Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains
Abstract
1. Introduction
2. Animal Wastes
3. Global Challenges versus Local Solutions: Case Studies
4. Environmental Legislation: Global Trends
5. Phosphorus Recovery Processes
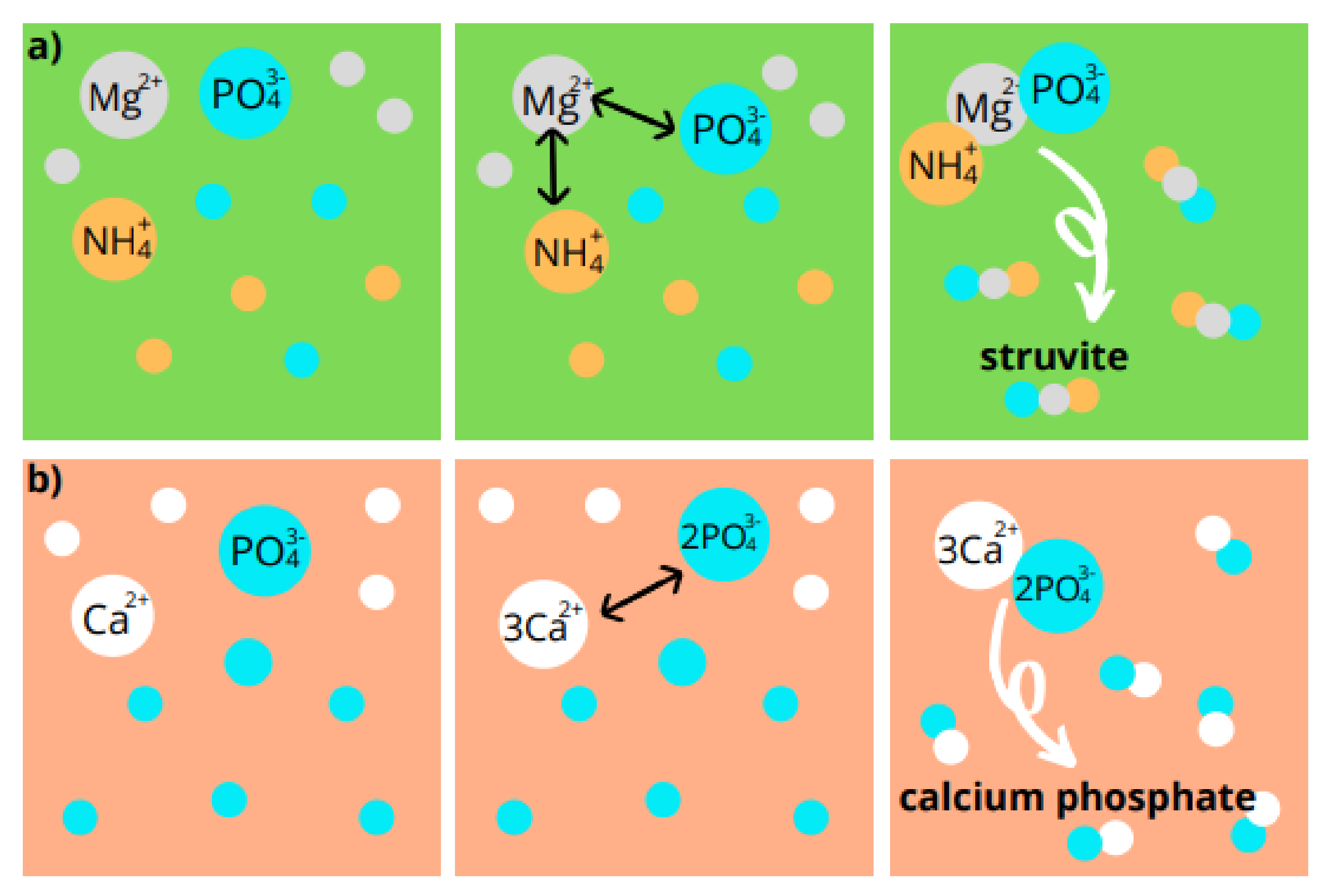
5.1. Chemical Precipitation
5.2. Crystallization of Struvite
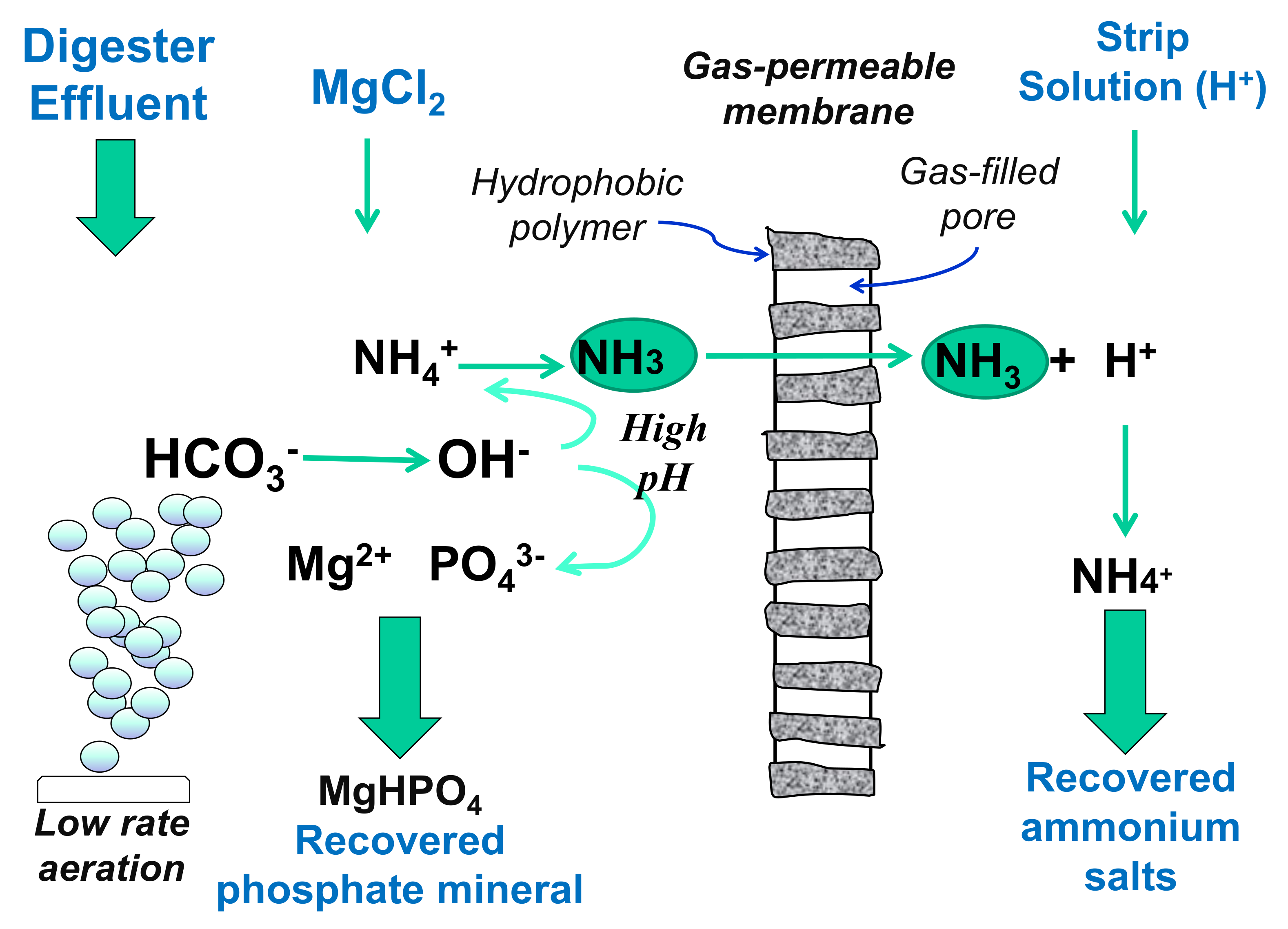
5.3. Newberyite Recovery
5.4. Thermal Treatment
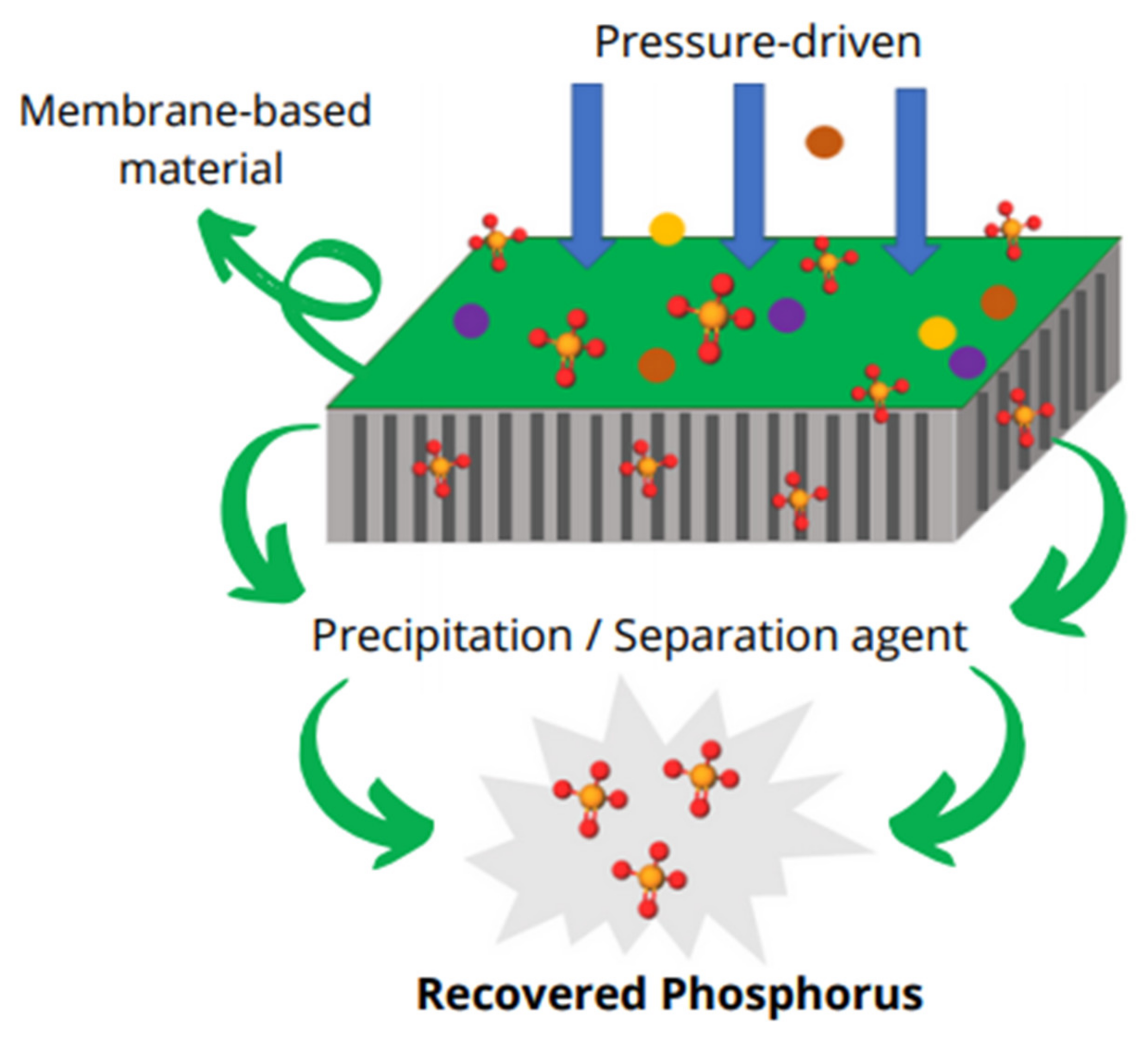
5.5. Nanofiltration (NF) Membrane Separation
5.6. Adsorption by Ion Exchange
5.7. Electro-Based Technologies: Coagulation and Flocculation
5.8. Biological Processes
5.8.1. Phosphorus Recovery by Composting
5.8.2. Microalgae
5.8.3. Enhanced Biological Phosphorus Removal
6. Technology Comparison, How to Choose Most Appropriate?
