Corrosion Resistance of Open Die Forged Austenitic Stainless Steel Samples Prepared with Different Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Open Die Forging—Upsetting
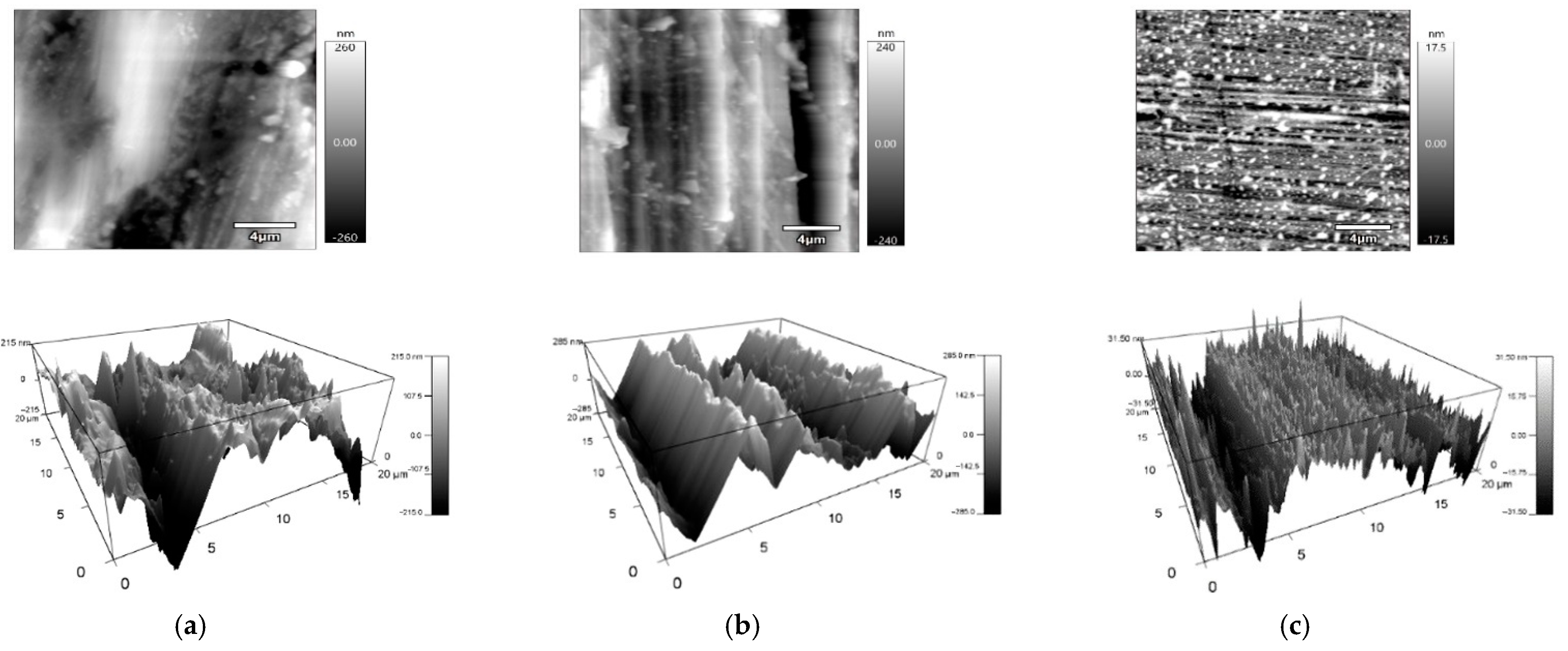
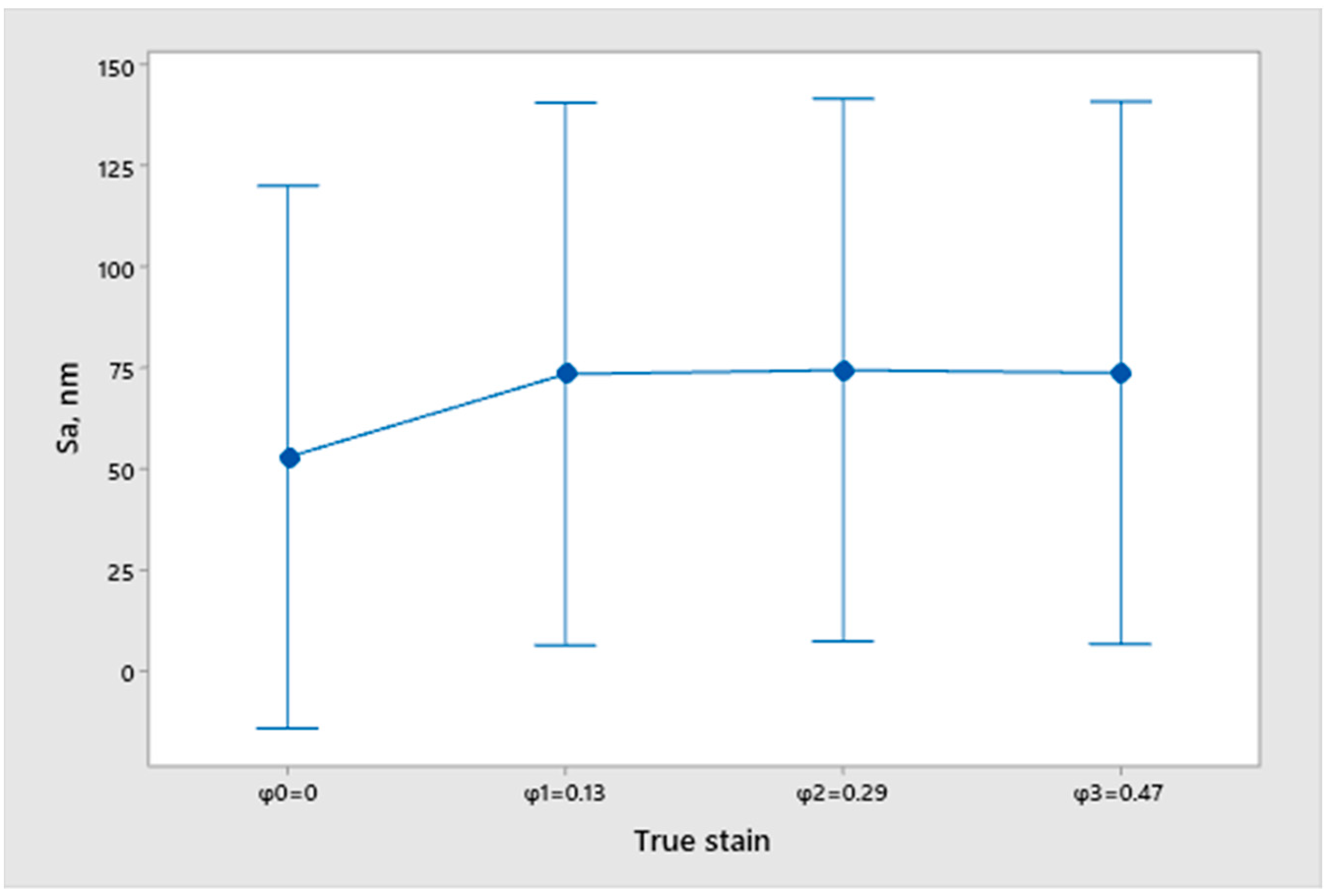
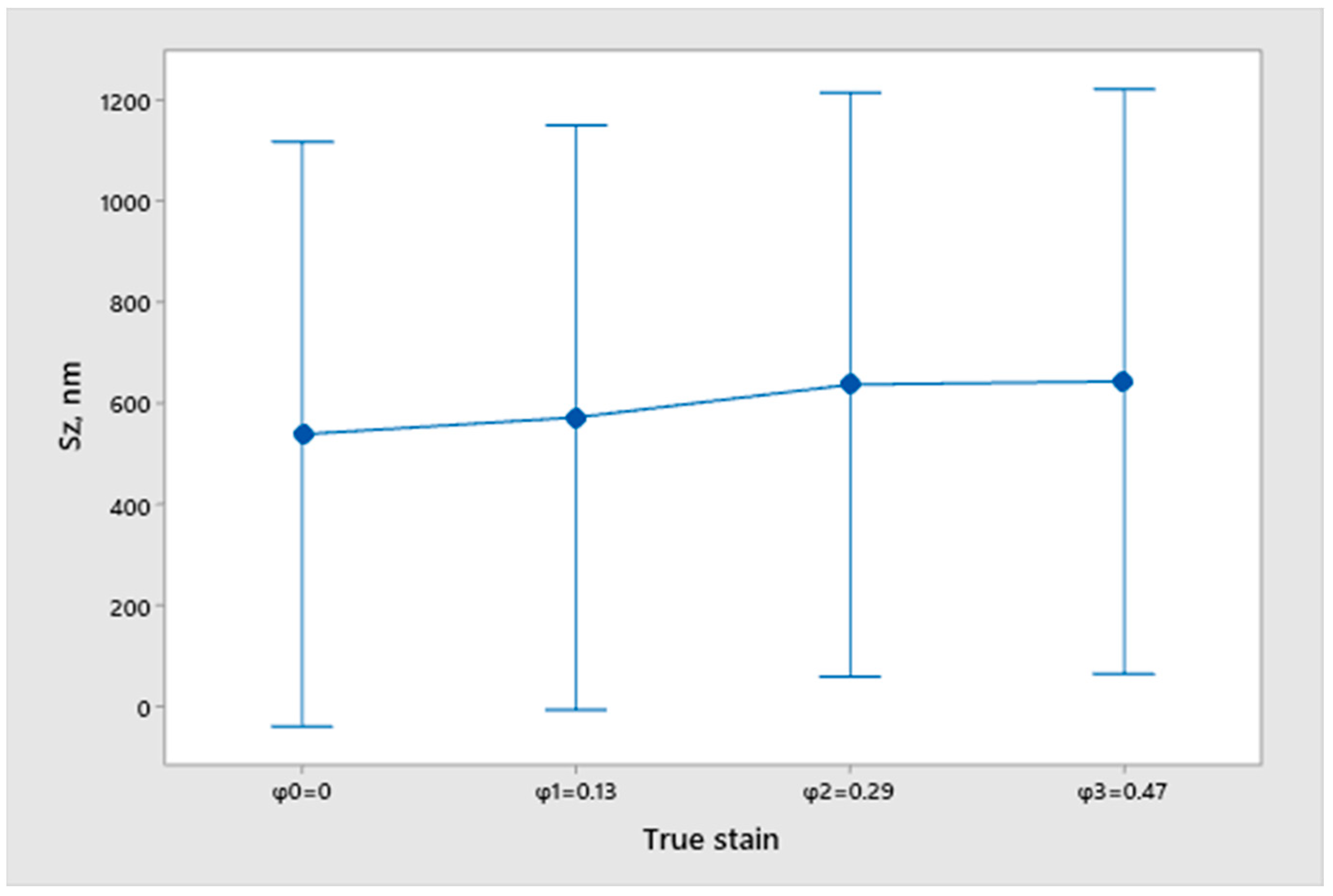
