Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample and Sesame Seeds
2.2. Soil Analysis
2.3. Isolation of Bacillus sp.
2.4. Screening of PGP Traits
2.4.1. Production of Hydrogen Cyanide (HCN)
2.4.2. Production of Ammonia
2.4.3. Production of Indole Acetic Acid (IAA)
2.4.4. Estimation of Phosphate Solubilization Activity
2.5. Screening and Production of Siderophore
2.6. Determiantion of Type of Siderophore
2.7. Polyphasic Identification of Potential Isolates
2.7.1. Biochemical Characterization
2.7.2. 16S rRNA Gene Sequence Analysis
2.8. Purification of Siderophore
2.8.1. Solvent Purification
2.8.2. Purification on Sep Pack C C18 Column
2.8.3. Purification on Amberlite-400 Resin Column
2.9. Characterization of Purified Siderophore
2.9.1. Thin Layer Chromatographic (TLC) Analysis
2.9.2. Fourier Transform Infra-Red (FTIR) Spectroscopic Analysis
2.9.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.9.4. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
2.10. Plant Growth-Promotion Studies under Greenhouse Conditions
- T0—Control uninocluated nutrient broth
- T1—with 200 mL of B. subtilis LSBS2 broth
- T2—with 200 mL of pure siderophore suspension (296 mg/L)
2.10.1. Estimation of Photosynthetic Pigments
2.10.2. Estimation of Total Iron Content in Sesame Seedling
2.10.3. Determination of Oil Content in Seeds
2.10.4. Estimation of Soil Nutrients
2.11. Statistical Analysis
3. Results
3.1. Soil Analysis
3.2. Isolation of Bacillus sp.
3.3. Characterization of Plant Growth Promoting (PGP) Activities
3.3.1. Production of Hydrogen Cyanide (HCN)
3.3.2. Production of Ammonia
3.3.3. Production of Indole Acetic Acid (IAA)
3.3.4. Phosphate Solubilization Analysis
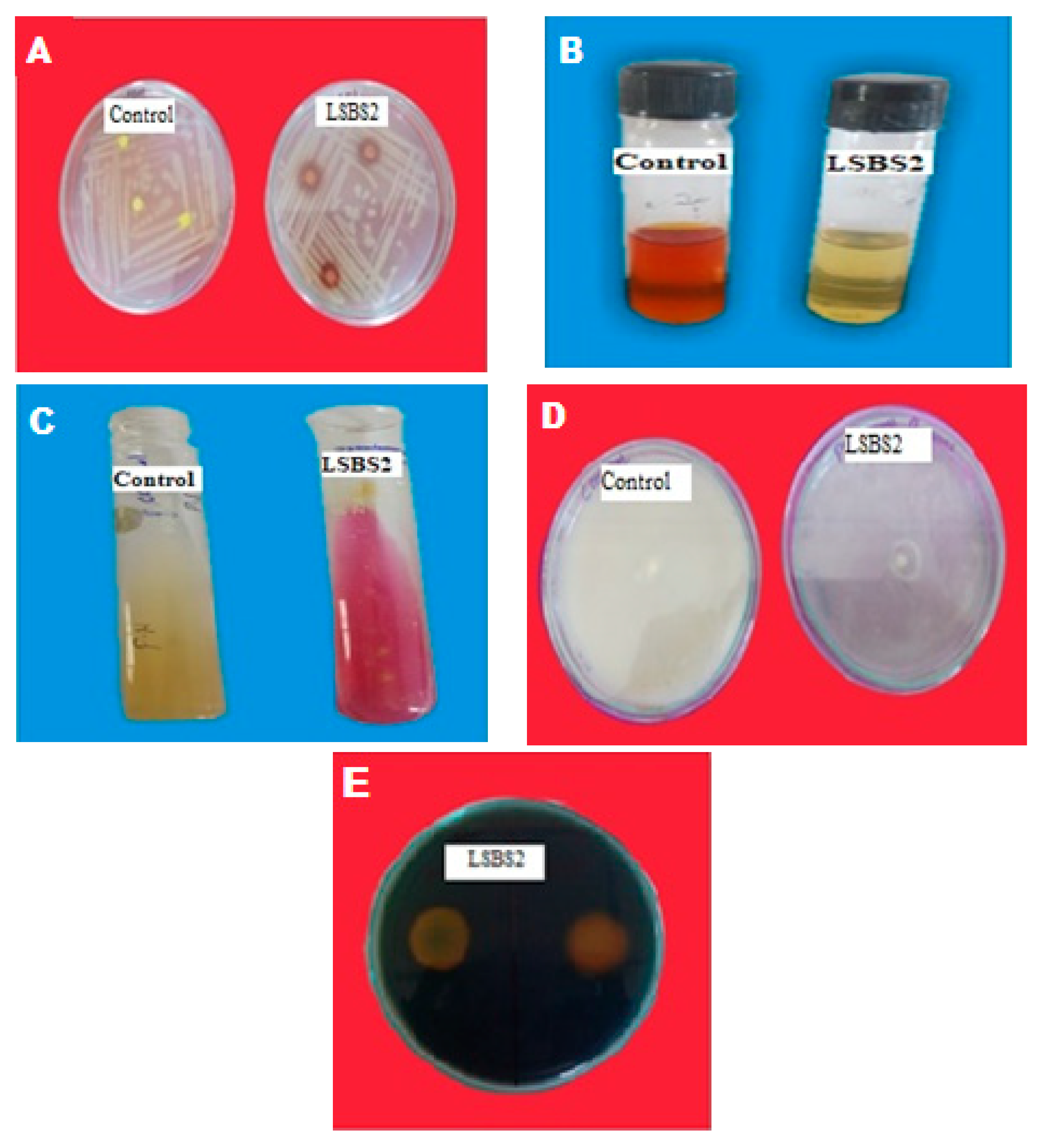
3.3.5. Screening and Production of Siderophore
3.4. Qualitative Detection of Siderophore
3.5. Polyphasic Identification of LSBS2
3.6. Purification of Siderophore
3.6.1. Solvent Purification
3.6.2. Purification on Sep-Pack C18 Column
3.6.3. Purification on Amberlite-400 Resin Column
3.7. Characterization of Purified Siderophore
3.7.1. Thin-Layer Chromatographic (TLC) Analysis
3.7.2. Infra-Red (IR) Spectroscopic Analysis
3.7.3. High-Performance Liquid Chromatography (HPLC) Analysis
3.7.4. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
3.8. Plant Growth-Promotion Studies under Greenhouse Conditions
3.8.1. Measurement of Plant Growth Parameters
3.8.2. Estimation of Total Iron, Seed Oil, and Photosynthetic Pigments
3.8.3. Measurement of Soil Nutrients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaron, G. Breeding for the improvement of the ideal plant type of sesame. Plant Breed. 2019, 124, 263–267. [Google Scholar]
- Singh, R.; Pandey, D.K.; Kumar, A. PGPR isolates from the rhizosphere of vegetable crop Momordica charantia: Characterization and application as biofertilizer. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1789–1802. [Google Scholar]
