The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Organic Fertilizer from Onion Peel Flour (OPF)
2.2. Soil Preconditioning
2.3. Experimental Design
2.4. Soil Analyses
2.4.1. Sample Collection
2.4.2. Moisture
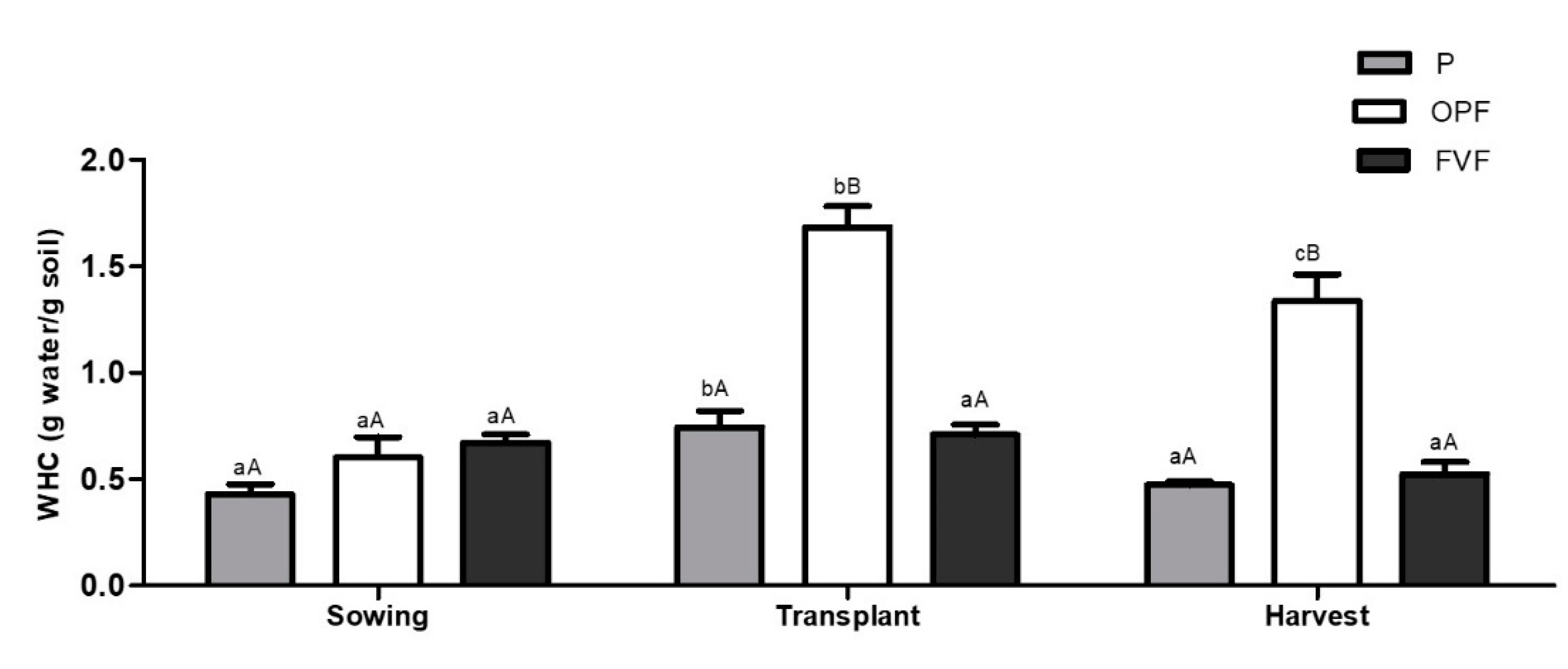
2.4.3. Water Retention Capacity
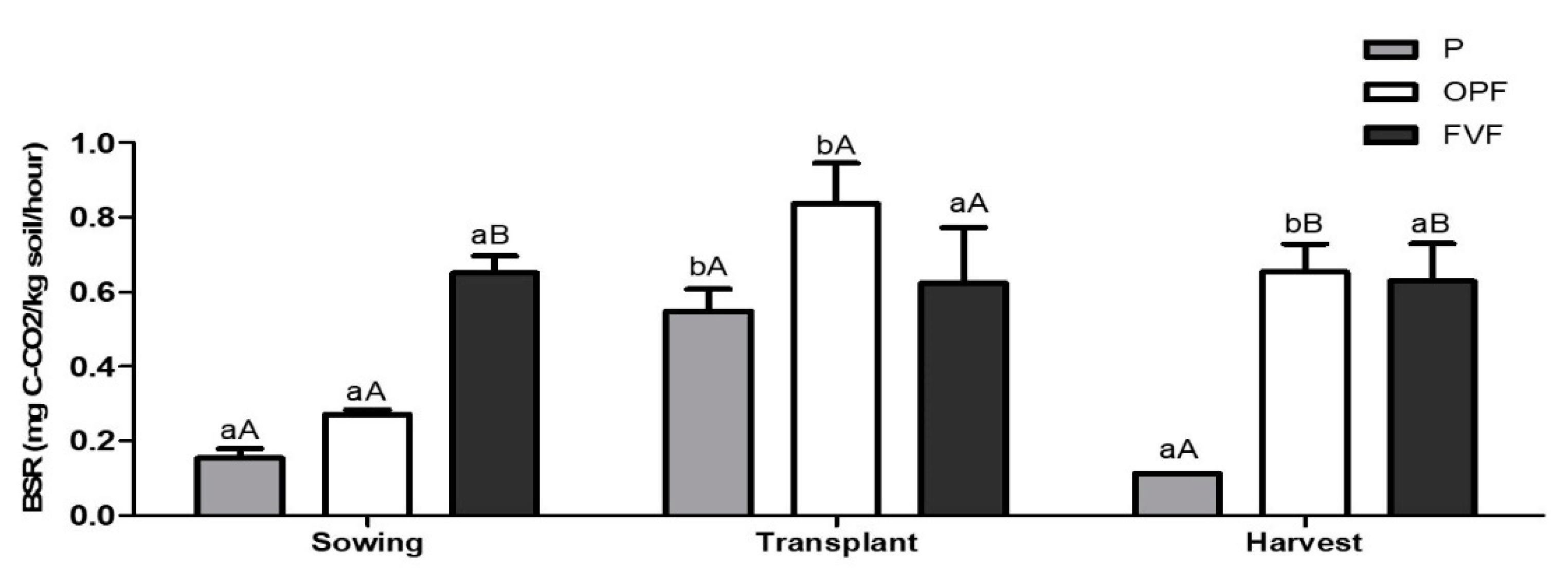
2.4.4. Soil Basal Respiration
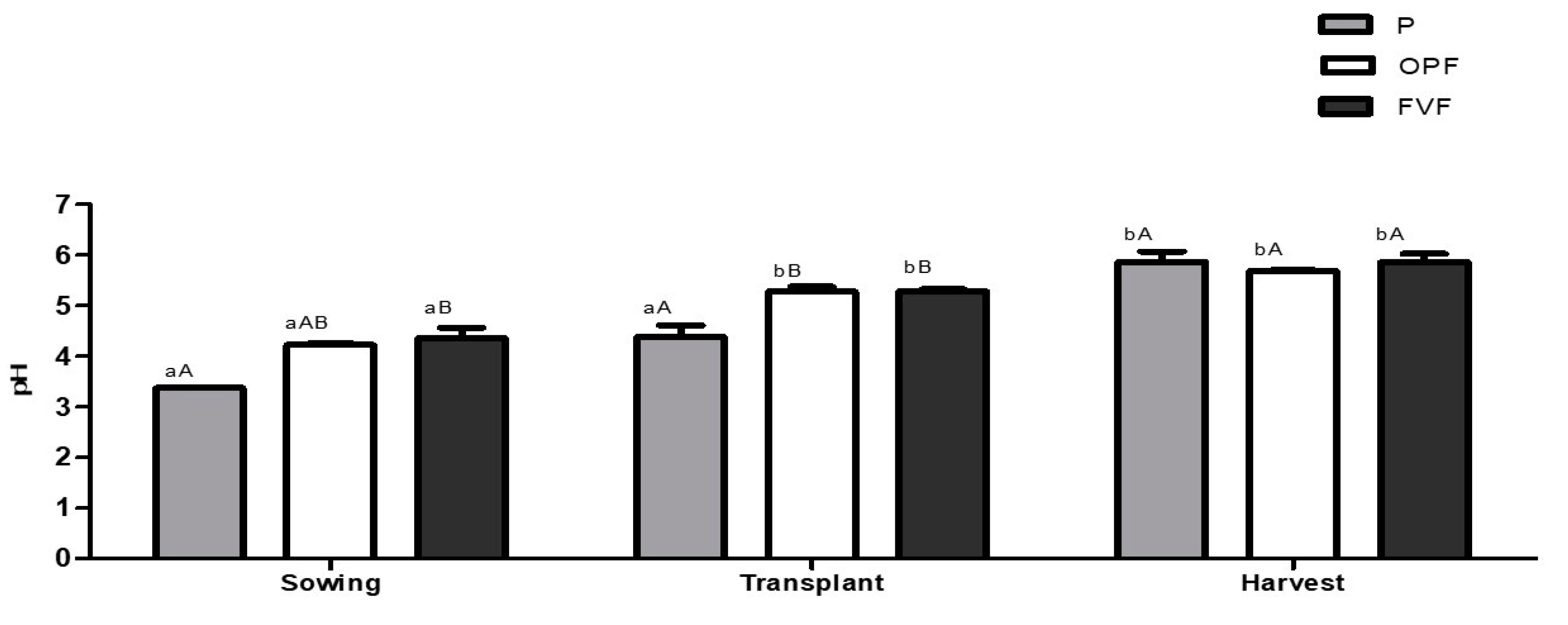
2.4.5. pH
2.4.6. Analysis of Lettuce Leaves
2.4.7. Sample Preparation
2.4.8. Antioxidant Activity
2.4.9. Statistical Treatment
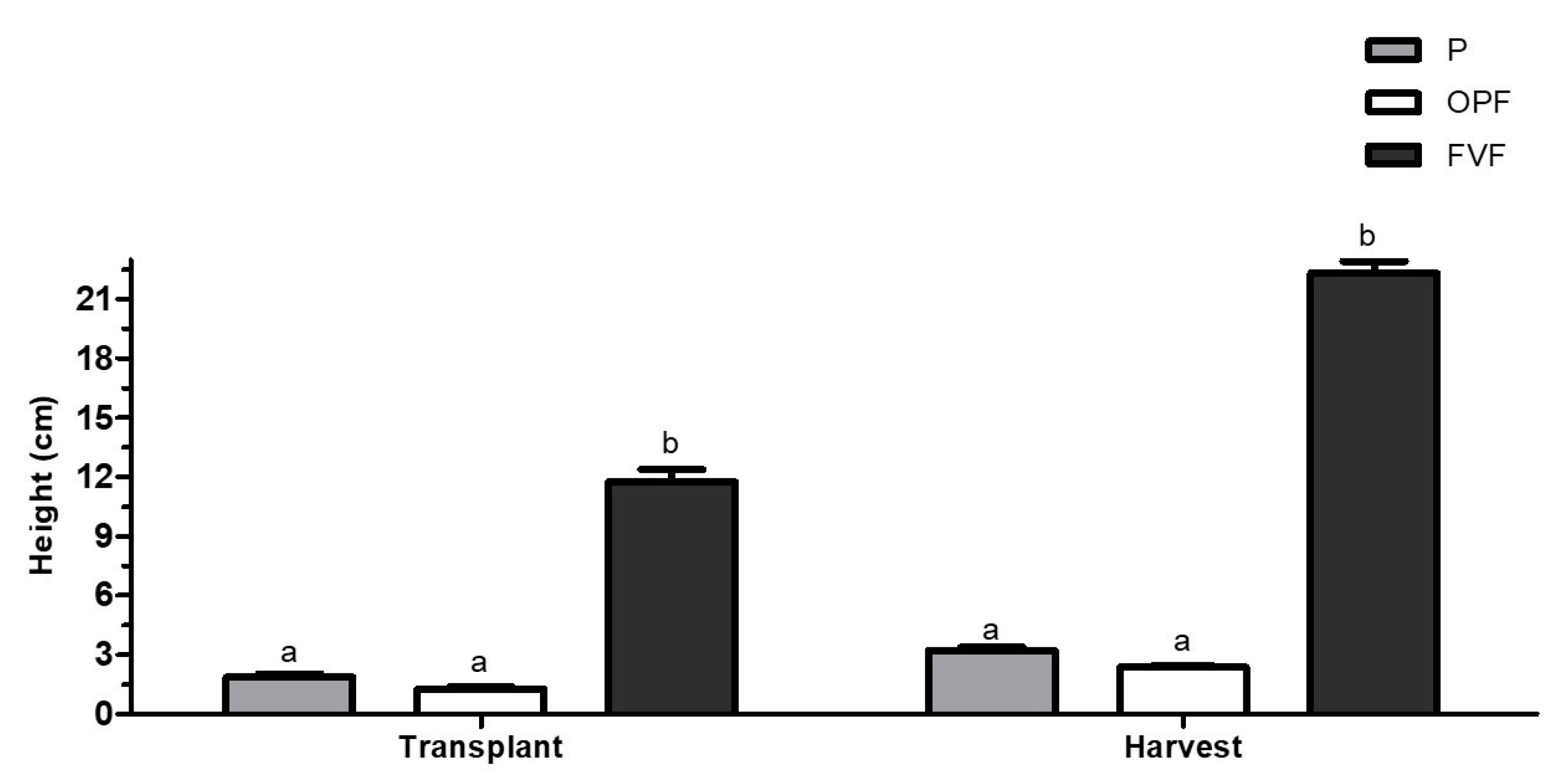
3. Results
3.1. Soil Quality
3.2. Lettuce Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Filho, R.C.d.S.; de Holanda, E.P.T.; de Oliveira, L.C.F.; da Silva, V.M.F. O aproveitamento de resíduos sólidos urbanos, por meio do processo de compostagem aeróbia enriquecida com casca de sururu para aproveitamento na construção civil. Ciênc. Exatas E Tecnol. 2017, 4, 125–134. [Google Scholar]
- Neethu, C.S.; Mujeeb Rahiman, K.M.; Rosmine, E.; Saramma, A.V.; Mohamed Hatha, A.A. Utilization of agro-industrial wastes for the production of lipase from Stenotrophomonas maltophilia isolated from Arctic and optimization of physical parameters. Biocatal. Agric. Biotechnol. 2015, 4, 703–709. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Onion skin extract as a protective agent against oxidative stress in Saccharomyces cerevisiae induced by cadmium. J. Food Biochem. 2019, 43. [Google Scholar] [CrossRef] [PubMed]
- Sayed, H.S.; Hassan, N.M.M.; Khalek, A. El The effect of using onion skin powder as a source of dietary fiber and antioxidants on properties of dried and fried noodles. Curr. Sci. Int. 2014, 3, 468–475. [Google Scholar]