2.2. Measurement of Surface Topography
- Sa—Arithmetic mean deviation;
- Sz—Maximum height;
- Sv—Maximum profile valley depth;
- Sp—Maximum profile peak height;
- Sku—Kurtosis;
- Ssk—Skewness; and
- Sq—Root mean square deviation.
2.3. Corrosion Resistance Testing
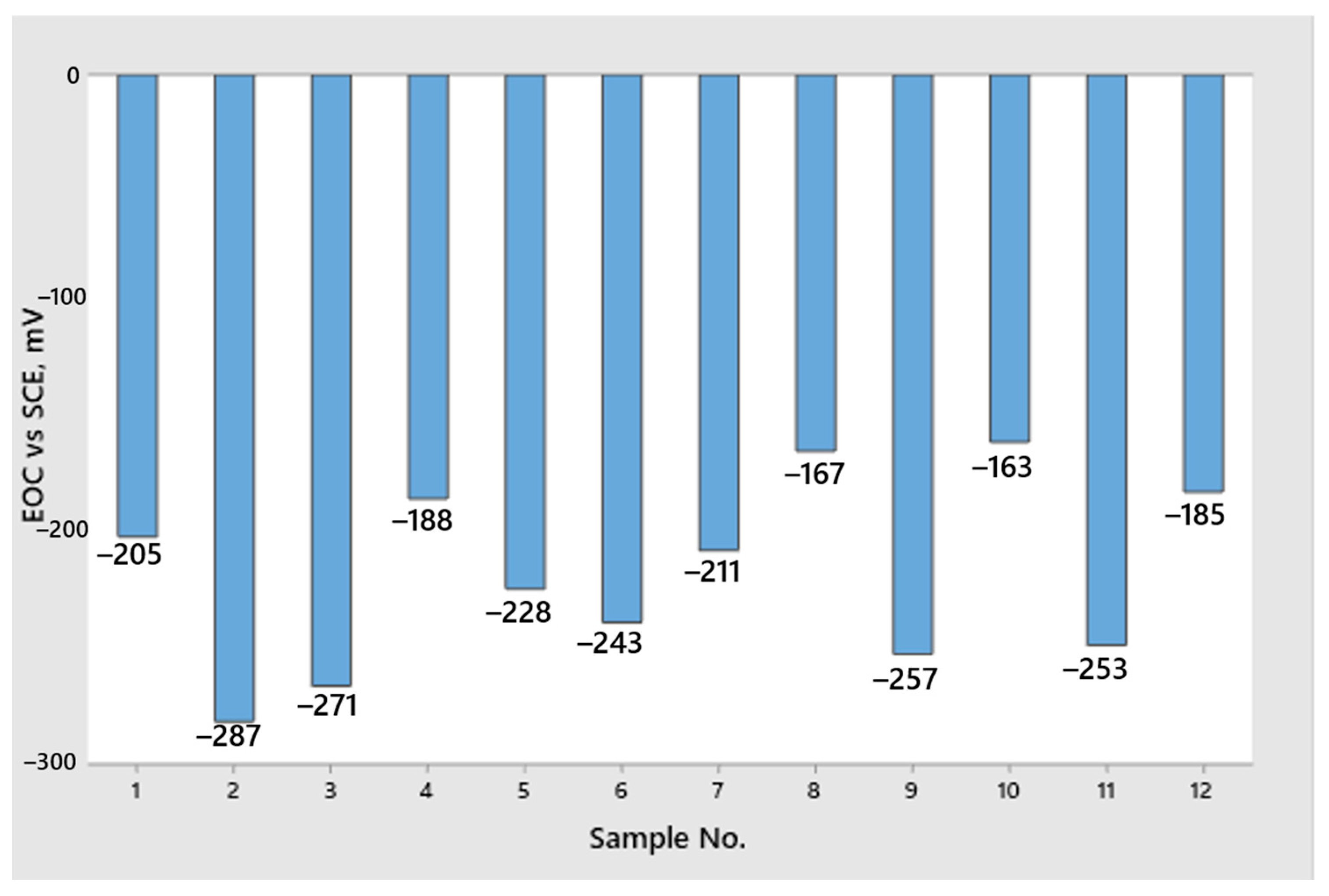