- Babu, A.N.; Jogaiah, S.; Ito, S.; Amruthesh, K.A.; Tran, L.-S.P. Improvement of growth, fruit weight, and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant Sci. 2015, 231, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, R.Z.; Ilyas, N.; Tabassum, B.; Hashem, A.; Abd Allah, E.F.; Jadhav, H.P. Role of Plant Growth-Promoting Rhizobacteria inFuture Climatic Scenario. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 175–189. [Google Scholar]
- Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Reddy, M.S.; Sayyed, R.Z. Plant growth-promoting rhizobacteria: An eco-friendly approach for sustainable agroecosystem. In Plant Soil-Microbes; Springer: Basel, Switzerland, 2016; pp. 182–201. [Google Scholar]
- Khan, A.; Sayyed, R.Z.; Seifi, S. Rhizobacteria: Legendary Soil Guards in Abiotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Vol 1 Abiotic Stress Management; Sayyed, R., Arora, N., Reddy, M., Eds.; Springer: Singapore, 2019; pp. 27–342. [Google Scholar]
- Reshma, P.; Naik, M.K.; Aiyaz, M.; Niranjana, S.R.; Chennappa, G.; Shaikh, S.S.; Sayyed, R.Z. Induced systemic resistance by 2,4diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. Indian J. Exp. Biol. 2018, 56, 207–212. [Google Scholar]
- Serrano, L.O. Biotechnology of siderophores in high-impact scientific fields. Biomol. Concepts 2017, 8, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.; Holmström, S.J. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gadhafi, K.R.; Hourston, J.E.; Gange, A.C. Developing soil microbial inoculants for pest management: Can one have too much that thing. J. Chem. Ecol. 2016, 42, 4. [Google Scholar] [CrossRef] [PubMed]
- Zope, V.; El Enshasy, H.; Sayyed, R.Z. Plant growth-promoting rhizobacteria: An overview in agricultural perspectives. In Plant Growth-Promoting Rhizobacteria for Sustainable Stress Management Vol II Rhizobacteria in Biotic Stress Management; Sayyed, R.Z., Ed.; Springer: Singapore, 2019; pp. 345–362. [Google Scholar]
- Fazeli-Nasab, B.; Sayyed, R.Z. Plant growth-promoting rhizobacteria and salinity stress: A Journey into the soil. In Plant Growth-promoting Rhizobacteria for Sustainable Stress Management Vol 1 Abiotic Stress Management; Sayyed, R., Arora, N., Reddy, M., Eds.; Springer: Singapore, 2019; pp. 21–34. [Google Scholar]
- Alef, K. Enrichment, isolation and counting of soil microorganism. In Methods in Applied Soil Microbilogy and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 123–192. [Google Scholar]
- Subbiah, B.V.; Asija, G.L. A rapid procedure for the determination of available nitrogen in the soil. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Hussain, F.; Malik, K.A. Evaluation of alkaline permanganate method and its modification as an index of soil nitrogen availability. Plant Soil. 1985, 84, 279–282. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture Circular 19: Washington, DC, USA, 1954; p. 939. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall of India Pvt, Ltd.: New Delhi, India, 1973; p. 498. [Google Scholar]