7. Life Cycle Assessment of the Recovery Systems and the Circular Economy
8. Future Perspectives: Technical Implications for Process
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Finzi, A.; Mattachini, G.; Lovarelli, D.; Riva, E.; Provolo, G. Technical, economic, and environmental assessment of a collective integrated treatment system for energy recovery and nutrient removal from livestock manure. Sustainability 2020, 12, 2756. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef] [PubMed]
- Sarvajayakesavalu, S.; Lu, Y.; Withers, P.J.A.; Pavinato, P.S.; Pan, G.; Chareonsudjai, P. Phosphorus recovery: A need for an integrated approach. Ecosyst. Health Sustain. 2018, 4, 48–57. [Google Scholar] [CrossRef]
- Ohtake, H.; Tsuneda, S. Phosphorus Recovery and Recycling; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Helin, J.; Weikard, H.P. A model for estimating phosphorus requirements of world food production. Agric. Syst. 2019, 176, 102666. [Google Scholar] [CrossRef]
- Ulrich, A.E.; Frossard, E. On the history of a reoccurring concept: Phosphorus scarcity. Sci. Total Environ. 2014, 490, 694–707. [Google Scholar] [CrossRef]
- FAO. World Fertilizer Outlook and Trends to 2022; FAO: Rome, Italy, 2019; Available online: http://www.fao.org/3/ca6746en/ca6746en.pdf (accessed on 24 May 2021).
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 2019, 6, 1–27. [Google Scholar] [CrossRef]
- Selman, M.; Greenhalgh, S. Eutrophication: Policies, Actions, and Strategies to Address Nutrient Pollution; World Resources Institute: Washington, DC, USA, 2009. [Google Scholar]
- Muisa, N.; Nhapi, I.; Ruziwa, W.; Manyuchi, M.M. Utilization of alum sludge as adsorbent for phosphorus removal in municipal wastewater: A review. J. Water Process Eng. 2020, 35, 101187. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US EPA). Office of Water and Office of Research and Development. National Rivers and Streams (841/R-16/007); US EPA: Washington, DC, USA, 2016. Available online: https://www.epa.gov/sites/production/files/2016-03/documents/nrsa_0809_march_2_final.pdf (accessed on 24 May 2021).
- Ministry of Ecology and Environment. Report on the State of the Ecology and Environment in China 2017; China Ministry of Ecology and Environment: Beijing, China, 2018. Available online: https://www.greengrowthknowledge.org/sites/default/files/downloads/policy-database/CHINA%29%202017%20Report%20on%20the%20Ecology%20and%20the%20Environment%20in%20China.pdf (accessed on 24 May 2021).