2.3.1. Testing of an Open Circuit Potential
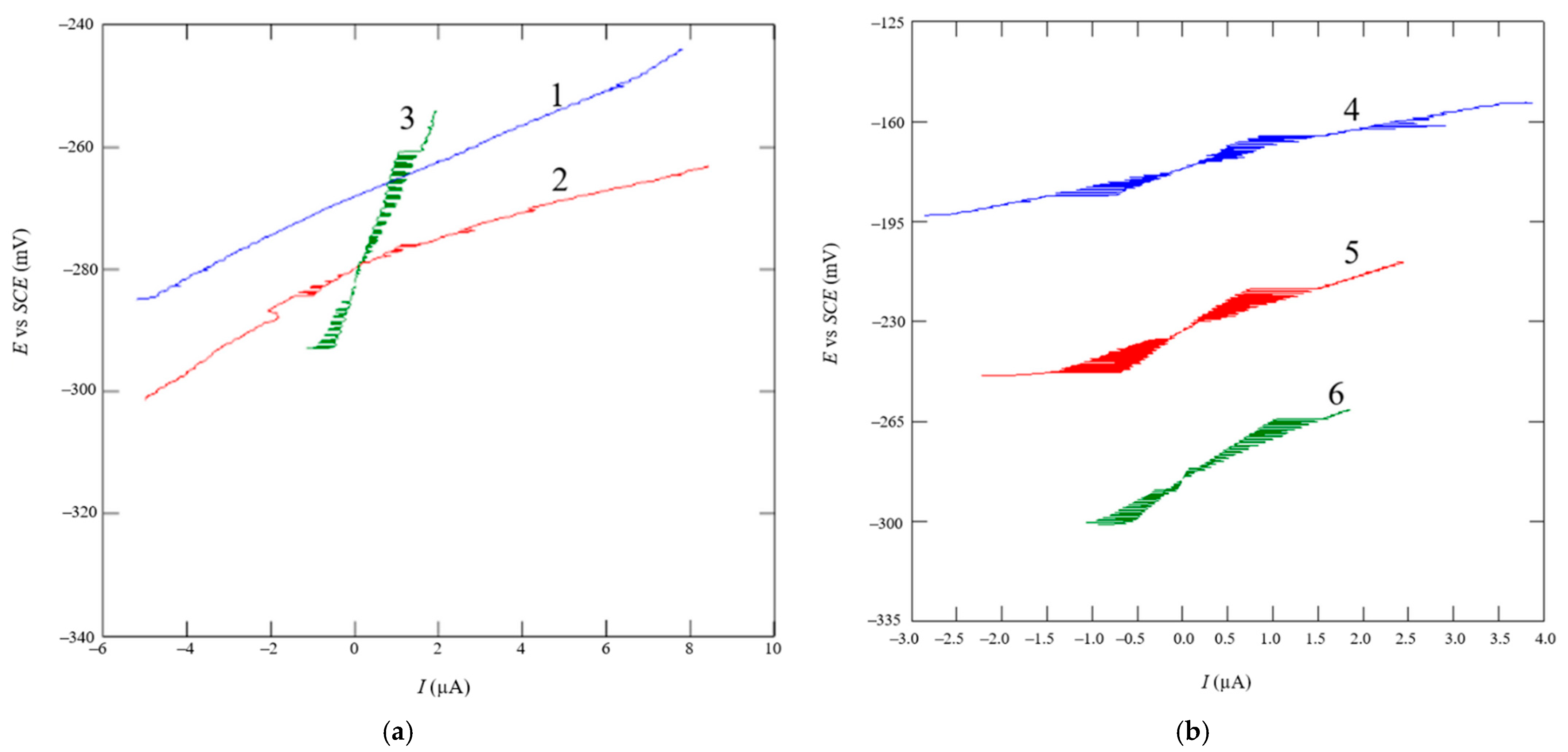
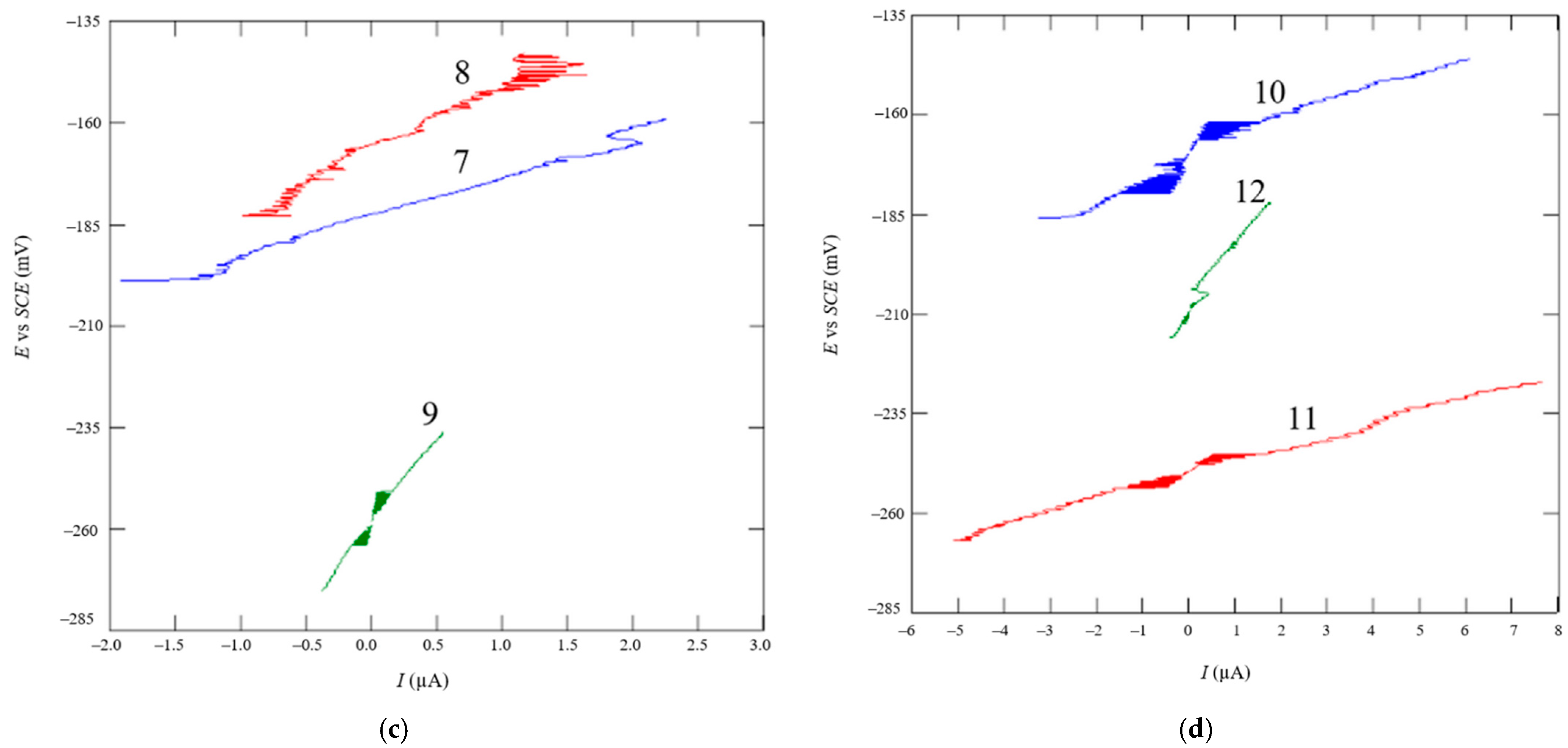
2.3.2. Linear Polarization
2.3.3. Tafel Extrapolation
- ρ—density, g/cm3;
- jcorr—corrosion current density, µA/cm2; and
- Ew—equivalent weight, g.