- Emsens, W.J.; Aggenbach, C.J.S.; Schoutens, K.; Smolders, A.J.P.; Zak, D.; van Diggelen, R. Soil Iron Content as a Predictor of Carbon and Nutrient Mobilization in Rewetted Fens. PLoS ONE 2016, 11, e0153166. [Google Scholar] [CrossRef] [PubMed]
- Ben David, A. Estimation method of serial dilution experiments. J. Microbiol. Methods 2014, 107, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Castric, P. Hydrogen cyanide, a secondary metabolite of Pseudomonas aeruginosa. Can. J. Microbiol. 1975, 21, 613. [Google Scholar] [CrossRef] [PubMed]
- Cappuccino, J.G.; Sherman, N. Microbiology: A Laboratory Manual, 8th ed.; Pearson: Madison, WI, USA, 2002; Volume 13, p. 978. [Google Scholar]
- Pandya, N.D.; Desai, P.V.; Jadhav, H.P.; Sayyed, R.Z. Plant growth-promoting potential of Aspergillus sp. NPF7, isolated from wheat rhizosphere in South Gujarat. Environ. Sustain. 2018, 1, 245–252. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Enhancing grain iron content of rice by the application of plant growth-promoting rhizobacteria. Plant Soil Environ. 2013, 59, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Schwyn, B.; Neiland, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Meyer, J.M.; Abdallah, M.A. The fluorescent pigments of Pseudomonas fluorescens: Biosynthesis, purification, and physicochemical properties. J. Gen. Microbiol. 1978, 107, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.M. Detection, isolation, and characterization of siderophore. Methods Enzymol. 1994, 235, 329–344. [Google Scholar]
- Arnow, L.E. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine tyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar] [CrossRef]
- Snow, G.A. Mycobactin: A growth factor for Mycobacterium johnei. II. Degradation and identification of fragments. J. Chem. Soc. 1954, 10, 2588–2596. [Google Scholar] [CrossRef]
- Holt, J.G. The Shorter Bergey’s Manual of Determinative Bacteriology; Wiliams and Wilkins: Baltimore, MD, USA, 1984. [Google Scholar]
- Gangurde, N.S.; Sayyed, R.Z.; Kiran, S.; Gulati, A. Development of eco-friendly bioplastic like PHB by distillery effluent microorganisms. Environ. Sci. Pollut. Res. 2013, 20, 488–497. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Pediyar, V.; Adam, K.A.; Badri, N.N.; Patole, M.; Shouche, Y.S. Aeromonas culicicola sp. nov., from the midgut of Culex quinquefasciatus. Int. J. Syst. Evol. Microbiol. 2002, 52, 1723–1728. [Google Scholar]
- Available online: http://www.ncbi.nlm.nih.gov (accessed on 2 March 2020).
- Sayyed, R.Z.; Chincholkar, S.B. Purification of siderophores of Alcaligenes faecalis on XAD. Bioresour. Technol. 2006, 97, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Mayer, J.M. Search for siderophores in microorganisms. In Microbes for Better Living, MICON 94 and 35th AMI Conference; Manja, K.R., Ed.; CFTRI: Mysore, India, 1994; pp. 9–12. [Google Scholar]