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 1–30. [Google Scholar] [CrossRef]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human impact on erodable phosphorus and eutrophication: A global perspective. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling—Closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef]
- Jansson, T.; Andersen, H.E.; Gustafsson, B.G.; Hasler, B.; Höglind, L.; Choi, H. Baltic Sea eutrophication status is not improved by the first pillar of the European Union Common Agricultural Policy. Reg. Environ. Chang. 2019, 19, 2465–2476. [Google Scholar] [CrossRef]
- Roy, E.D. Phosphorus recovery and recycling with ecological engineering: A review. Ecol. Eng. 2017, 98, 213–227. [Google Scholar] [CrossRef]
- Cieślik, B.; Konieczka, P. A review of phosphorus recovery methods at various steps of wastewater treatment and sewage sludge management. The concept of “no solid waste generation” and analytical methods. J. Clean. Prod. 2017, 142, 1728–1740. [Google Scholar] [CrossRef]
- Vanotti, M.B.; García-González, M.C.; Szögi, A.A.; Harrison, J.H.; Smith, W.B.; Moral, R. Removing and Recovering Nitrogen and Phosphorus from Animal Manure. In Animal Manure: Production, Characteristics, Environmental Concerns, and Management; Waldrip, H.M., Pagliari, P.H., He, Z., Eds.; American Society of Agronomy: Madison, WI, USA, 2020; Volume 67, pp. 275–321. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development (OECD); Food and Agricultural Organization (FAO). Agricultural Outlook 2019–2028; FAO: Rome, Italy, 2019; p. 44. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K.; et al. Biological nutrient removal and recovery from solid and liquid livestock manure: Recent advance and perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef]
- Vanden Nest, T.; Amery, F.; Fryda, L.; Boogaerts, C.; Bilbao, J.; Vandecasteele, B. Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci. Total Environ. 2021, 750, 141699. [Google Scholar] [CrossRef]
- Sheppard, S.C. Elemental Composition of Swine Manure from 1997 to 2017: Changes Relevant to Environmental Consequences. J. Environ. Qual. 2019, 48, 164–170. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Castillo, A.; Pagés-Díaz, J.; Guerrero, L. Carbon, nitrogen and phosphorus recovery from liquid swine wastes: A review. J. Chem. Technol. Biotechnol. 2020, 95, 2335–2347. [Google Scholar] [CrossRef]
- Hadin, Å.; Eriksson, O. Horse manure as feedstock for anaerobic digestion. Waste Manag. 2016, 56, 506–518. [Google Scholar] [CrossRef]
- Karunanithi, R.; Szögi, A.A.; Bolan, N.; Naidu, R.; Loganathan, P.; Hunt, P.G.; Vanotti, M.B.; Saint, C.P.; Ok, Y.S.; Krishnamoorthy, S. Phosphorus Recovery and Reuse from Waste Streams; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; p. 131. [Google Scholar] [CrossRef]
- Lemming, C.; Oberson, A.; Magid, J.; Bruun, S.; Scheutz, C.; Frossard, E.; Jensen, L.S. Residual phosphorus availability after long-term soil application of organic waste. Agric. Ecosyst. Environ. 2019, 270–271, 65–75. [Google Scholar] [CrossRef]
- Li, B.; Dinkler, K.; Zhao, N.; Sobhi, M.; Merkle, W.; Liu, S.; Dong, R.; Oechsner, H.; Guo, J. Influence of anaerobic digestion on the labile phosphorus in pig, chicken, and dairy manure. Sci. Total Environ. 2020, 737, 140234. [Google Scholar] [CrossRef]
- Grigatti, M.; Boanini, E.; Bolzonella, D.; Sciubba, L.; Mancarella, S.; Ciavatta, C.; Marzadori, C. Organic wastes as alternative sources of phosphorus for plant nutrition in a calcareous soil. Waste Manag. 2019, 93, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Miele, M.; Steinmetz, R.L.R. Advanced swine manure treatment and utilization in Brazil. Bioresour. Technol. 2009, 100, 5485–5489. [Google Scholar] [CrossRef] [PubMed]
- Magrí, A.; Carreras-Sempere, M.; Biel, C.; Colprim, J. Recovery of phosphorus from waste water profiting from biological nitrogen treatment: Upstream, concomitant or downstream precipitation alternatives. Agronomy 2020, 10, 1039. [Google Scholar] [CrossRef]
