Exploring the Use of Polyols, Corn, and Beet Juice for Decreasing the Freezing Point of Brine Solution for Deicing of Pavements
Abstract
:1. Introduction
2. Experimental Study
2.1. Materials
2.2. Sample Preparation
2.2.1. Polyols-Brine Solution
2.2.2. Corn Juice-NaCl Solution
2.2.3. Beet-Juice-NaCl Solution
2.3. Freezing Point Depression Test
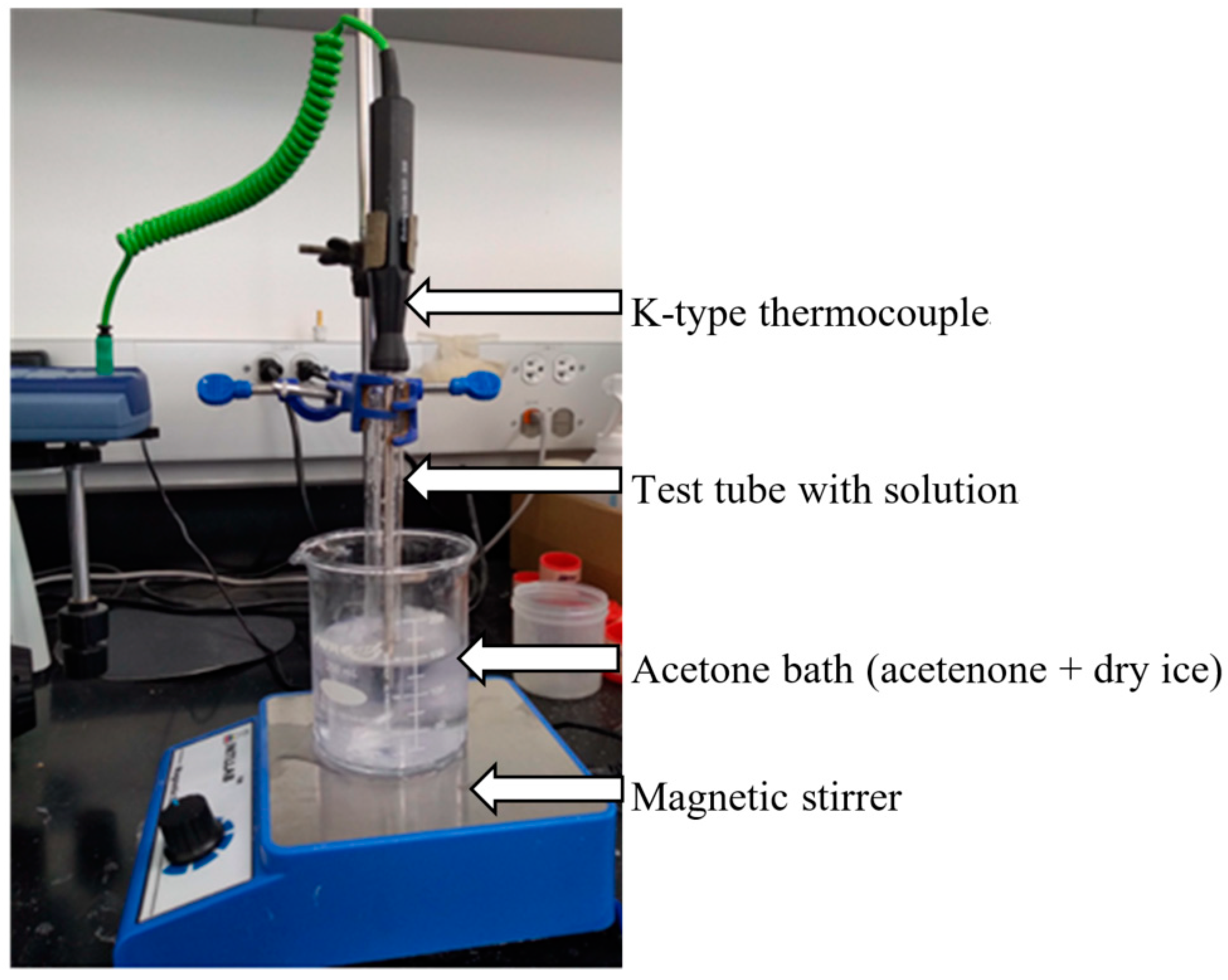
2.3.1. In-House Experimental Setup
2.3.2. Differential Scanning Calorimetry (DSC) Analysis
2.4. Measuring Soluble Solids in Corn and Beet Juice
3. Description of Typical a Freezing Point Depression Curve and DSC Thermogram
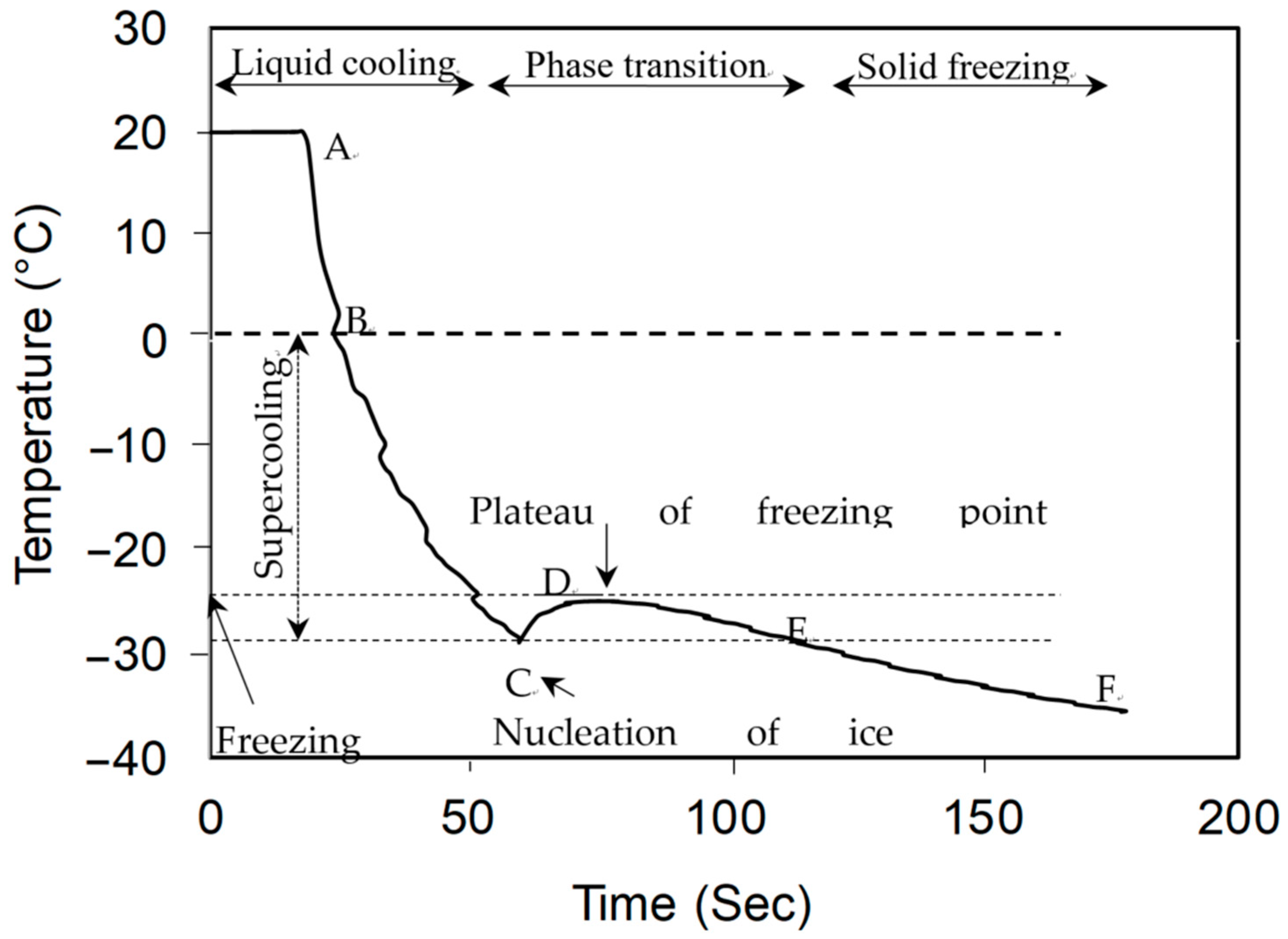