- Budzikiewicz, H. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol. Rev. 1993, 10, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Iron and its role in microbial physiology. In Microbial Iron Metabolism a Comprehensive Creative; Neilands, J.B., Ed.; Academic Press: New York, NY, USA, 1974; pp. 449–467. [Google Scholar]
- Tank, N.; Rajendran, N.; Patel, B.; Saraf, M. Evaluation and biochemical characterization of a distinctive pyoverdin from a pseudomonas isolated from chickpea rhizosphere. Braz. J. Microbiol. 2012, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Manninen, O.T.; Mattila-Sandholm, T. Methods for detection of Pseudomonas siderophores. J. Microbiol. Methods 1993, 19, 223–234. [Google Scholar] [CrossRef]
- Zajdowicz, S.; Haller, J.C.; Krafft, A.E.; Hunsucker, S.W.; Mant, C.T.; Duncan, M.W. Purification and Structural Characterization of Siderophore (Corynebactin) from Corynebacterium diphtheriae. PLoS ONE 2012, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markley, J.L.; Bax, A.; Arata, Y.; Hilbers, C.W.; Kaptein, R.; Sykes, B.D.; Wright, P.E.; Wüthrich, K. Recommendations for the presentation of NMR structures of proteins and nucleic acids. IUPAC-IUBMB-IUPAB Inter-Union Task Group on the Standardization of Data Bases of Protein and Nucleic Acid Structures Determined by NMR Spectroscopy. J. Biomol. NMR 1998, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 30. [Google Scholar] [CrossRef]
- Bansal, U.K.; Satija, D.R.; Ahuja, K.L. The oil composition of diverse groundnut (Arachis hypogaea L.) genotypes relation to different environments. J. Sci. Food Agric. 1993, 63, 17–19. [Google Scholar] [CrossRef]
- Arslan, C.; Uzun, B.; Ulger, S.; Illhan, C. Determination of Oil Content and Fatty acid composition of Sesame mutants suited for intensive management conditions. J. Am. Oil Chem. Soc. 2007, 84, 917–920. [Google Scholar] [CrossRef]
- Labconco, C. A Guide to Kjeldahl Nitrogen Determination Methods and Apparatus; Labconco Corporation: Houston, TX, USA, 1998. [Google Scholar]
- Sims, J. Soil test phosphorus: Principles and methods. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters. South. Coop. Ser. Bull. 2009, 408, 9–19. [Google Scholar]
- Upadhyay, A.; Sahu, R. Determination of potassium in soil and plant. In Laboratory Manual on Advances in Agro-Technologies for Improving Soil, Plant and Atmosphere Systems; CAFT: Jabalpur, India, 2012; pp. 23–35. [Google Scholar]
- Sahrawat, K. Determination of calcium, magnesium, zinc, and manganese in plant tissue using a dilute HCl extraction method. Commun. Soil Sci. Plant Anal. 1987, 18, 947–962. [Google Scholar] [CrossRef]