- Djodjic, F.; Montas, H.; Shirmohammadi, A.; Bergström, L.; Ulén, B. A Decision Support System for Phosphorus Management at a Watershed Scale. J. Environ. Qual. 2002, 31, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D. Soil phosphorus saturation ratio for risk assessment in land use systems. Front. Environ. Sci. 2014, 2, 1–4. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Nicoloso, R.d.S.; Mumbach, G.L.; de Souza Junior, A.A.; Dall’orsoletta, D.J.; Schmitt, D.E.; Smyth, T.J. Establishing Environmental soil phosphorus thresholds to decrease the risk of losses to water in soils from Rio Grande do Sul, brazil. Rev. Bras. Cienc. Solo 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Kleinman, P.J.A. The Persistent Environmental Relevance of Soil Phosphorus Sorption Saturation. Curr. Pollut. Rep. 2017, 3, 141–150. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhu, J.G.; Wang, X.R.; Wu, Z.H.; Zhang, Z. Case study on nitrogen and phosphorus emissions from paddy field in Taihu region. Environ. Geochem. Health 2004, 26, 209–219. [Google Scholar] [CrossRef]
- Zhang, B.; Shrestha, N.K.; Rudra, R.; Shukla, R.; Daggupati, P.; Goel, P.K.; Dickinson, W.T.; Allataifeh, N. Threshold storm approach for locating phosphorus problem areas: An application in three agricultural watersheds in the Canadian Lake Erie basin. J. Great Lakes Res. 2020, 46, 132–143. [Google Scholar] [CrossRef]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U. S. freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef]
- Pretty, J.N.; Mason, C.F.; Nedwell, D.B.; Hine, R.E.; Leaf, S.; Dils, R. Environmental costs of freshwater eutrophication in England and Wales. Environ. Sci. Technol. 2003, 37, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, H.; Boekhold, A. Phosphorus saturation in soils and groundwaters. Land Degrad. Dev. 1993, 4, 233–243. [Google Scholar] [CrossRef]
- Jia, W.; Yan, Z.; Chadwick, D.R.; Kang, L.; Duan, Z.; Bai, Z.; Chen, Q. Integrating soil testing phosphorus into Environmentally based manure management in peri-urban regions: A case study in the Beijing area. Agric. Ecosyst. Environ. 2015, 209, 47–59. [Google Scholar] [CrossRef]
- Sharma, L.K.; Bali, S.K.; Zaeen, A.A. A case study of potential reasons of increased soil phosphorus levels in the Northeast United States. Agronomy 2017, 7, 85. [Google Scholar] [CrossRef]
- Weikard, H.P.; Seyhan, D. Distribution of phosphorus resources between rich and poor countries: The effect of recycling. Ecol. Econ. 2009, 68, 1749–1755. [Google Scholar] [CrossRef]
- Powers, S.M.; Chowdhury, R.B.; MacDonald, G.K.; Metson, G.S.; Beusen, A.H.W.; Bouwman, A.F.; Hampton, S.E.; Mayer, B.K.; McCrackin, M.L.; Vaccari, D.A. Global Opportunities to Increase Agricultural Independence Through Phosphorus Recycling. Earth’s Futur. 2019, 7, 370–383. [Google Scholar] [CrossRef]
- Hanserud, O.S.; Brod, E.; Øgaard, A.F.; Müller, D.B.; Brattebø, H. A multi-regional soil phosphorus balance for exploring secondary fertilizer potential: The case of Norway. Nutr. Cycl. Agroecosyst. 2016, 104, 307–320. [Google Scholar] [CrossRef]
- Bomans, E.; Fransen, K.; Gobin, A.; Mertens, J.; Michiels, P.; Vandendriessche, H.; Vogels, N. Addressing Phosphorus Related Problems in Farm Practice: Final Report to the European Comission; European Union: Brussels, Belgium, 2005; Available online: https://ec.europa.eu/environment/natres/pdf/phosphorus/AgriPhosphorusReport%20final.pdf (accessed on 24 May 2021).
- Kazadi Mbamba, C.; Lindblom, E.; Flores-Alsina, X.; Tait, S.; Anderson, S.; Saagi, R.; Batstone, D.J.; Gernaey, K.V.; Jeppsson, U. Plant-wide model-based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Res. 2019, 155, 12–25. [Google Scholar] [CrossRef]
- Melia, P.M.; Cundy, A.B.; Sohi, S.P.; Hooda, P.S.; Busquets, R. Trends in the recovery of phosphorus in bioavailable forms from wastewater. Chemosphere 2017, 186, 381–395. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems. Front. Environ. Sci. 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of phosphorus recovery from wastewater by crystallization processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Fundação Estadual de Engenharia do Meio Ambiente (FEEMA). Norma Técnica 202.R-10; FEEMA: Rio de Janeiro, Brazil, 1986. [Google Scholar]
- Conselho Estadual do Meio Ambiente (Consema). Resolução n° 128; Consema: Rio Grande do Sul, Brazil, 2006. Available online: https://www.sema.rs.gov.br/upload/arquivos/201611/30155644-resolucao-128-06-efluentes.pdf (accessed on 24 May 2021).
