Development of Growth Model for Grafted Hot Pepper Seedlings as Affected by Air Temperature and Light Intensity
Abstract
1. Introduction
2. Materials and Methods
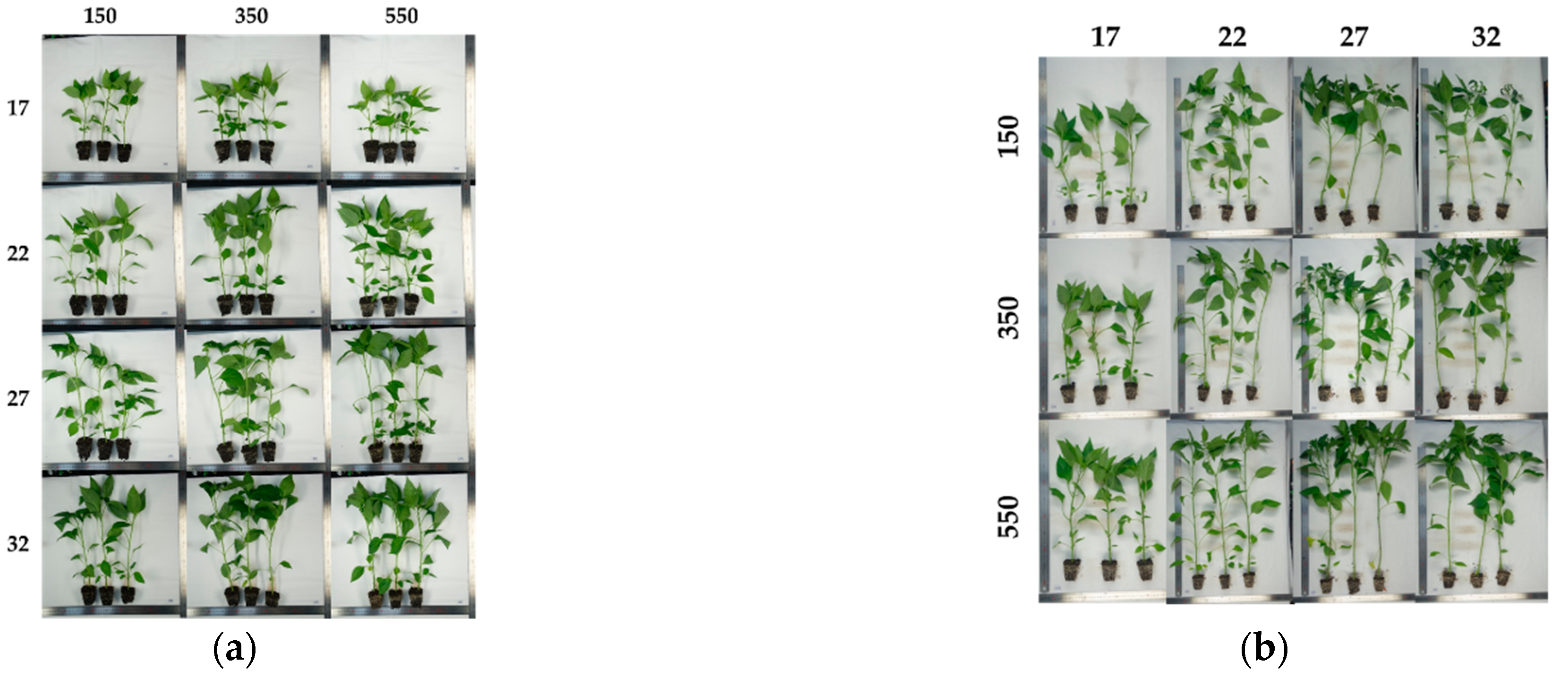
2.1. Plant Materials, Environments Treatment, and Growth Data Collections
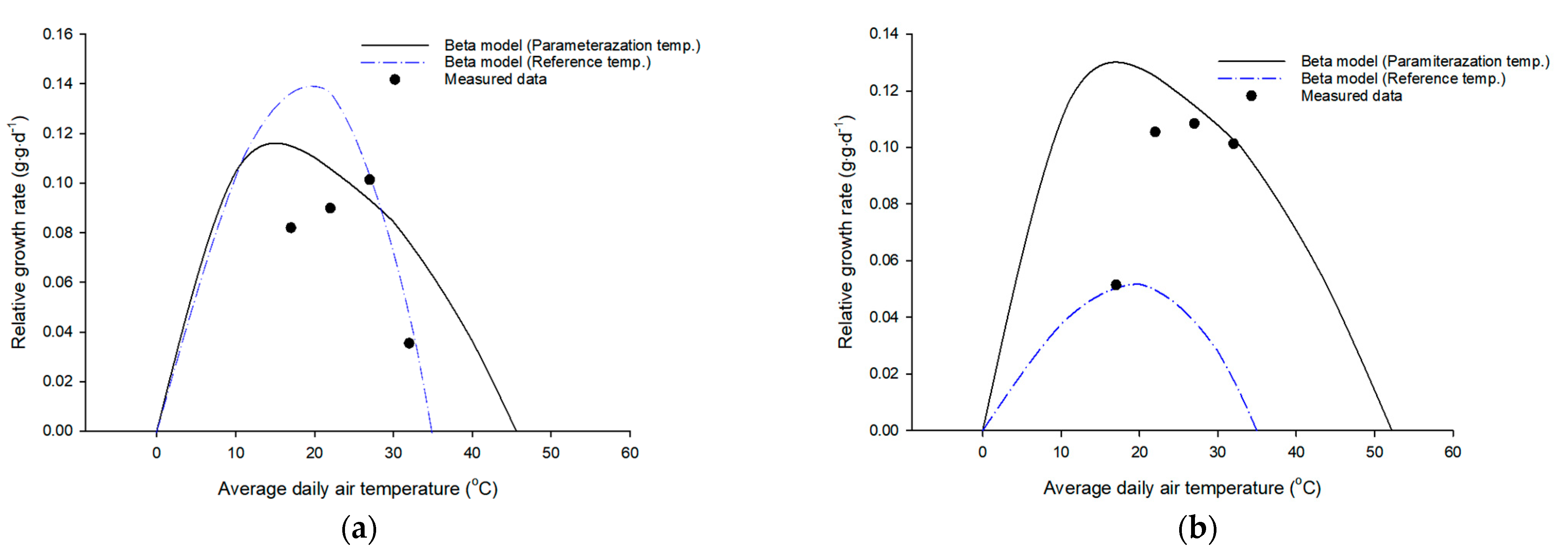
2.2. Growth Analysis and Development of Growth Models
2.3. Experiment Design and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.W.; Jang, Y.; Kim, Y.C.; Chun, C. Seedling raising technology of vegetable. In History of Korea Horticulture; Korea Society for Horticultural Science: Suwon, Korea, 2013; pp. 127–133. [Google Scholar]
- Lee, J.M.; Kuboda, C.; Tsao, S.J.; Bie, Z.; Hoyos Echevarria, P.; Morra, L.; Oda, M. Current status of fruit vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Bie, Z.; Nawaz, M.A.; Huang, Y.; Lee, J.M.; Golla, G. Introduction to vegetable grafting. In Vegetable Grafting, Principles and Practices; Colla, G., Alfocea, F.P., Schwarz, D., Eds.; CABI Publishing: Oxfordshire, UK, 2017; pp. 1–21. [Google Scholar]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci. Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- An, S. A Survey on Grafted Seedlings Production Practices and Investigation of Cucumber Scions and Figleaf Gourd Rootstocks Production Environment in a Plant Factory with Artificial Lighting; Wonkwang University: Iksan, Korea, 2021. [Google Scholar]
- Bisbis, M.B.; Gruda, N.S.; Blake, M.M. Securing horticulture in a changing climate-a mini review. Horticulturae 2019, 5, 56. [Google Scholar] [CrossRef]
- Sánchez-Molina, J.A.; Pérez, N.; Rodríguez, F.; Guzmán, J.L.; López, J.C. Support system for decision making in the management of the greenhouse environmental based on growth model for sweet pepper. Agric. Syst. 2015, 139, 144–152. [Google Scholar] [CrossRef]
- Marcelis, L.; Heuvelink, E.; Goudriaan, J. Modelling biomass production and yield of horticultural crops: A review. Sci. Hortic. 1998, 74, 83–111. [Google Scholar] [CrossRef]
- Heuvelink, E. Evaluation of a dynamic simulation model for tomato crop growth and development. Ann. Bot. 1999, 83, 413–422. [Google Scholar] [CrossRef]
- Rodríguez, F.; Berenguel, M.; Guzmán, J.; Ramírez-Arias, A. Modeling and Control of Greenhouse Crop Growth; Springer: Cham, Switzerland, 1999. [Google Scholar]