3. Results and Discussion
4. Conclusions
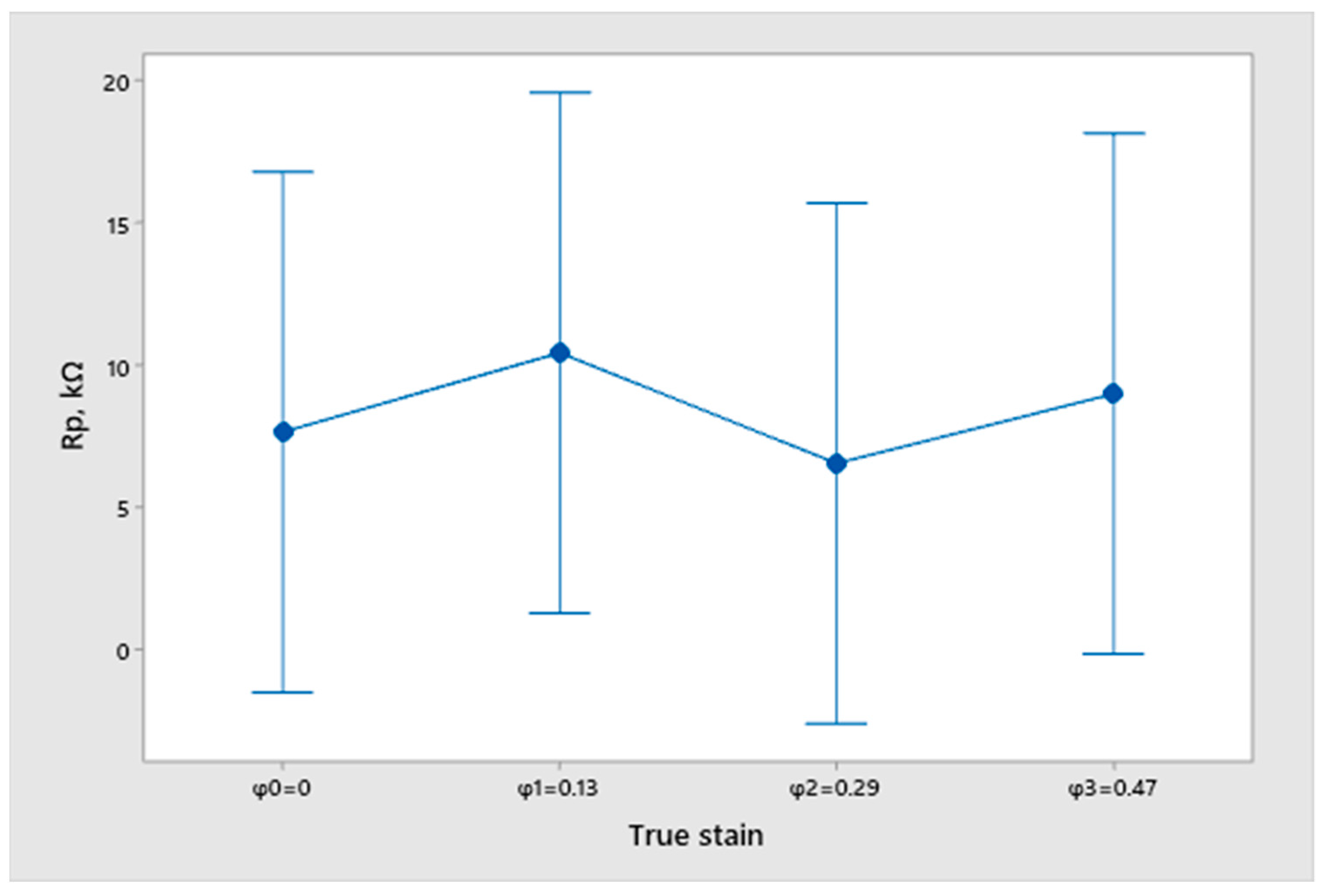
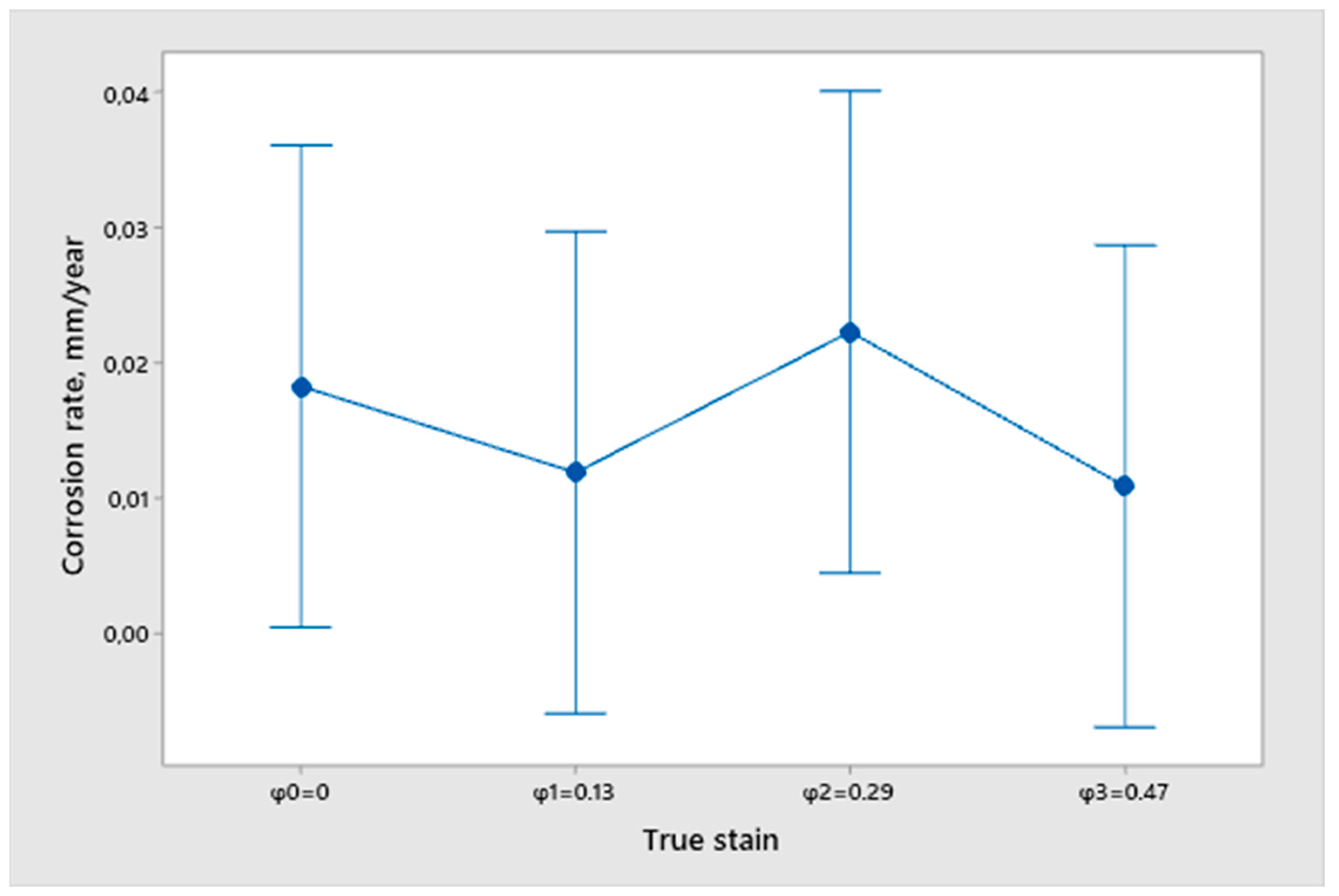
- From the open circuit potential testing, we can conclude that none of the samples are fully corrosion resistant and that all samples have a certain dissolution in the 3.5% NaCl solution. However, we cannot obtain any correlation between the true strain value and the differences in the surfacing with the measured potential of EOC. Therefore, we cannot conclude that the deformation degree and quality of sample finishing has an influence on corrosion resistance.