- Jogaiah, S.; Roopa, K.S.; Pushpalatha, H.G.; Shekar Shetty, H. Evaluation of plant growth-promoting rhizobacteria for their efficiency to promote growth and induce systemic resistance in pearl millet against downy mildew disease. Arch. Phytopathol. Plant Prot. 2010, 43, 368–378. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Jogaiah, S. Defense mechanism and diverse actions of fungal biological control agents against plant biotic stresses. In Plant Biotechnology: Progress in Genomic Era; Khurana, S.M., Paul, G., Rajarshi, K., Eds.; Springer International PubNew: Delhi, India, 2019; pp. 461–481. [Google Scholar]
- Sasirekha, S.; Srividya, S. Siderophore production by Pseudomonas aeruginosa FP6, a biocontrol strain for Rhizoctonia solani and Colletotrichum gloeosporioides causing diseases in chili. Agric. Nat. Res. 2016, 50, 255–256. [Google Scholar]
- Sayyed, R.Z.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; Enshasy, H.E. Siderophore Production in Groundnut Rhizosphere isolate, Achromobacter sp. RZS2 Influenced By Physicochemical Factors and Metal Ions. Environ. Sustain. 2019, 1, 295–301. [Google Scholar] [CrossRef]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; Enshasy, H.E.; Malek, R.A.; Syed, A.; et al. Co-inoculation of rhizobacteria and biochar application improves growth and nutrient in soybean and enriches soil nutrients and enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Suriani, N.; Suprapta, D.; Novizar, N.; Parwanayoni, N.; Darmadi, A.; Dewi, D.; Sudatri, N.; Ahmad, F.; Sayyed, R.Z.; Syed, A.; et al. A Mixture of Piper Leaves Extracts and Rhizobacteria for Sustainable Plant Growth Promotion & Biocontrol of Blast Pathogen of Organic Bali Rice. Sustainability 2020, 12, 8490. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Sudisha, J.; Niranjana, S.R.; Umesha, S.; Prakash, H.S.; Shekar Shetty, H. Transmission of seed-borne infection of muskmelon by Didymella bryoniae and effect of seed treatments on disease incidence and fruit yield. Biol. Cont. 2006, 37, 196–205. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Badgujar, M.D.; Sonawane, H.M.; Mhaske, M.M.; Chincholkar, S.B. Production of microbial iron chelators (siderophores) by fluorescent pseudomonas. Indian J. Biotechnol. 2005, 4, 484–490. [Google Scholar]
- Sayyed, R.Z.; Gangurde, N.S.; Chincholkar, S.B. Siderophore production by A. faecalis and its application for growth promotion in A. hypogaea. Indian J. Biotechnol. 2010, 9, 302–307. [Google Scholar]
- Pahari, A.; Dangar, T.K.; Mishra, B.B. Siderophore quantification of bacteria from Sundarbans and its effect on the growth of brinjal (Solanum melongena L.). Bioscan 2016, 11, 2147–2151. [Google Scholar]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Modified chrome azurol S method for detection and estimation of siderophores having an affinity for metal ions other than iron. Environ. Sustain. 2018, 1, 81–87. [Google Scholar] [CrossRef]