- Sánchez-Molina, J.; Reinoso, J.; Acién, F.; Rodríguez, F.; López, J. Development of a biomass-based system for nocturnal temperature and diurnal CO2 concentration control in greenhouses. Biomass Bioenerg. 2014, 67, 60–71. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Legris, M.; Nieto, C.; Sellaro, R.; Prat, S.; Casal, J.J. Perception and signalling of light and temperature cues in plants. Plant J. 2017, 90, 683–697. [Google Scholar] [CrossRef]
- Hunt, W.F.; Halligan, G. Growth and developmental responses of perennial ryegrass grown at constant temperature. I. Influence of light and temperature on growth and net assimilation. Aust. J. Plant Physiol. 1981, 8, 181–190. [Google Scholar] [CrossRef]
- Brewster, J.L. The response of growth rate to temperature in seedlings of several Allium crop species. Ann. Appl. Biol. 1979, 93, 351–357. [Google Scholar] [CrossRef]
- Brewster, J.L.; Sutherland, R.A. The rapid determination in controlled environments of parameters for predicting seedling growth rates in natural conditions. Ann. Appl. Biol. 1993, 122, 123–133. [Google Scholar] [CrossRef]
- Kittas, C.; Rigakis, N.; Katsoulas, N.; Bartzanas, T. Influence of shading screens on microclimate, growth and productivity of tomato. Acta Hortic. 2009, 807, 97–102. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C. Bell pepper (Capsicum annum L.) crop as affected by shade level: Microenvironment, plant Growth, leaf gas exchange, and leaf mineral nutrient concentration. HortScience 2013, 48, 175–182. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology. Ecophysiological and Stress Physiology of Functional Groups; Springer: Berlin, Germany, 1995. [Google Scholar]
- Potter, T.I.; Rood, S.B.; Zanewich, K.P. Light intensity, gibberellin content and the resolution of shoot growth in Brassica. Planta 1999, 207, 505–511. [Google Scholar] [CrossRef]
- Lee, J.M. Vegetable Sciences Crop Details; Hyangmunsa: Seoul, Korea, 2015; pp. 108–126. [Google Scholar]
- Heo, Y.; Park, E.G.; Son, B.G.; Choi, Y.W.; Lee, Y.J.; Park, Y.H.; Suh, J.M.; Cho, J.H.; Hong, C.O.; Lee, S.G.; et al. The Influence of abnormally high temperature on growth and yield of hot pepper (Capsicum annuum L.). J. Agric. Life Sci. 2013, 47, 9–15. [Google Scholar]
- Kim, S.H.; You, H.; Park, E.G.; Son, B.G.; Cho, Y.W.; Lee, Y.J.; Park, Y.H.; Suh, J.M.; Cho, J.H.; Hong, C.O.; et al. The influence of temperature, amino acid and polyamin on pollen germination of pepper (Capsicum annuum L.). J. Agric. Life Sci. 2013, 47, 1–8. [Google Scholar]