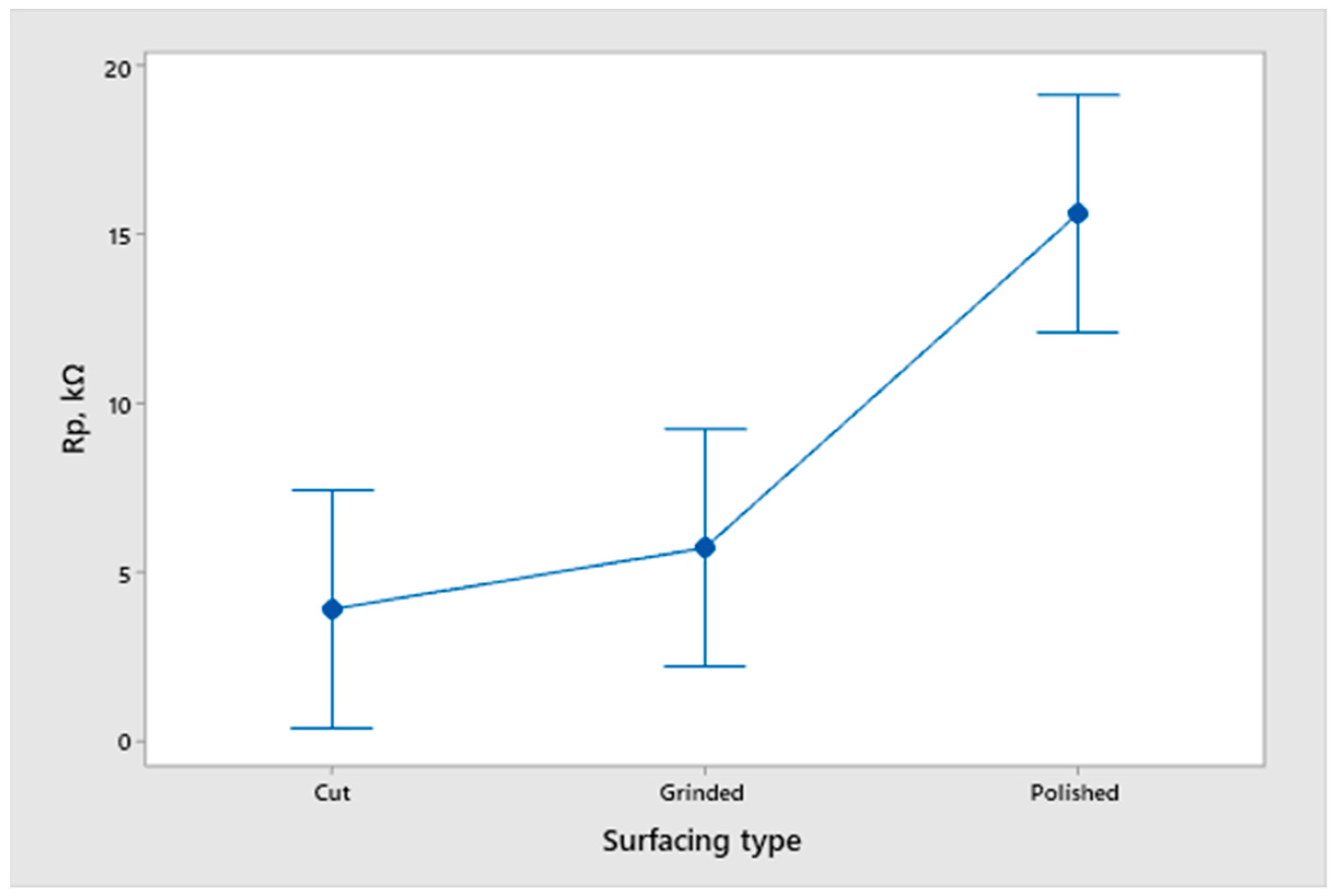
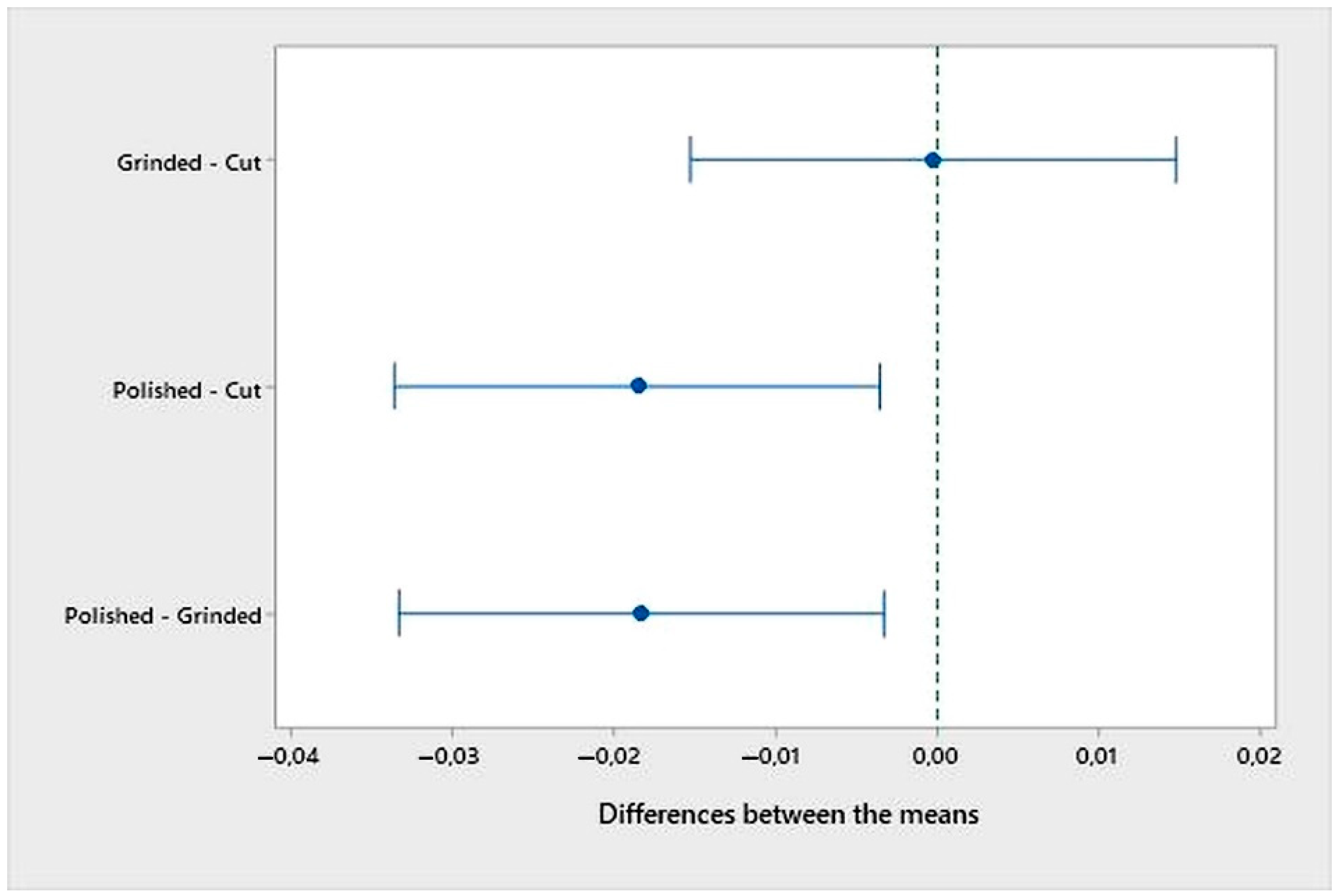
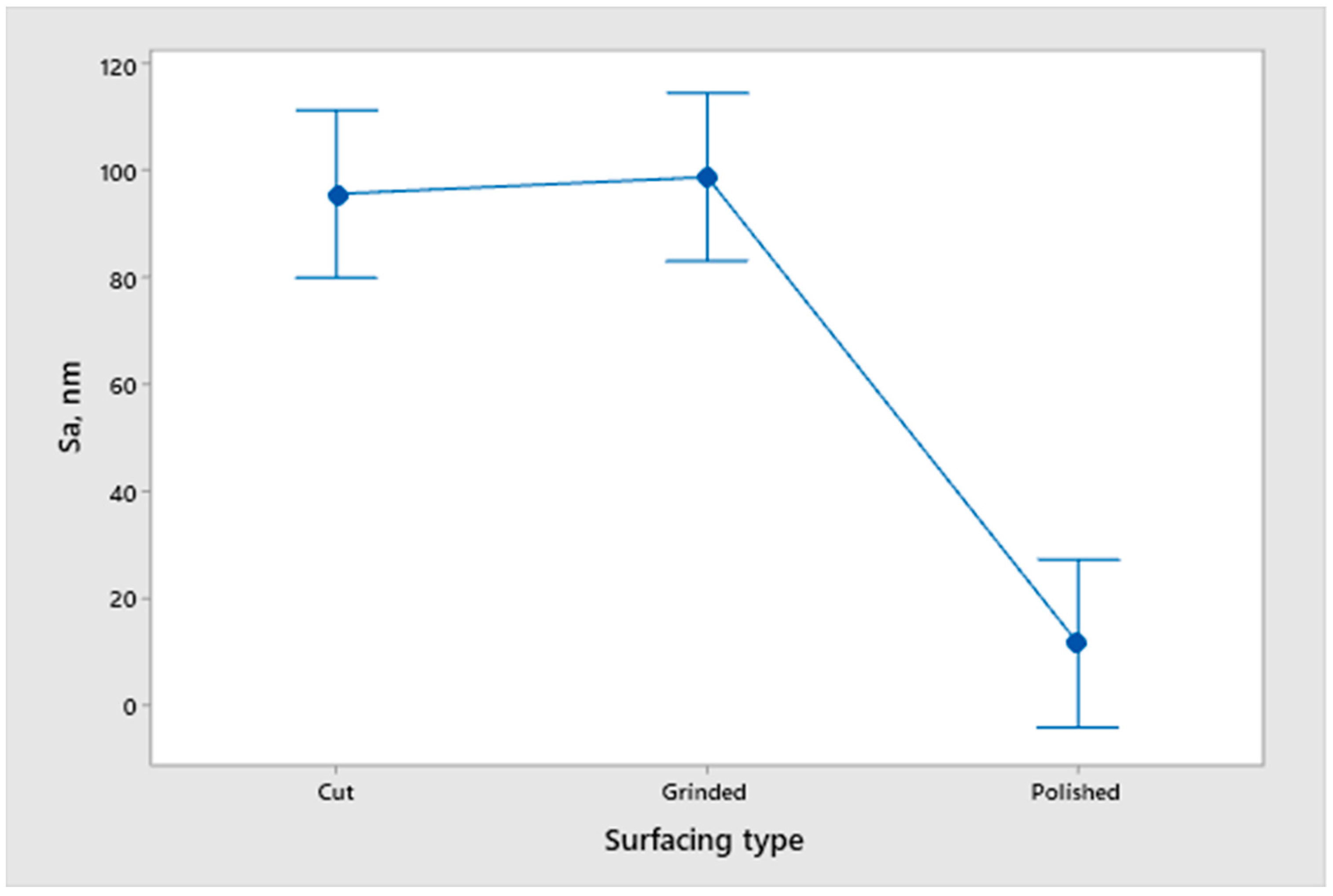
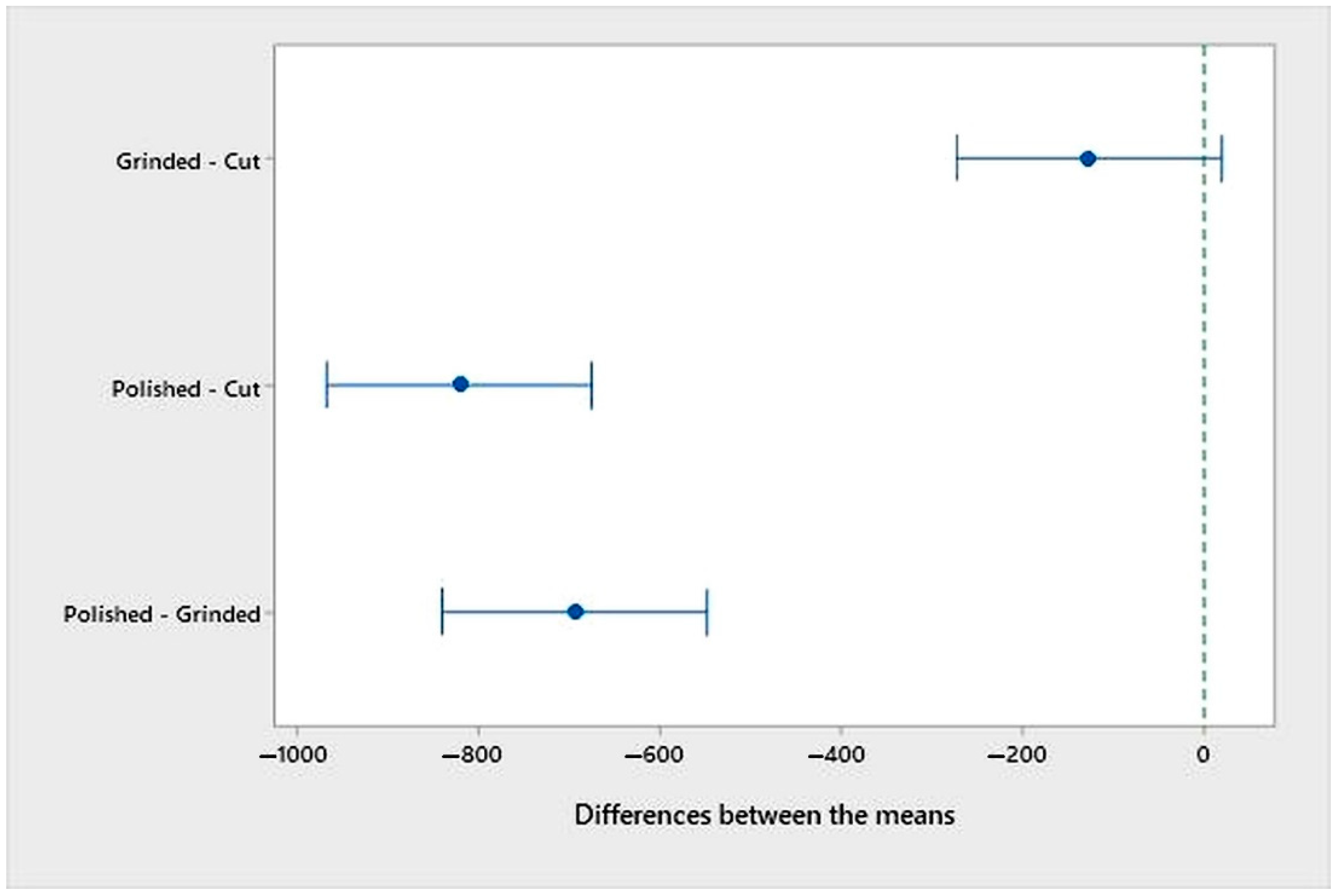
- An analysis of the variance (ANOVA method) and Multiple Comparisons t-Test (Fisher LSD) was conducted for the results of the Linear polarization method and the Tafel extrapolation method. It can be concluded that the deformation degree has no statistically significant influence on corrosion resistance and corrosion rate. Multiple Comparisons t-Test (Fisher LSD) showed that the mean of the corrosion resistance and the mean of the corrosion rate for the polished surfacing type are significantly different from the means for ground and cut surfacing types. The difference between cut surfacing types and ground surfacing types is not statistically significant for either corrosion resistance or corrosion rate when alpha is set at 0.05.
- Photographs of all surfaces before and after corrosion resistance testing do not show any visible changes on the surfaces.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danchenko, V.N. Metal Forming; NMetAU: Dnepropetrovsk, Ukraine, 2007. [Google Scholar]
