy | Western North Pacific | Muroto Cape, Kochi, Japan | 33.25 N | 134.166667 W | [24] |
| TSP-C2-Sy | Western North Pacific | Tsushima Island, Nagasaki, Japan | 34.183333 N | 129.283333 E | [24] |
| - | Western North Pacific | Jiaozhou Bay, China | 36.02575 N | 120.290231 E | [38] |
| - | Western North Pacific | Zhujiang River estuary, China | 22.483333 N | 113.75 E | [40] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, N.S.; Kim, E.S.; Lee, J.A.; Kim, K.M.; Kwak, M.S.; Yoon, M.; Hong, J.W. First Report of the Dinoflagellate Genus Effrenium in the East Sea of Korea: Morphological, Genetic, and Fatty Acid Characteristics. Sustainability 2020, 12, 3928. https://doi.org/10.3390/su12093928
Kang NS, Kim ES, Lee JA, Kim KM, Kwak MS, Yoon M, Hong JW. First Report of the Dinoflagellate Genus Effrenium in the East Sea of Korea: Morphological, Genetic, and Fatty Acid Characteristics. Sustainability. 2020; 12(9):3928. https://doi.org/10.3390/su12093928
Chicago/Turabian StyleKang, Nam Seon, Eun Song Kim, Jung A Lee, Kyeong Mi Kim, Min Seok Kwak, Moongeun Yoon, and Ji Won Hong. 2020. "First Report of the Dinoflagellate Genus Effrenium in the East Sea of Korea: Morphological, Genetic, and Fatty Acid Characteristics" Sustainability 12, no. 9: 3928. https://doi.org/10.3390/su12093928
APA StyleKang, N. S., Kim, E. S., Lee, J. A., Kim, K. M., Kwak, M. S., Yoon, M., & Hong, J. W. (2020). First Report of the Dinoflagellate Genus Effrenium in the East Sea of Korea: Morphological, Genetic, and Fatty Acid Characteristics. Sustainability, 12(9), 3928. https://doi.org/10.3390/su12093928