- Song, E.Y.; Moon, K.H.; Son, I.C.; Wi, S.H.; Kim, C.H.; Lim, C.K.; Oh, S.J. Impact of elevating temperature based on climate change scenarios on growth and fruit quality of red pepper. Kor. J. Agric. For. Meteorol. 2015, 17, 248–253. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, S.K.; Lee, H.J.; Lee, H.S.; Lee, J.H. Impact of moderate and extreme climate change scenarios on growth, morphological features, photosynthesis, and fruit production of hot pepper. Ecol. Evol. 2018, 8, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Bjökman, O. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Falk, S.; Leverenz, J.W.; Samuelsson, G.; Öquist, G. Changes in photosystem II fluorescence in Chlamydomonas reinhardtii exposed to increasing levels of irradiance in relationship to the photosynthetic response to light. Photosynth. Res. 1992, 31, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Grosse, H. Interactions between sucrose synthesis and CO2 fixation IV. Temperature-dependent adjustment of the relation between sucrose synthesis and CO2 fixation. J. Plant Physiol. 1988, 133, 392–400. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Öquist, G.; Hurry, V.M.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Bruggink, G.T.; Heuvelink, E. Influence of light on the growth of young tomato, cucumber and sweet pepper plants in the greenhouse: Effects on relative growth rate, net assimilation rate and leaf area ratio. Sci. Hortic. 1987, 31, 161–174. [Google Scholar] [CrossRef]
- Heuvelink, E. Influence of day and night temperature on the growth of young tomato plants. Sci. Hortic. 1989, 38, 11–22. [Google Scholar] [CrossRef]
- Heuvelink, E.; Dorais, M. Crop growth and yield. In Tomatoes, 1st ed.; Heuvelink, E., Ed.; CABI Publishing: Oxfordshire, UK, 2011; pp. 85–144. [Google Scholar]
- Lentz, W. Model applications in horticulture: A review. Sci. Hortic. 1998, 74, 151–174. [Google Scholar] [CrossRef]
- Nilwik, H.J.M. Growth analysis of sweet pepper (Capsicum annuum L.) 2. Interacting effects of irradiance, temperature and plant age in controlled conditions. Ann. Bot. 1981, 48, 137–146. [Google Scholar] [CrossRef]