- Kingkam, W.; Zhao, C.Z.; Li, H.; Zhang, H.X.; Li, Z.M. Hot Deformation and Corrosion Resistance of High-Strength Low-Alloy Steel. Acta Metall. Sin. 2019, 32, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Guo, W.; Liang, T. The effect of pre-deformation on corrosion resistance of the passive film formed on 2205 duplex stainless steel. J. Alloy. Compd. 2016, 686, 176–183. [Google Scholar] [CrossRef]
- Wu, G.; Singh, P.M. Effect of Plastic Deformation on Pitting Mechanism of SS304. Metall. Mater. Trans. A 2019, 50, 4750–4757. [Google Scholar] [CrossRef]
- Peguet, L.; Malki, B.; Baroux, B. Influence of cold working on the pitting corrosion resistance of stainless steels. Corros. Sci. 2007, 49, 1933–1948. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, T.-C.; Huang, R.-T.; Tsay, L.-W. Stress Corrosion Cracking Susceptibility of 304L Substrate and 308L Weld Metal Exposed to a Salt Spray. Materials 2017, 10, 187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, H.-M.; Chen, T.-C.; Chang, J.-M. The Effects of Cold Work on the Incipient Pitting Morphology Evolution of 304L Stainless Steels. Corrosion 2021, 77, 339–349. [Google Scholar] [CrossRef]