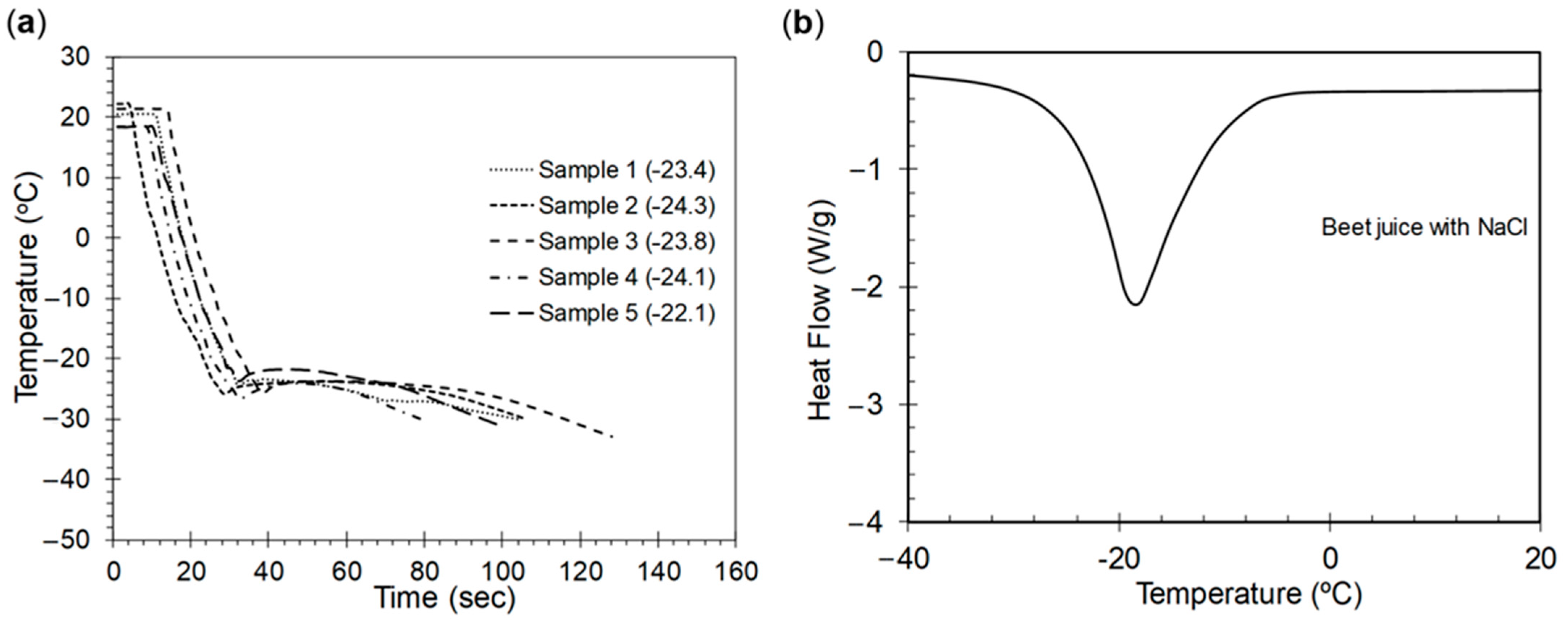
3.1. Freezing Point Depression Curve
3.2. Freezing Point and DSC Thermogram
4. Results and Discussion
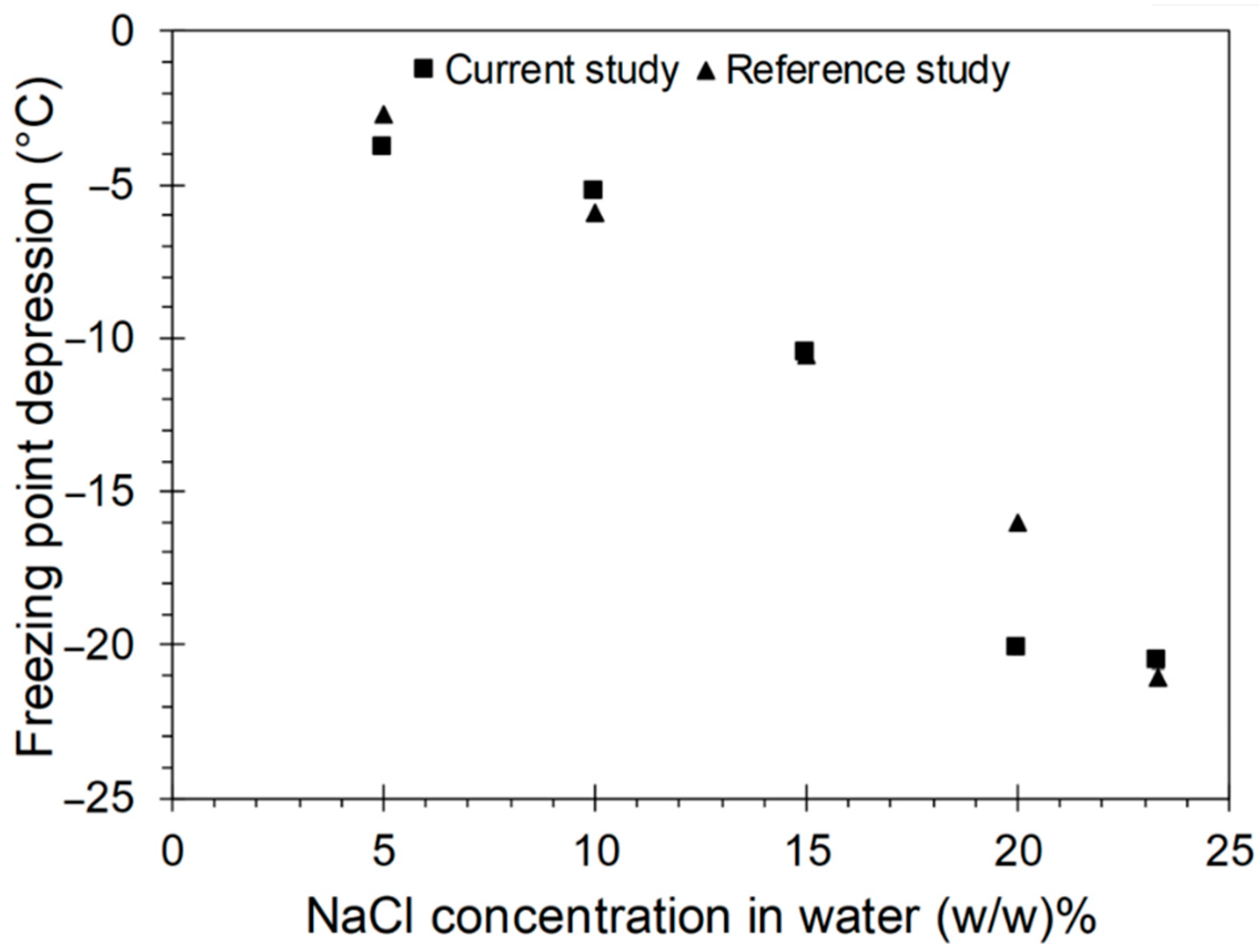
4.1. Validation of the Experimental Setup
4.2. Freezing Points of Polyol-Brine Solutions
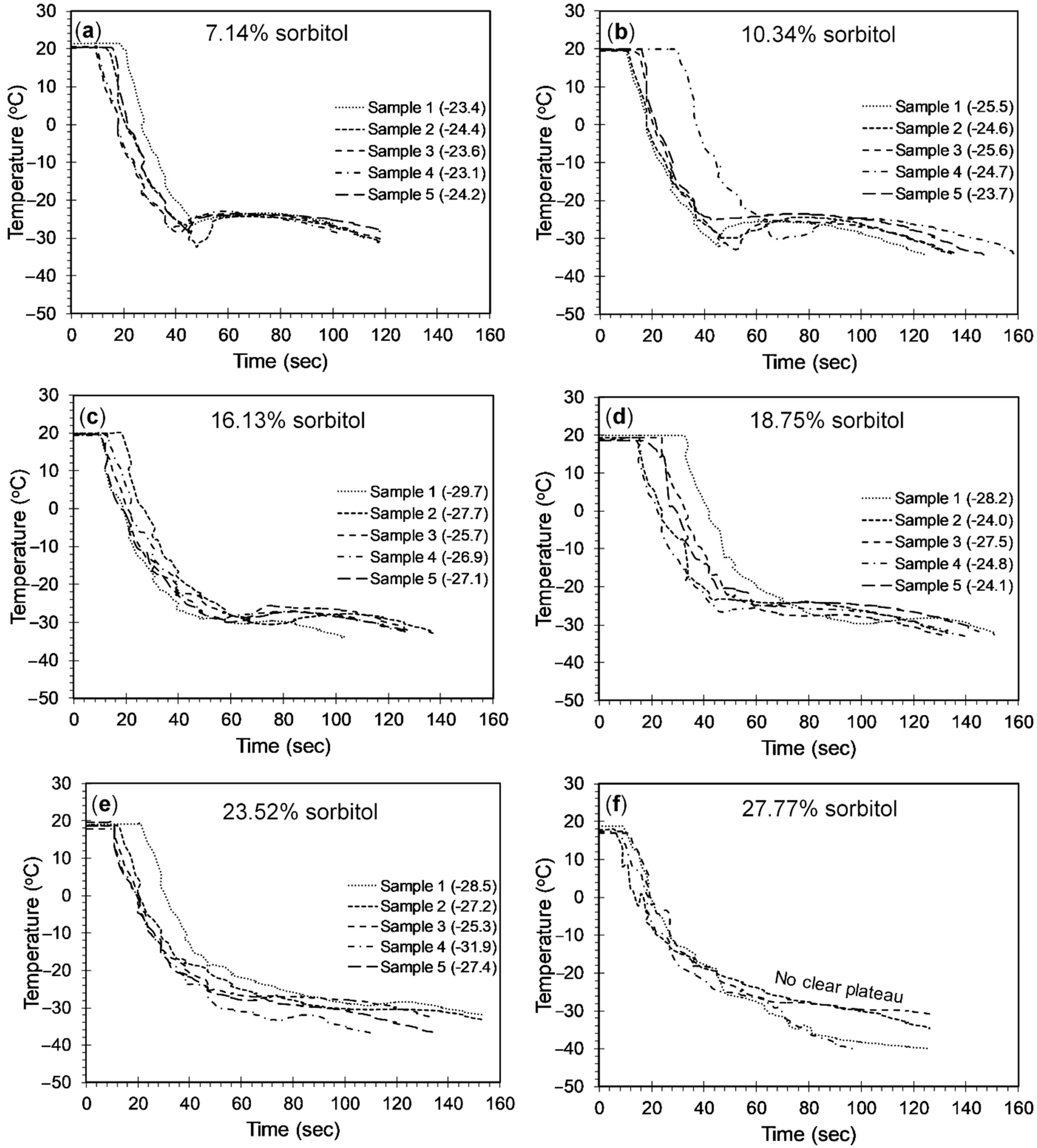