- Bruggink, G.T. Influence of light on the growth of young tomato, cucumber and sweet pepper plants in the greenhouse: Calculating the effect of differences in light integral. Sci. Hortic. 1987, 31, 175–183. [Google Scholar] [CrossRef]
- Buwalda, F.; Van Henten, E.J.; De Gelder, A.; Bontsema, J.; Hemming, J. Toward an optimal control strategy for sweet pepper cultivation 1. A dynamic crop model. Acta Hortic. 2006, 718, 367–374. [Google Scholar] [CrossRef]


| Air Temp (A) (°C) | PPF (B) (μmol·m−2·s−1) | Plant Height (cm/Plant) | No. of Leaves (/Plant) | Leaf Area (cm2/Plant) | Fresh Weight (g/Plant) | Dry Weight (g/Plant) |
|---|---|---|---|---|---|---|
| 17 | 150 | 31.8 d z | 12.8 f | 240.2 e | 8.7 f | 0.71 f |
| 350 | 32.2 d | 13.8 fe | 225.4 e | 9.4 ef | 0.89 ef | |
| 550 | 30.1 d | 13.3 fe | 215.1 e | 9.8 def | 1.01 ef | |
| 22 | 150 | 49.5 c | 17.8 de | 326.0 cd | 12.0 de | 1.07 ef |
| 350 | 56.9 b | 22.2 cd | 393.6 bc | 18.2 bc | 1.87 cd | |
| 550 | 60.0 b | 23.4 c | 366.9 cd | 17.9 bc | 2.15 bc | |
| 27 | 150 | 57.0 b | 31.2 b | 382.9 bc | 17.1 c | 1.64 d |
| 350 | 59.0 b | 33.6 b | 406.3 abc | 19.0 abc | 1.94 cd | |
| 550 | 64.7 a | 34.1 b | 416.6 abc | 21.2 a | 2.44 ab | |
| 32 | 150 | 47.2 c | 30.0 b | 291.1 de | 12.3 d | 1.22 e |
| 350 | 58.6 b | 34.3 b | 469.8 ab | 20.6 ab | 2.11 bc | |
| 550 | 58.8 b | 39.0 a | 491.9 a | 20.6 ab | 2.68 a | |
| Significance y | ||||||
| A | *** | *** | *** | *** | *** | |
| B | *** | ** | ** | *** | *** | |
| A × B | *** | NS | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwack, Y.; An, S.; Kim, S.K. Development of Growth Model for Grafted Hot Pepper Seedlings as Affected by Air Temperature and Light Intensity. Sustainability 2021, 13, 5895. https://doi.org/10.3390/su13115895
Kwack Y, An S, Kim SK. Development of Growth Model for Grafted Hot Pepper Seedlings as Affected by Air Temperature and Light Intensity. Sustainability. 2021; 13(11):5895. https://doi.org/10.3390/su13115895
Chicago/Turabian StyleKwack, Yurina, Sewoong An, and Sung Kyeom Kim. 2021. "Development of Growth Model for Grafted Hot Pepper Seedlings as Affected by Air Temperature and Light Intensity" Sustainability 13, no. 11: 5895. https://doi.org/10.3390/su13115895
APA StyleKwack, Y., An, S., & Kim, S. K. (2021). Development of Growth Model for Grafted Hot Pepper Seedlings as Affected by Air Temperature and Light Intensity. Sustainability, 13(11), 5895. https://doi.org/10.3390/su13115895