- Bösing, I.; Herrmann, M.; Bobrov, I.; Thöming, J.; Kuhfuss, B.; Epp, J.; Baune, M. The influence of microstructure deformation on the corrosion resistance of cold formed stainless steel. MATEC Web Conf. 2018, 190, 04002. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, R.K.; Das, G. Influence of Forging and Annealing on the Microstructure and Corrosion Behavior of Austenitic Stainless Steel. J. Inst. Eng. 2020, 101, 105–109. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 1–4. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F. Influence of Microstructure on Corrosion Behaviour of a Ni-Based Superalloy in 3.5 wt.% NaCl. Electrochim. Acta 2007, 52, 7193–7202. [Google Scholar] [CrossRef]
- Xu, C.C.; Hu, G. Effect of deformation-induced martensite on the pit propagation behavior of 304 stainless steel. Anti-Corros. Methods Mater. 2004, 51, 381–388. [Google Scholar] [CrossRef]
- Dacapo: Corrosion Resistance Table. Available online: https://dacapo.com/assets/download/tech/corrosion_resistance_table.pdf (accessed on 30 March 2021).
- Guanyu Tube: Corrosion Resistance Table of Stainless Steel Nickel Monel Inconel. Available online: https://tubingchina.com/Corrosion-Resistance-Table.htm (accessed on 30 March 2021).