- Reddy, J.P.; Rhim, J.W. Extraction and Characterization of Cellulose Microfibers from Agricultural Wastes of Onion and Garlic. J. Nat. Fibers 2018, 15, 465–473. [Google Scholar] [CrossRef]
- Lee, K.A.; Kim, K.T.; Nah, S.Y.; Chung, M.S.; Cho, S.W.; Paik, H.D. Antimicrobial and antioxidative effects of onion peel extracted by the subcritical water. Food Sci. Biotechnol. 2011, 20, 543–548. [Google Scholar] [CrossRef]
- Martins, R.C.; Chiapetta, S.C.; De Paula, F.D.; Gonçalves, É.C.B.A. Avaliação da vida de prateleira de bebida isotônica elaborada com suco concentrado de frutas e hortaliças congeladas por 30 dias. Aliment. E Nutr. 2011, 22, 623–629. [Google Scholar]
- Roberta, M.S.A.; Mariana, S.L.F.; Édira, C.B.A.G. Functional capacity of flour obtained from residues of fruit and vegetables. Int. Food Res. J. 2014, 21, 1675–1681. [Google Scholar]
- Brito, T.B.; Carrajola, J.F.; Gonçalves, E.C.B.A.; Martelli-Tosi, M.; Ferreira, M.S.L. Fruit and vegetable residues flours with different granulometry range as raw material for pectin-enriched biodegradable film preparation. Food Res. Int. 2019, 121, 412–421. [Google Scholar] [CrossRef]
- Santos, M.C.P.; Gonçalves, É.C.B.A. Effect of different extracting solvents on antioxidant activity and phenolic compounds of a fruit and vegetable residue flour. Sci. Agropecu. 2016, 7, 7–14. [Google Scholar] [CrossRef]
- Gonçalves, E.C.B.A.; Lozano-Sanchez, J.; Gomes, S.; Ferreira, M.S.L.; Cameron, L.C.; Segura-Carretero, A. Byproduct Generated During the Elaboration Process of Isotonic Beverage as a Natural Source of Bioactive Compounds. J. Food Sci. 2018, 83, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H. The Combined Use of Chemical and Organic Fertilizers And/Or Biofertilizer for Crop Growth And Soil Fertility? In International Workshop on Sustained Management; Department of Soil and Environmental Sciences, National Chung Hsing University: Taichung, Taiwan, 2006; pp. 1–11. [Google Scholar]