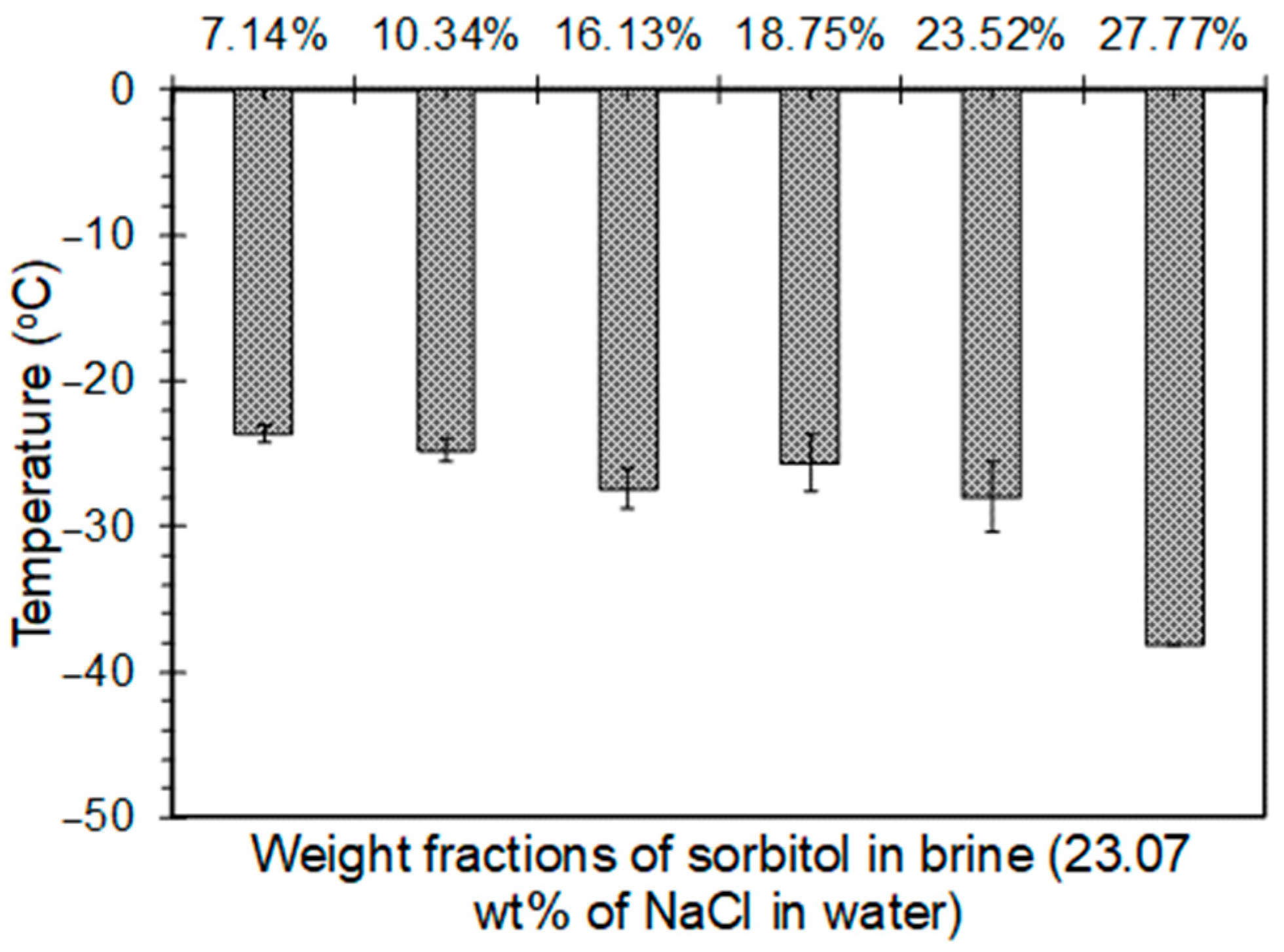
4.2.1. Sorbitol-Brine Solutions
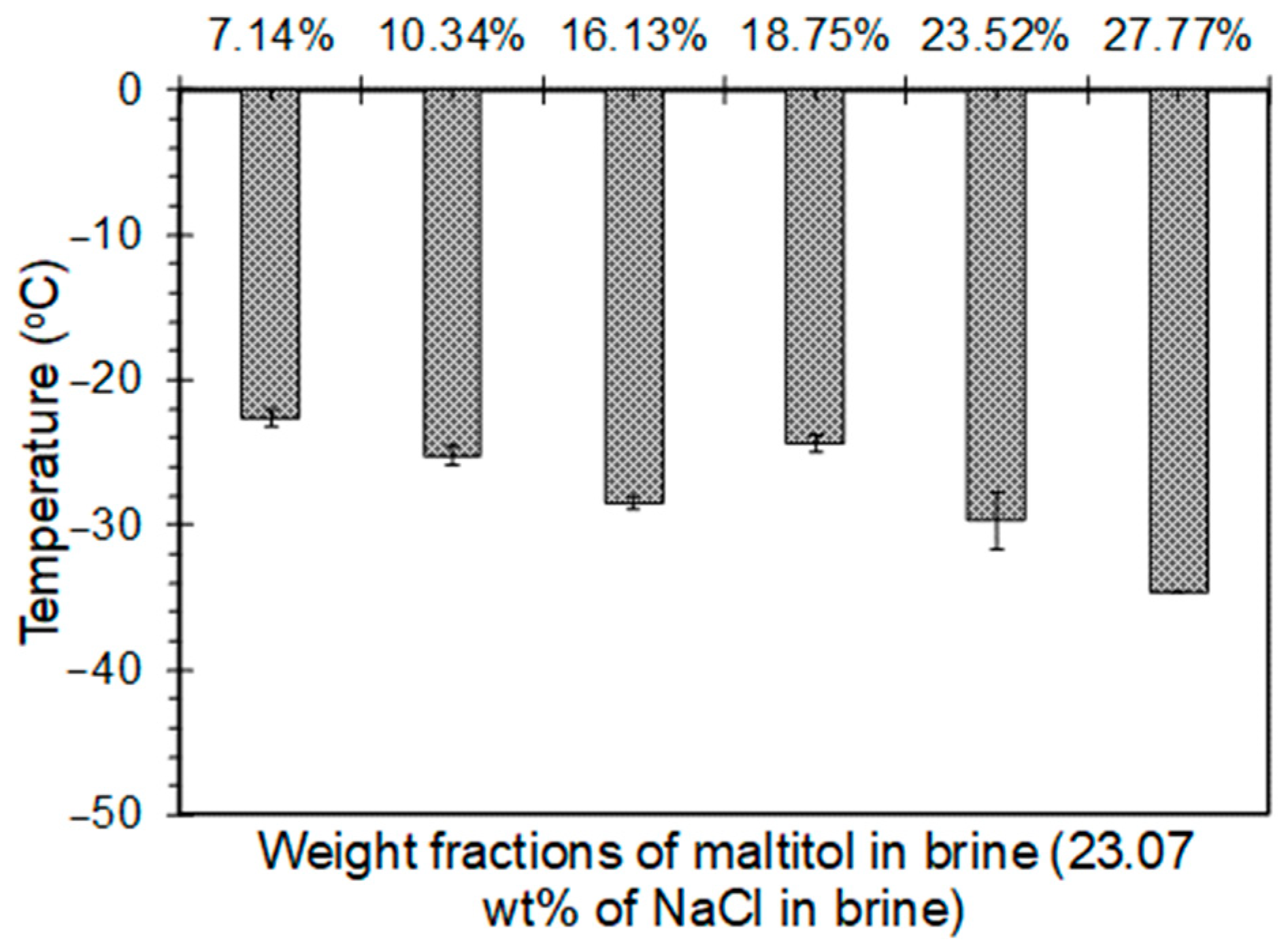
4.2.2. Maltitol-Brine Solutions
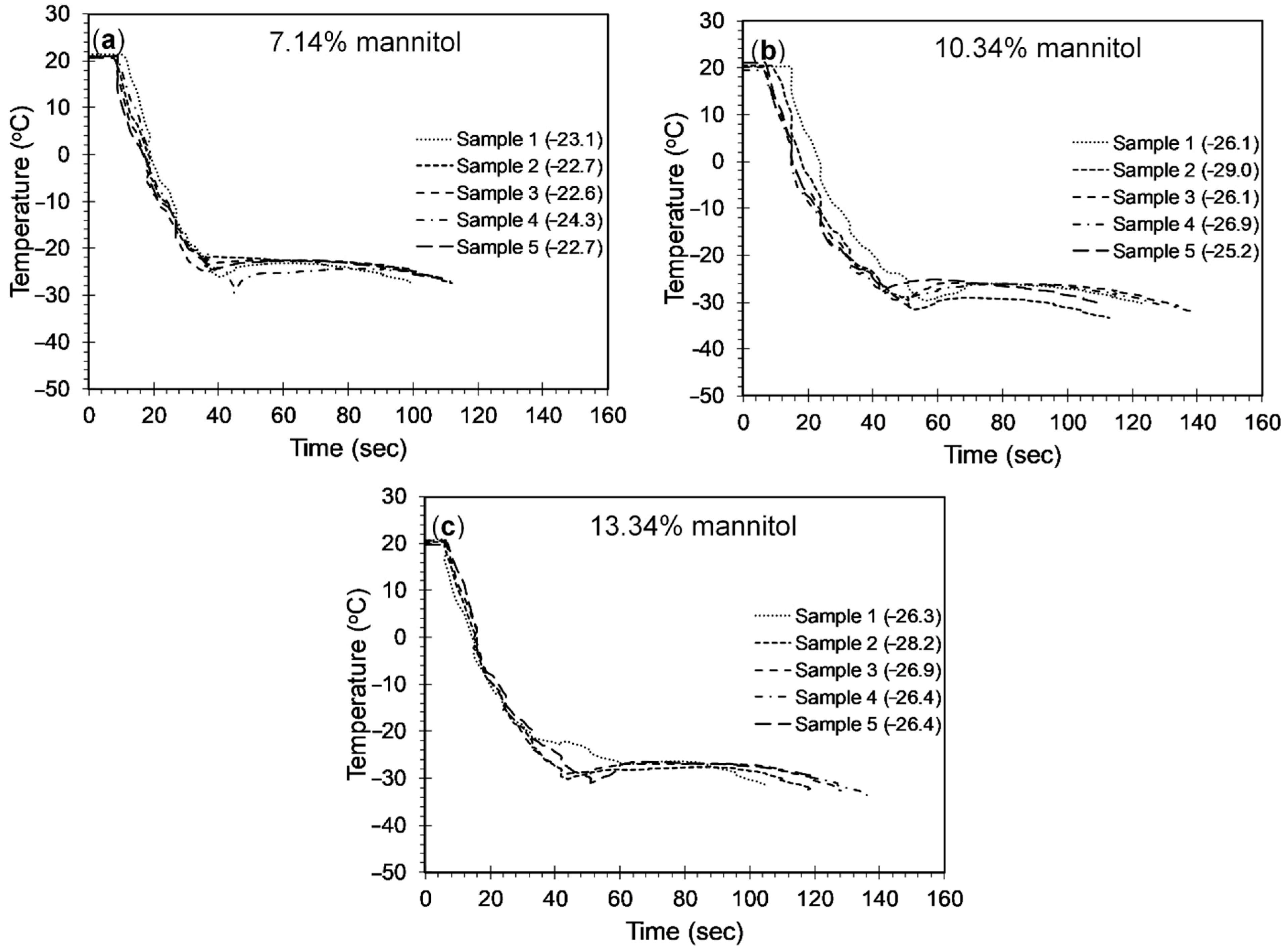
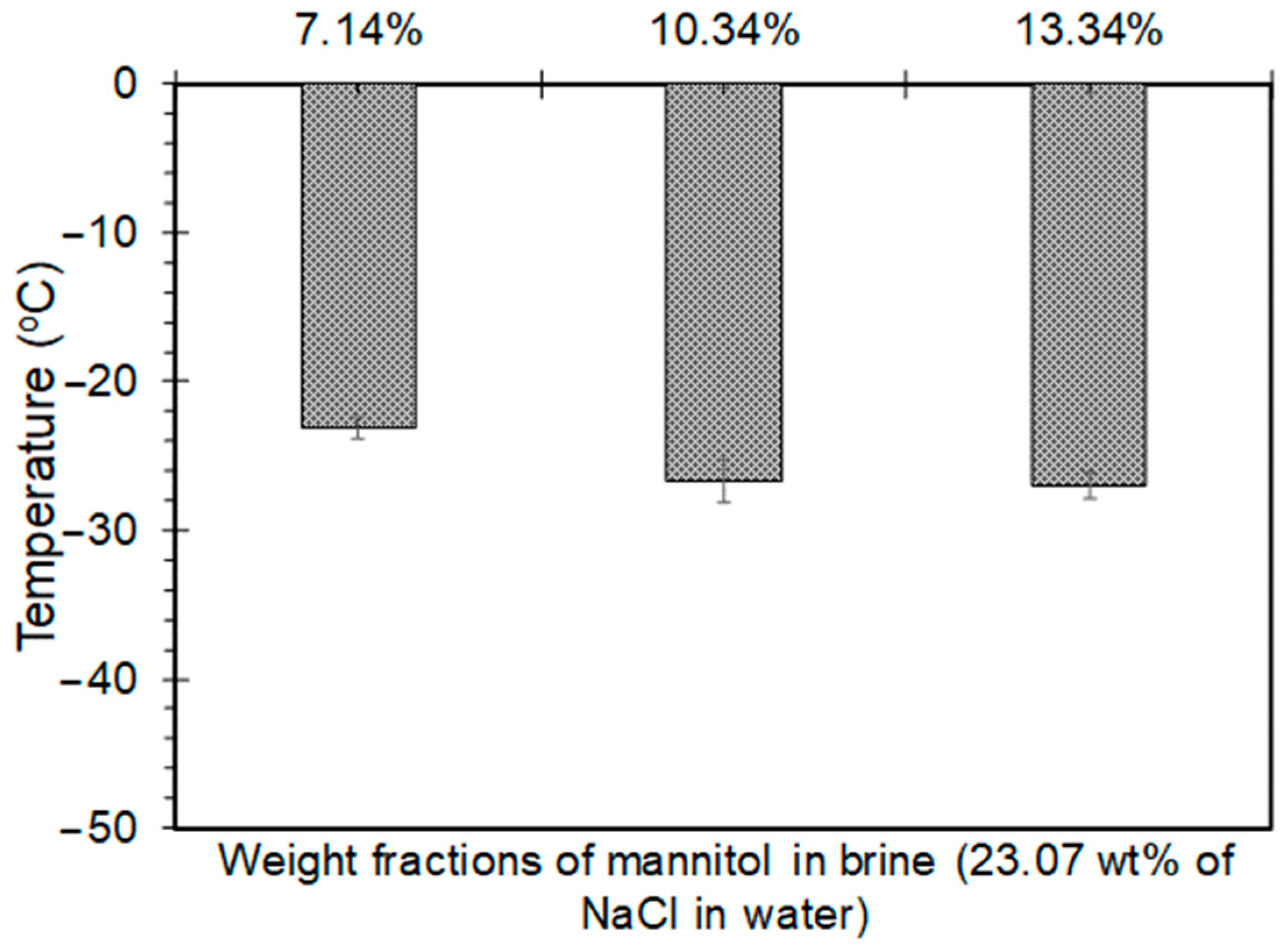
4.2.3. Mannitol-Brine Solutions
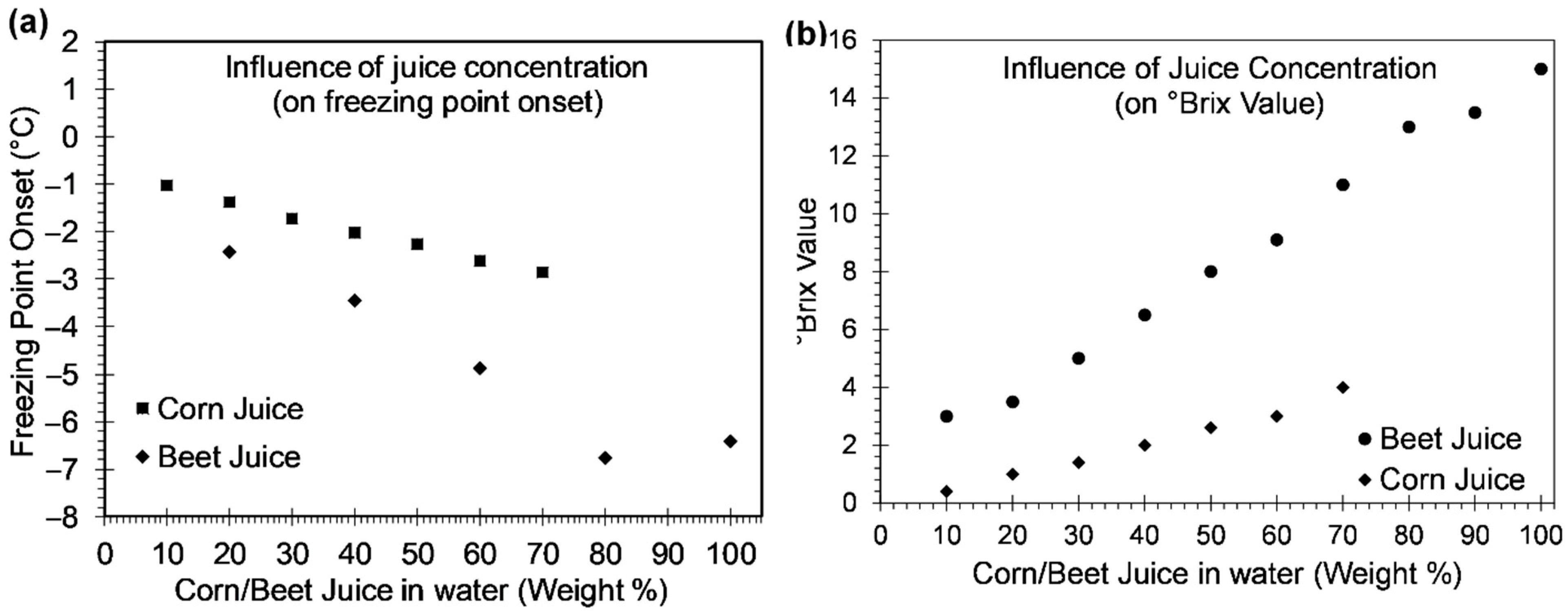
4.3. Freezing Points of Corn and Beet Juice
Influence of NaCl on the Freezing Point of Corn and Beet Juice
5. Conclusions
- Addition of polyols to the 23.3 wt% brine solution was observed to lower the freezing point below −21.1 °C. The freezing point decreased until the solubility limit of polyols was reached. While a 15.13%, 26.11%, and 15.08% drop in the freezing point was observed when the weight fraction was increased from 7.14% to 16.13% for sorbitol, maltitol, and mannitol, respectively, a 35.39% and 19.46% drop was observed in the freezing point depression when the weight fraction was increased from 23.52% to 27.77% for sorbitol and maltitol, respectively.