- Mehri, I.; Khessairi, A.; Yousra, T.; Neila, S.; Imen, D.; Marie, M.J.; Abdennasseur, H. Effect of dose response of zinc and manganese on siderophores induction. Am. J. Environ. Sci. 2012, 8, 143–151. [Google Scholar]
- De Los Santos-Villalobos, S. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol. 2012, 28, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Suganya, A.; Umaa Rani, K.; Ramesh, B. Screening and Partial Purification of Hydroxamate Type Siderophore from Pseudomonas sp. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2380–2385. [Google Scholar] [CrossRef] [Green Version]
- Siebner-Freibach, H.; Yariv, S.; Lapides, Y.; Hadar, Y.; Chen, Y. Thermo-FTIR spectroscopic study of the siderophore ferrioxamine B: Spectral analysis and stereochemical implications of iron chelation, pH, and temperature. J. Agri. Food Chem. 2005, 53, 3434–3443. [Google Scholar] [CrossRef]
- Bruns, H.; Crüsemann, M.; Letzel, A.C.; Alanjary, M.; McInerney, J.O.; Jensen, P.R.; Schulz, S.; Moore, B.S.; Ziemer, N. Function-related replacement of bacterial siderophore pathways. ISME J. 2018, 12, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Joseph, B.; Patra, R.R.; Lawrence, R. Characterization of plant growth-promoting rhizobacteria associated with chickpea (Cicerarietinum L.). Int. J. Plant Prod. 2007, 2, 141–152. [Google Scholar]
- Vleesschauwer, D.; Höfte, M. Rhizobacteria-induced systemic resistance. Adv. Bot. Res. 2009, 51, 223–281. [Google Scholar]
- Kalam, S.; Basu, A.; Iqbal Ahmad, R.Z.; El-Enshasy, S.H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Sayyed, R.; Saraf, M. Bacterial Determinants and Plant Defense Induction: Their Role as a Bio-Control Agent in Agriculture Plant Soil Microbes, Plant Soil Microbes; Springer: Basel, Switzerland, 2016; pp. 187–204. [Google Scholar]
- Verma, V.C.; Singh, S.K.; Prakash, S. Bio-control and plant growth promotion potential of siderophore producing endophytic Streptomyces from Azadirachta indica A. Juss. J. Basic. Microbiol. 2011, 51, 550–556. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Naphade, B.S.; Chincholkar, S.B. Consortium of A. feacalis & Pseudomonas for growth promotion in Groundnut. Asian JMB BT Environ. Sci. 2007, 11, 48–51. [Google Scholar]
- Sayyed, R.Z.; Chincholkar, S.B. Siderophore producing A. feacalis more biocontrol potential vis-a-vis chemical fungicide. Curr. Microbiol. 2009, 58, 47–51. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; El Enshasy, H.A.; Hanapi, Z.; Ilyas, N.; Elgorban, A.M.; Bahkali, A.H.; Marraiki, N. Evaluation of Klebsiella variicola for Amelioration of Soil Salinity and Improvement in the Growth and Nutrients Uptake in Wheat and Maize under Salinity Stress Conditions. Agronomy 2021, 11, 927. [Google Scholar] [CrossRef]
- Kumar, V.; Menon, S.; Agarwal, H.; Gopalakrishnan, D. Characterization and optimization of bacterium isolated from soil samples for the production of siderophores. Resour. Effic. Technol. 2017, 3, 434–439. [Google Scholar]