- Pellejero, G.; Miglierina, A.; Aschkar, G.; Turcato, M.; Jiménez-Ballesta, R. Effects of the onion residue compost as an organic fertilizer in a vegetable culture in the Lower Valley of the Rio Negro. Int. J. Recycl. Org. Waste Agric. 2017, 6, 159–166. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Yang, X.; Cui, X.; Zhao, L.; Guo, D.; Feng, L.; Wei, S.; Zhao, C.; Huang, D. Exogenous glycine nitrogen enhances accumulation of glycosylated flavonoids and antioxidant activity in lettuce (Lactuca sativa L.). Front. Plant Sci. 2017, 8, 1–16. [Google Scholar] [CrossRef]
- Schneider, M.; Goss, K.U. Temperature dependence of the water retention curve for dry soils. Water Resour. Res. 2011, 47, 1–5. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Kapoulas, N.; Koukounaras, A.; Ilić, Z.S. Nutritional quality of lettuce and onion as companion plants from organic and conventional production in north Greece. Sci. Hortic. 2017, 219, 310–318. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E. Life cycle assessment of organic versus conventional agriculture. A case study of lettuce cultivation in Greece. J. Clean. Prod. 2016, 112, 2462–2471. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Santos, M.C.P.; Moro, T.M.A.; Basto, G.J.; Andrade, R.M.S.; Gonçalves, É.C.B.A. Formulation and characterization of functional foods based on fruit and vegetable residue flour. J. Food Sci. Technol. 2015, 52, 822–830. [Google Scholar] [CrossRef]
- Maria, R.; Lana, Q. Composto Orgânico De Lixo Urbano E Vermiculita Como Substrato Para a Produção De Mudas De Alface, Tomate E Couve-Flor Organic Compost of Urban Waste and Vermiculite as Substrate for Lettuce, Tomato and Cauliflower Seedling Production. Biosci. J. 2004, 20, 67–74. [Google Scholar]