- Evgeny, B.; Hughes, T.; Eskin, D. Effect of surface roughness on corrosion behaviour of low carbon steel in inhibited 4 M hydrochloric acid under laminar and turbulent flow conditions. Corros. Sci. 2016, 103, 196–205. [Google Scholar] [CrossRef]
- ASTM G5-94(2011)e1. Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. Annu. Book ASTM Stand. 2004, 3, 48–58. [Google Scholar]
- Tkacz, J.; Minda, J.; Fintová, S.; Wasserbauer, J. Comparison of Electrochemical Methods for the Evaluation of Cast AZ91 Magnesium Alloy. Materials 2016, 9, 925. [Google Scholar] [CrossRef] [Green Version]









| Chemical Element | C (%) | Mn (%) | P (%) | S (%) | Si (%) | Cr (%) | Ni (%) | Mo (%) |
|---|---|---|---|---|---|---|---|---|
| 0.08 | 2.00 | 0.045 | 0.030 | 0.75 | 1.06 | 8.05 | 0.29 |
| Deformation | Average Sample Height h, mm | True Strain φ | Final Outer Diameter d0, mm | Final Inner Diameter d1, mm | Forming Force F, kN |
|---|---|---|---|---|---|
| No Deformation | 40.0 | - | 20 | 7 | - |
| Stage 1 | 35.1 | 0.13 | 21 | 7 | 400 |
| Stage 2 | 29.9 | 0.29 | 23 | 8 | 500 |
| Stage 3 | 25.2 | 0.47 | 26 | 9 | 550 |
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| True Strain | φ0 = 0 | φ1 = 0.13 | φ2 = 0.29 | φ3 = 0.47 | ||||||||
| Surfacing | C | G | P | C | G | P | C | G | P | C | G | P |
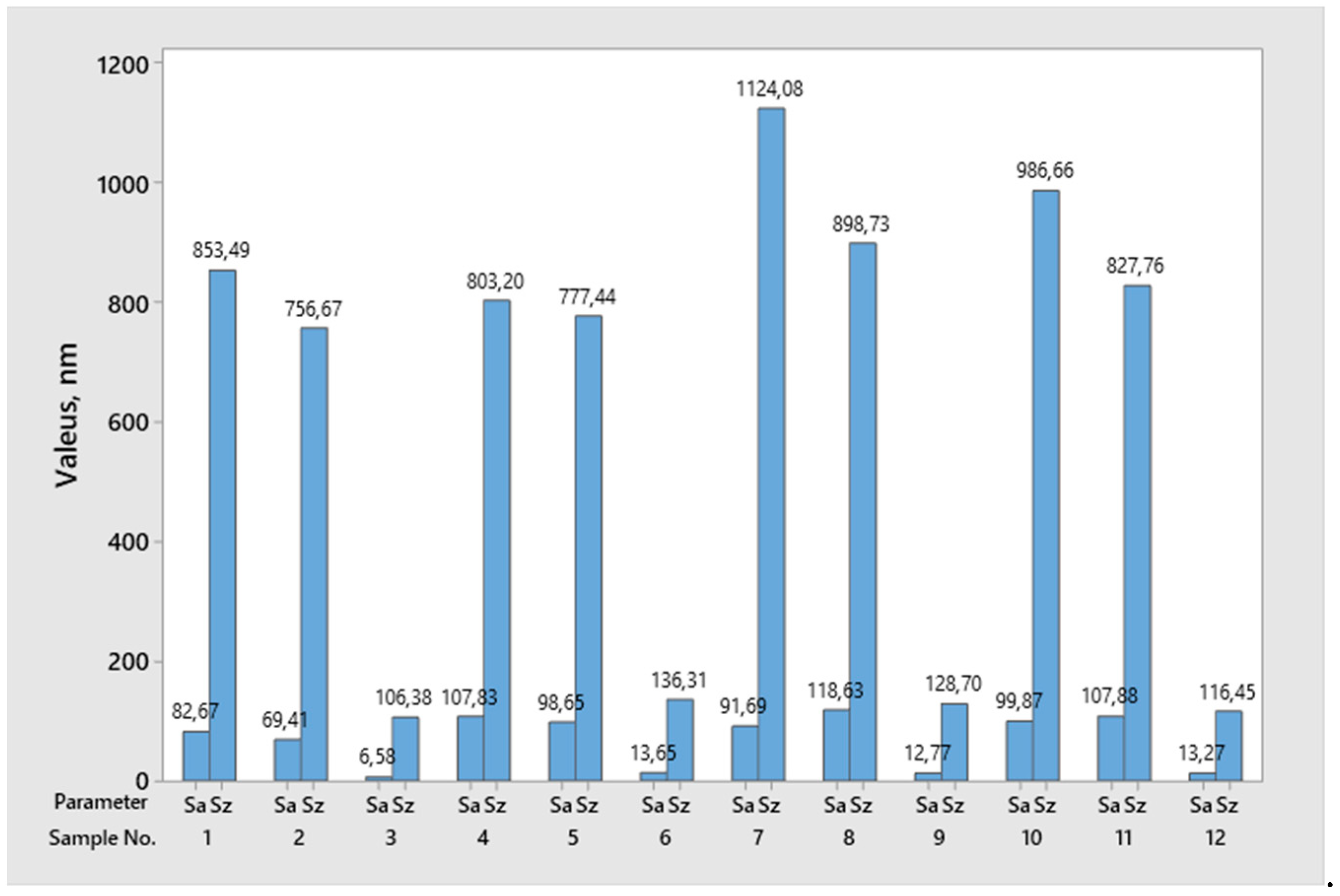
| Height Area Roughness Parameters | Sa | Sz | Sv | Sp | Sku | Ssk | Sq | |
|---|---|---|---|---|---|---|---|---|
| Surface Type | nm | nm | nm | nm | - | - | nm | |
| Cut Surface | Arithmetic Mean | 105.5 | 1265.9 | 628.5 | 637.5 | 3.9 | −0.2 | 202.2 |
| Standard Deviation | 10.8 | 692.5 | 258.3 | 485.4 | 2.1 | 0.6 | 162.8 | |
| Ground Surface | Arithmetic Mean | 98.6 | 730.1 | 314.9 | 415.3 | 2.7 | 0.1 | 119.3 |
| Standard Deviation | 21.1 | 86.1 | 53.1 | 63.0 | 0.3 | 0.2 | 24.3 | |
| Polished Surface | Arithmetic Mean | 11.6 | 122.0 | 54.4 | 67.5 | 3.5 | −0.1 | 14.6 |
| Standard Deviation | 3.3 | 13.2 | 11.3 | 12.4 | 0.7 | 0.5 | 4.0 | |
| Sample No. | Before Corrosion Resistance Testing | After Corrosion Resistance Testing | Sample No. | Before Corrosion Resistance Testing | After Corrosion Resistance Testing |
|---|---|---|---|---|---|
| 1 |  |  | 7 |  |  |
| 2 |  |  | 8 |  |  |
| 3 |  |  | 9 |  |  |
| 4 |  |  | 10 |  |  |
| 5 |  |  | 11 |  |  |
| 6 |  |  | 12 |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keran, Z.; Stojanović, I.; Novak, A.H.; Runje, B.; Razumić, A.; Vidović, D. Corrosion Resistance of Open Die Forged Austenitic Stainless Steel Samples Prepared with Different Surfaces. Sustainability 2021, 13, 5871. https://doi.org/10.3390/su13115871
Keran Z, Stojanović I, Novak AH, Runje B, Razumić A, Vidović D. Corrosion Resistance of Open Die Forged Austenitic Stainless Steel Samples Prepared with Different Surfaces. Sustainability. 2021; 13(11):5871. https://doi.org/10.3390/su13115871
Chicago/Turabian StyleKeran, Zdenka, Ivan Stojanović, Amalija Horvatić Novak, Biserka Runje, Andrej Razumić, and Denis Vidović. 2021. "Corrosion Resistance of Open Die Forged Austenitic Stainless Steel Samples Prepared with Different Surfaces" Sustainability 13, no. 11: 5871. https://doi.org/10.3390/su13115871
APA StyleKeran, Z., Stojanović, I., Novak, A. H., Runje, B., Razumić, A., & Vidović, D. (2021). Corrosion Resistance of Open Die Forged Austenitic Stainless Steel Samples Prepared with Different Surfaces. Sustainability, 13(11), 5871. https://doi.org/10.3390/su13115871