- Ramos-Solano, B.; García, J.A.L.; Garcia-Villaraco, A.; Algar, E.; Garcia-Cristobal, J.; Mañero, F.J.G. Siderophore and chitinase producing isolates from the rhizosphere of Nicotiana glauca Graham enhance growth and induce systemic resistance in Solanum lycopersicum L. Plant Soil. 2010, 334, 189–197. [Google Scholar] [CrossRef]
- Akhtar, N.; Ilyas, N.; Yasmin, H.; Sayyed, R.Z.; Hasnain, Z.A.; Elsayed, E.; El Enshasy, H.A. Role of Bacillus cereus in Improving the Growth and Phytoextractability of Brassica nigra (L.) K. Koch in Chromium Contaminated Soil. Molecules 2021, 26, 1569. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Bacillus: A biological tool for crop improvement through bio-molecular changes in adverse environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Ravari, B.S.; Heidarzadeh, N. Isolation and characterization of rhizosphere auxin producing Bacilli and evaluation of their potency on wheat growth improvement. Arch. Agron. Soil Sci. 2014, 60, 895–905. [Google Scholar] [CrossRef]
- Tahir Hafiz, A.S.; Qin, G.; Huijun, W.; Waseem, R.; Alwina, H.; Liming, W.; Colman Massawe, V.; Xuewen, G. Plant Growth Promotion by Volatile Organic Compounds Produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar]
- Qiao, J.; Yu, X.; Liang, X.; Liu, Y.; Borriss, R.; Liu, Y. Addition of plant-growth-promoting Bacillus subtilis PTS-394 on tomato rhizosphere has no durable impact on the composition of root microbiome. BMC Microbiol. 2017, 17, 131. [Google Scholar] [CrossRef]
- Trivedi, P.; Pandey, A. Plant growth promotion abilities and formulation of Bacillus megaterium strain B 388 (MTCC6521) isolated from a temperate Himalayan location. Indian J. Microbiol. 2008, 48, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D. Bacillus species in the soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [Green Version]
- Varma, P.K.; Suresh, V.Y.; Teja, M.R.; Kumar, K.V.K. Potentiality of native Bacillus species in enhancing sesame seed germination and their antagonism against Macrophomina phaseolina under in vitro conditions. J. Oilseeds Res. 2017, 34, 98–102. [Google Scholar]







| Properties Measured | Rhizosphere Soil from Sesamum |
|---|---|
| Sand (%) | 37.9 |
| Silt (%) | 19.86 |
| Clay (%) | 21.4 |
| pH | 7.2 |
| Organic carbon (%) | 0.315 |
| Electric conductivity (dS/m) | 0.17 |
| Water-holding capacity (%) | 41.4 |
| Nitrogen (mg/ha) | 48.5 |
| Phosphorus (mg/ha) | 12.3 |
| Potassium (mg/ha) | 107 |
| Magnesium (me/100 g) | 2.52 |
| Sulphur (mg/ha) | 8.1 |
| Calcium (mg/ha) | 6.1 |
| Zinc (ppm) | 0.37 |
| Iron (ppm) | 1.26 |
| Manganese (ppm) | 0.58 |
| Sodium (me/100 g) | 0.77 |
| Cupper (ppm) | 1.52 |
| Fraction | Color | CAS Test | Absorbance | Absorbance Maxima (nm) | Yield (mg/L) |
|---|---|---|---|---|---|
| 1 | Brown | Negative | 3.011, 2.911, 3.211 | 291, 195, 305 | 00 |
| 2 | Dark Yellow | Negative | 2.911, 1.871, 3.013 | 295, 201, 311 | 00 |