- de Oliveira, E.Q.; de Souza, R.J.; da Cruz, M.d.C.M.; Marques, V.B.; França, A.C. Produtividade de alface e rúcula, em sistema consorciado, sob adubação orgânica e mineral. Hortic. Bras. 2010, 28, 36–40. [Google Scholar] [CrossRef][Green Version]
- Loss, A.; Pereira, M.G.; Beutler, S.J.; Perin, A.; dos Anjos, L.H.C. Carbono mineralizável, carbono orgânico e nitrogênio em macroagregados de Latossolo sob diferentes sistemas de uso do solo no Cerrado Goiano. Semin. Ciênc. Agrár. 2013, 34, 2153–2168. [Google Scholar] [CrossRef]
- Andréa, M.M.; Hollweg, M.J.M. Comparação de métodos para determinação de biomassa microbiana em dois solos. Rev. Bras. Ciênc. Solo 2004, 28, 981–986. [Google Scholar] [CrossRef]
- Gombert, A.K.; Pinto, A.L.; Castilho, L.R.; Freire, D.M.G. Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substrate. Process Biochem. 1999, 35, 85–90. [Google Scholar] [CrossRef]
- Da Silva Almeida, A.E.; Neto, F.B.; Costa, L.R.; Da Silva, M.L.; De Lima, J.S.S.; Barros Júnior, A.P. Eficiência agronômica do consórcio alface-rúcula fertilizado com flor-de-seda. Rev. Caatinga 2015, 28, 79–85. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Gao, H.; Shao, M. Effects of temperature changes on soil hydraulic properties. Soil Tillage Res. 2015, 153, 145–154. [Google Scholar] [CrossRef]
- Tran, A.T.P.; Chang, I.; Cho, G.C. Soil water retention and vegetation survivability improvement using microbial biopolymers in drylands. Geomech. Eng. 2019, 17, 475–483. [Google Scholar] [CrossRef]