- The minimum freezing points achieved by sorbitol, maltitol, and mannitol, when combined with 23.3 wt% brine solution, were −38.1 °C, −35.6 °C, and −26.7 °C, respectively, at weight fractions of 27.77%, 27.77%, and 13.33%, respectively.
- The freezing points of corn and beet juice were observed to decrease with an increase in their weight concentration. This phenomenon could be attributed to the higher quantity of the total soluble solids in the higher concentration juices. While corn juice exhibited a freezing point of −2.87 °C at 70% weight fraction of corn kernels, 100% concentration beet juice exhibited a freezing point of −6.8 °C.
- Addition of NaCl to corn and beet juice further depressed their freezing points. The 70 wt% corn and 100% concentration beet juice with 23.3 wt% NaCl lowered the freezing points to −23.5 °C, and −28 °C, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Izumi, I.; Nakamura, T.; Sack, R.L. Snow Engineering: Recent Advances. In Proceedings of the Third International Conference, Sendai, Japan, 26–31 May 1996; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Kao, J.C.; Sung, W.-P. Civil, Architecture and Environmental Engineering Volume 2. In Proceedings of the International Conference ICCAE, Taipei, Taiwan, 4–6 November 2016. [Google Scholar]
- National Research Council (US). Deicing, Highway Deicing: Comparing Salt and Calcium Magnesium Acetate; Transportation Research Board: Washington, DC, USA, 1991. [Google Scholar]
- FHWA. How Do Weather Events Impact Roads?—FHWA Road Weather Management. 2018. Available online: https://ops.fhwa.dot.gov/weather/q1_roadimpact.htm (accessed on 7 March 2021).
- Fischel, M. Evaluation of Selected Deicers Based on a Review of the Literature; Colorado Department of Transportation, Research Branch: Denver, CO, USA, 2009. [Google Scholar]
- Akin, M.; Huang, J.; Shi, X.; Veneziano, D.; Williams, D. Snow Removal at Extreme Temperatures; Western Transportation Institute, Montana State University: Bozeman, MT, USA, 2013. [Google Scholar]
- Kimbrough, D. Salting roads: The solution for winter driving. ChemMatters 2006, 24, 14–16. Available online: file:///C:/Users/MDPI/AppData/Local/Temp/chemmatters-feb2006-salting-roads-1.pdf (accessed on 7 March 2021).
- Sajid, H.U.; Kiran, R.; Qi, X.; Bajwa, D.S.; Battocchi, D. Employing corn derived products to reduce the corrosivity of pavement deicing materials. Constr. Build. Mater. 2020, 263. [Google Scholar] [CrossRef]
- Sajid, H.U.; Naik, D.L.; Kiran, R. Improving the ice-melting capacity of traditional deicers. Constr. Build. Mater. 2021, 271. [Google Scholar] [CrossRef]
- Goldberg, I. Functional Foods: Designer Foods, Pharmafoods, Nutraceuticals; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Uraji, T.; Kohno, H.; Yoshimura, H.; Shimoyamada, M.; Watanabe, K. Freezing Point Depression of Polyol-Aqueous Solutions in the High Concentration Range. Food Sci. Technol. Int. Tokyo 1996, 2, 38–42. [Google Scholar] [CrossRef]
- Aliyu, B.K.; Osheku, C.A.; Oyedeji, E.O.; Adetoro, M.A.L.; Okon, A.A.; Idoko, C.M. Validating a novel theoretical expression for burn tine and average thrust in solid rocket motor design. Adv. Res. 2015, 5, 1–11. [Google Scholar]
- Dewick, P.M. Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal Chemistry and Biological Chemistry; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Guddat, S.; Thevis, M.; Schänzer, W. Identification and Quantification of the Osmodiuretic Mannitol in Urine for Sports Drug Testing Using Gas Chromatography-Mass Spectrometry. Eur. J. Mass Spectrom. 2008, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Carbohydrate Chemistry for Food Scientists; Elsevier BV: New York, NY, USA, 2019. [Google Scholar]