| 3 | Faint Brown | Negative | 1.931, 1.951, 2.401 | 271, 232, 301 | 00 |
| Filtrate | Golden Yellow | Positive | 3.161 | 272 | 200 |
| Water Wash | Yellow | Positive | 3.161 | 272 | 20 |
| Fraction | Color | CAS Test | Absorbance (nm) | λ max (nm) | Yield (mg/L) |
|---|---|---|---|---|---|
| 1 | Dark Golden yellow | Positive | 4.010 | 224 | 296 |
| 2 | Light Golden yellow | Positive | 3.957 | 224 | 50 |
| 3 | Yellow | Negative | 2.112, 1.782 | 298, 302 | 00 |
| 4 | Faint yellow | Negative | 0.975, 0.893 | 404, 396 | 00 |
| 5 | Greenish-yellow | Negative | 0.243, 0.0471 | 462, 664 | 00 |
| Treatment | Leaf Length (cm) | Shoot Length (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) | Number of Pods |
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Control | 7.3 ± 0.9 | 29.6 ± 1.24 | 6.66 ± 0.47 | 2.6 ± 0.1 | 1.3 ± 0.6 | 7 ± 1.0 |
| LSBS2 | 9.2 ± 0.7 | 35.4 ± 1.24 | 8 ± 0.81 | 3.2 ± 0.3 | 2.0 ± 0.4 | 12 ± 0.5 |
| Pure siderophore | 8.2 ± 0.5 | 31.3 ± 0.93 | 7.1 ± 0.81 | 2.9 ± 0.2 | 1.9.0 ± 0.3 | 9 ± 0.4 |
| Treatment | Iron Content * | Total Seed Oil Content (%) * | Photosynthetic Pigments * | |||
|---|---|---|---|---|---|---|
| Leaf (µg/g) | Shoot (µg/g) | Seed (µg/g) | Carotenoids (mg/g) | Chlorophyll (mg/g) | ||
| Control | 420.46 ± 5.76 | 512.4 ± 4.34 | 433.38 ± 2.45 | 36.16 ± 2.75 | 0.04 ± 0.04 | 0.056 ± 0.056 |
| LSBS2 | 484.02 ± 5.28 | 563.00 ± 2.31 | 562.60 ± 4.30 | 47.3 ± 2.06 | 0.26 ± 0.02 | 1.078 ± 0.229 |
| Pure siderophore | 441.02 ± 4.078 | 537.00 ± 1.92 | 541.51 ± 2.21 | 39.9 ± 1.24 | 0.17 ± 0.02 | 0.901 ± 0.231 |
| Nutrients Measured | Untreated Soil | Soil Treated with B. subtilis LSBS2 |
|---|---|---|
| Nitrogen (mg/kg) | 48.5 ± 0.5 | 62.96 ± 0.55 ** |
| Phosphorus | 12.36 ± 0.3 | 19.2 ± 0.15 * |
| Potassium | 107 ± 1.0 | 153 ± 1.0 ** |
| Magnesium | 2.52 ± 0.03 | 3.89 ± 0.60 * |
| Calcium | 6.1 ± 0.1 | 11.37 ± 0.45 ** |
| Zinc (ppm) | 0.37 ± 0.45 | 0.21 ± 0.01 |
| Iron (ppm) | 1.26 ± 0.01 | 6.41 ± 0.05 *** |
| Manganese (ppm) | 0.58 ± 0.01 | 0.6 ± 0.1 * |
| Sodium (mg/kg) | 0.77 ± 0.01 | 3.72 ± 0.04 *** |
| Cupper (ppm) | 1.52 ± 0.02 | 2.96 ± 0.15 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustainability 2021, 13, 5394. https://doi.org/10.3390/su13105394
Nithyapriya S, Lalitha S, Sayyed RZ, Reddy MS, Dailin DJ, El Enshasy HA, Luh Suriani N, Herlambang S. Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame. Sustainability. 2021; 13(10):5394. https://doi.org/10.3390/su13105394
Chicago/Turabian StyleNithyapriya, S., Sundaram Lalitha, R. Z. Sayyed, M. S. Reddy, Daniel Joe Dailin, Hesham A. El Enshasy, Ni Luh Suriani, and Susila Herlambang. 2021. "Production, Purification, and Characterization of Bacillibactin Siderophore of Bacillus subtilis and Its Application for Improvement in Plant Growth and Oil Content in Sesame" Sustainability 13, no. 10: 5394. https://doi.org/10.3390/su13105394