- Greenwood, D.J.; McKee, J.M.T.; Fuller, D.P.; Burns, I.G.; Mulholland, B.J. A novel method of supplying nutrients permits predictable shoot growth and root: Shoot ratios of pre-transplant bedding plants. Ann. Bot. 2007, 99, 171–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daud, Z.; Hatta, M.Z.M.; Kassim, A.S.M.; Awang, H.; Aripin, A.M. Exploring of agro waste (pineapple leaf, corn stalk, and napier grass) by chemical composition and morphological study. BioResources 2014, 9, 872–880. [Google Scholar] [CrossRef]
- Cardoso, E.J.B.N.; Andreote, F.D. Microbiologia do Solo, 2nd ed.; ESALQ: Piracicaba, Brazil, 2016. [Google Scholar]
- Yan, K.; Jarvis, C.; Gu, J.; Yan, Y. Production and catalytic transformation of levulinic acid: A platform for speciality chemicals and fuels. Renew. Sustain. Energy Rev. 2015, 51, 986–997. [Google Scholar] [CrossRef]
- Rahmati, M.; Pohlmeier, A.; Abasiyan, S.M.A.; Weihermüller, L.; Vereecken, H. Water retention and pore size distribution of a biopolymeric-amended loam soil. Vadose Zone J. 2019, 18. [Google Scholar] [CrossRef]
- Nascimento, P.; Marim, R.; Carvalho, G.; Mali, S. Nanocellulose produced from rice hulls and its effect on the properties of biodegradable starch films. Mater. Res. 2016, 19, 167–174. [Google Scholar] [CrossRef]
- Powell, S.M.; Ferguson, S.H.; Snape, I.; Siciliano, S.D. Fertilization stimulates anaerobic fuel degradation of antarctic soils by denitrifying microorganisms. Environ. Sci. Technol. 2006, 40, 2011–2017. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Sexstone, A.J.; Parkin, T.B.; Revsbech, N.P. Anaerobic processes in soil. Plant Soil 1984, 76, 197–212. [Google Scholar] [CrossRef]
- Rawls, W.J.; Pachepsky, Y.A.; Ritchie, J.C.; Sobecki, T.M.; Bloodworth, H. Effect of soil organic carbon on soil water retention. Geoderma 2003, 116, 61–76. [Google Scholar] [CrossRef]