- Kalsoom, U.; Bennett, I.; Boyce, M.C. A review of extraction and analysis: Methods for studying osmoregulants in plants. J. Chromatogr. Sep. Tech. 2016, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Zumbé, A.; Lee, A.; Storey, D. Polyols in confectionery: The route to sugar-free, reduced sugar and reduced calorie confectionery. Br. J. Nutr. 2001, 85, S31–S45. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, S.; Minsk, L.D.; Blackburn, R.R.; Fleege, E.J. Manual of Practice for an Effective Anti-Icing Program: A Guide for Highway Winter Maintenance Personnel; Federal Highway Administration: Washington, DC, USA, 1996. [Google Scholar]
- Villa-Vélez, H.A.; Telis-Romero, J.; Higuita, D.M.C.; Telis, V.R.N. Effect of maltodextrin on the freezing point and thermal conductivity of uvaia pulp (Eugenia piriformis Cambess). Ciência Agrotecnol. 2012, 36, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Telis, V.; Telis-Romero, J.; Sobral, P.; Gabas, A. Freezing Point and Thermal Conductivity of Tropical Fruit Pulps: Mango and Papaya. Int. J. Food Prop. 2007, 10, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Groenewoud, W. Characterisation of Polymers by Thermal Analysis; Elsevier BV: New York, NY, USA, 2001. [Google Scholar]
- Rahman, M.; Guizani, N.; Al-Khaseibi, M.; Al-Hinai, S.A.; Al-Maskri, S.S.; Al-Hamhami, K. Analysis of cooling curve to determine the end point of freezing. Food Hydrocoll. 2002, 16, 653–659. [Google Scholar] [CrossRef]
- Stewart, M.I. Heat Transfer Theory. In Surface Production Operations; Gulf Professional Publishing: Boston, MA, USA, 2014; pp. 39–97. [Google Scholar]
- Louhenkilpi, S. Continuous Casting of Steel. In Treatise on Process; Metallurgy, S., Ed.; Elsevier: Boston, MA, USA, 2014; pp. 373–434. [Google Scholar]
- Srivastava, L.M. Plant. Growth and Development: Hormones and Environment; Elsevier: NewYork, NY, USA, 2002. [Google Scholar]
- Belch, A.C.; Berkowitz, M.; McCammon, J.A. Solvation structure of a sodium chloride ion pair in water. J. Am. Chem. Soc. 1986, 108, 1755–1761. [Google Scholar] [CrossRef]
- Kelley, M.; Donley, A.; Clark, S.; Clark, A. Structure and Dynamics of NaCl Ion Pairing in Solutions of Water and Methanol. J. Phys. Chem. B 2015, 119, 15652–15661. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.H.; Sherkat, F. Handbook of Food Science, Technology, and Engineering-4 Volume Set; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Pappas, L.S. Ice Creams & Sorbets: Cool Recipes; Chronicle Books LLC: Sanfrancisco, CA, USA, 2006. [Google Scholar]
- Jayawardena, J.; Vanniarachchi, M.; Wansapala, M. Freezing point depression of different Sucrose solutions and coconut water. Int. J. Food Sci. Nutr. 2017, 2, 68–71. [Google Scholar]
- Auleda, J.; Raventós, M.; Sánchez, J.; Hernández, E. Estimation of the freezing point of concentrated fruit juices for application in freeze concentration. J. Food Eng. 2011, 105, 289–294. [Google Scholar] [CrossRef]
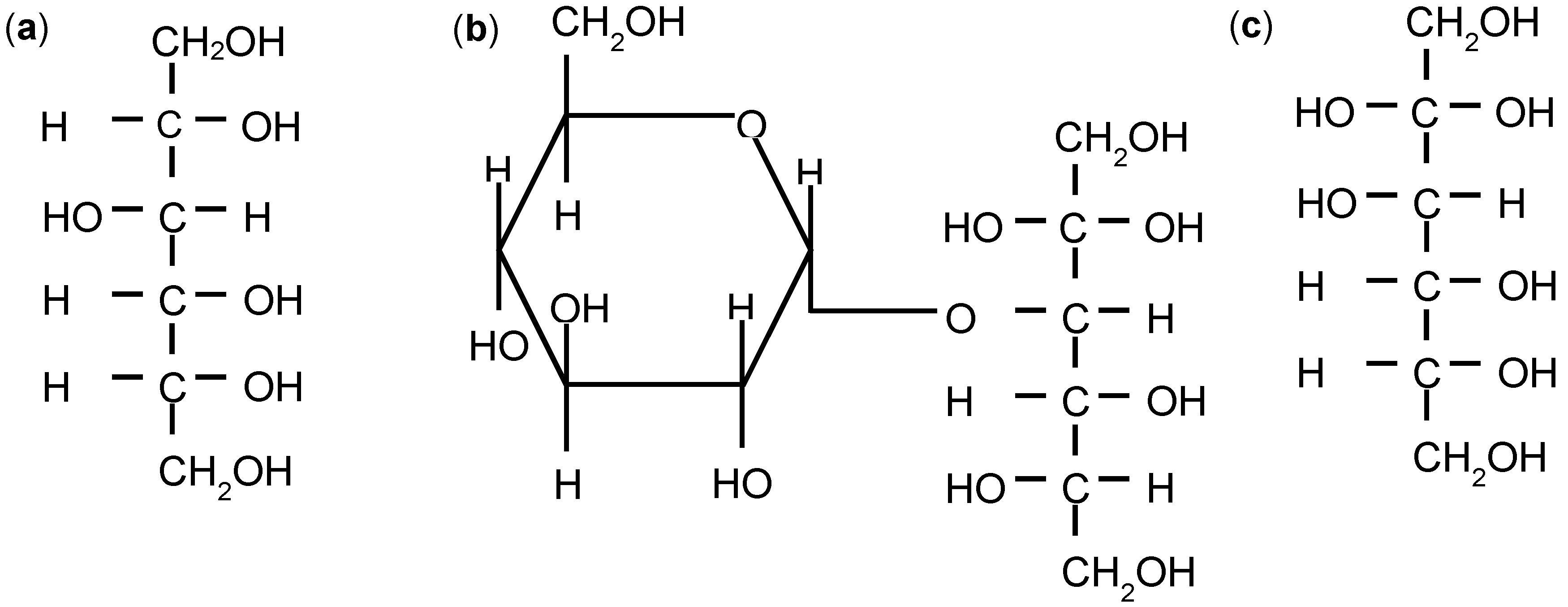



| Sorbitol | Maltitol | Mannitol | |
|---|---|---|---|
| Molecular formula | C6H14O6 | C12H24O11 | C6H14O6 |
| Molecular weight (g/mol) | 182.172 | 344.313 | 182.172 |
| Water solubility (g/100 mL of water at 25 °C) [17] | 246.6 | 171.2 | 18.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, T.; Lavadiya, D.N.; Kiran, R. Exploring the Use of Polyols, Corn, and Beet Juice for Decreasing the Freezing Point of Brine Solution for Deicing of Pavements. Sustainability 2021, 13, 5765. https://doi.org/10.3390/su13115765
Abbas T, Lavadiya DN, Kiran R. Exploring the Use of Polyols, Corn, and Beet Juice for Decreasing the Freezing Point of Brine Solution for Deicing of Pavements. Sustainability. 2021; 13(11):5765. https://doi.org/10.3390/su13115765
Chicago/Turabian StyleAbbas, Tauqeer, Dayakar Naik Lavadiya, and Ravi Kiran. 2021. "Exploring the Use of Polyols, Corn, and Beet Juice for Decreasing the Freezing Point of Brine Solution for Deicing of Pavements" Sustainability 13, no. 11: 5765. https://doi.org/10.3390/su13115765
APA StyleAbbas, T., Lavadiya, D. N., & Kiran, R. (2021). Exploring the Use of Polyols, Corn, and Beet Juice for Decreasing the Freezing Point of Brine Solution for Deicing of Pavements. Sustainability, 13(11), 5765. https://doi.org/10.3390/su13115765