- Templeton, D.W.; Sluiter, A.D.; Hayward, T.K.; Hames, B.R.; Thomas, S.R. Assessing corn stover composition and sources of variability via NIRS. Cellulose 2009, 16, 621–639. [Google Scholar] [CrossRef]
- Xue, R.; Shen, Y.; Marschner, P. Low soil water content during plant growth influences soil respiration and microbial biomass after plant removal and rewetting. J. Soil Sci. Plant Nutr. 2016, 16. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.L.; Anh Mai, T.L.; Brandão, M.; Anaya de la Rosa, R.; Kristiansen, P.; Joseph, S. Climate-change and health effects of using rice husk for biochar-compost: Comparing three pyrolysis systems. J. Clean. Prod. 2017, 162, 260–272. [Google Scholar] [CrossRef]
- Christodoulou, E.; Agapiou, A.; Anastopoulos, I.; Omirou, M.; Ioannides, I.M. The effects of different soil nutrient management schemes in nitrogen cycling. J. Environ. Manag. 2019, 243, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, N.; Xu, M.; Wang, S.; Zhang, J.; Cai, Z.; Cheng, Y. The influence of long-term animal manure and crop residue application on abiotic and biotic N immobilization in an acidified agricultural soil. Geoderma 2019, 337, 710–717. [Google Scholar] [CrossRef]
- Melo, E.d.A.; Maciel, M.I.S.; Lima, V.L.A.G.; Leal, F.L.L.; Caetano, A.C.d.S.; Nascimento, R.J. Capacidade antioxidante de hortaliças usualmente consumidas. Ciência E Tecnol. Aliment. 2006, 26, 639–644. [Google Scholar] [CrossRef]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of nutrient deficiency and abiotic environmental stresses on yield, phenolic compounds and antiradical activity in lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- López, A.; Javier, G.A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical composition and antioxidant capacity of lettuce: Comparative study of regular-sized (Romaine) and baby-sized (Little Gem and Mini Romaine) types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Zhao, X.; Iwamoto, T.; Carey, E.E. Antioxidant capacity of leafy vegetables as affected by high tunnel environment, fertilisation and growth stage. J. Sci. Food Agric. 2007, 87, 2692–2699. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e162. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalheiro, T.R.T.; Alcoforado, R.d.O.; Silva, V.S.d.A.; Coimbra, P.P.S.; Mendes, N.d.S.; Cavalcanti, E.D.C.; Jurelevicius, D.d.A.; Gonçalves, É.C.B.d.A. The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity. Sustainability 2021, 13, 128. https://doi.org/10.3390/su13010128
Cavalheiro TRT, Alcoforado RdO, Silva VSdA, Coimbra PPS, Mendes NdS, Cavalcanti EDC, Jurelevicius DdA, Gonçalves ÉCBdA. The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity. Sustainability. 2021; 13(1):128. https://doi.org/10.3390/su13010128
Chicago/Turabian StyleCavalheiro, Tamara Righetti Tupini, Raquel de Oliveira Alcoforado, Vinicius Soares de Abreu Silva, Pedro Paulo Saldanha Coimbra, Nathânia de Sá Mendes, Elisa D´avila Costa Cavalcanti, Diogo de Azevedo Jurelevicius, and Édira Castello Branco de Andrade Gonçalves. 2021. "The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity" Sustainability 13, no. 1: 128. https://doi.org/10.3390/su13010128
APA StyleCavalheiro, T. R. T., Alcoforado, R. d. O., Silva, V. S. d. A., Coimbra, P. P. S., Mendes, N. d. S., Cavalcanti, E. D. C., Jurelevicius, D. d. A., & Gonçalves, É. C. B. d. A. (2021). The Impact of Organic Fertilizer Produced with Vegetable Residues in Lettuce (Lactuca sativa L.) Cultivation and Antioxidant Activity. Sustainability, 13(1), 128. https://doi.org/10.3390/su13010128