- Assembléia Legislativa de Santa Catarina (ALSC). Lei Estadual no14.675—Código Estadual do Meio Ambiente; Governo do Estado de Santa Catarina: Santa Catarina, Brazil, 2009.
- Conselho Nacional do Meio Ambiente (Conama). Resolução n° 430; Conama: Brasilia, Brazil, 2011. Available online: http://www2.mma.gov.br/port/conama/legiabre.cfm?codlegi=646 (accessed on 24 May 2021).
- Luca, L.; Pricopie, A.; Barbu, M.; Ifrim, G.; Caraman, S. Control strategies of phosphorus removal in wastewater treatment plants. Control Comput. 2019, 74, 236–241. [Google Scholar] [CrossRef]
- Metcalf & Eddy Inc. Wastewater Engineering: Treatment and Reuse, 4th ed.; Tchobanoglous, G., Burton, F.L., Stensel, D.H., Eds.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- United States Environmental Protection Agency (US EPA). Discharge Monitoring Report (DMR) Pollutant Loading Tool; US EPA: Washington, DC, USA, 2015.
- Bashar, R.; Gungor, K.; Karthikeyan, K.G.; Barak, P. Cost effectiveness of phosphorus removal processes in municipal wastewater treatment. Chemosphere 2018, 197, 280–290. [Google Scholar] [CrossRef]
- Hong Li, R.; Mao Wang, X.; Yan Li, X. A membrane bioreactor with iron dosing and acidogenic co-fermentation for enhanced phosphorus removal and recovery in wastewater treatment. Water Res. 2018, 129, 402–412. [Google Scholar] [CrossRef]
- European Commission. On the Review of the List of Critical Raw Materials for the EU and the Implementation of the Raw Materials Initiative; European Union: Brussels, Belgium, 2014; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52014DC0297&from=EN (accessed on 24 May 2021).
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef]
- Guedes, P.; Couto, N.; Mateus, E.P.; Ribeiro, A.B. Phosphorus Recovery in Sewage Sludge by Electrokinetic Based Technologies: A Multivariate and Circular Economy View. Waste Biomass Valorization 2017, 8, 1587–1596. [Google Scholar] [CrossRef]
- Günther, S.; Grunert, M.; Müller, S. Overview of recent advances in phosphorus recovery for fertilizer production. Eng. Life Sci. 2018, 18, 434–439. [Google Scholar] [CrossRef]
- Jafarinejad, S. Forward osmosis membrane technology for nutrient removal/recovery from wastewater: Recent advances, proposed designs, and future directions. Chemosphere 2021, 263, 128116. [Google Scholar] [CrossRef]
- Lee, C.G.; Alvarez, P.J.J.; Kim, H.G.; Jeong, S.; Lee, S.; Lee, K.B.; Lee, S.H.; Choi, J.W. Phosphorous recovery from sewage sludge using calcium silicate hydrates. Chemosphere 2018, 193, 1087–1093. [Google Scholar] [CrossRef]
- Volpin, F.; Chekli, L.; Phuntsho, S.; Cho, J.; Ghaffour, N.; Vrouwenvelder, J.S.; Kyong Shon, H. Simultaneous phosphorous and nitrogen recovery from source-separated urine: A novel application for fertiliser drawn forward osmosis. Chemosphere 2018, 203, 482–489. [Google Scholar] [CrossRef]
- Suzuki, K.; Tanaka, Y.; Kuroda, K.; Hanajima, D.; Fukumoto, Y. Recovery of phosphorous from swine wastewater through crystallization. Bioresour. Technol. 2005, 96, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Meesschaert, B.; Monballiu, A.; Ghyselbrecht, K.; Van Goethem, C.; Halleux, H.; Pinoy, L. Pilot scale recovery of phosphorus as calcium phosphate from nitrified UASB effluent of a potato processor and subsequent reuse in the wet process for phosphoric acid production. J. Environ. Chem. Eng. 2020, 8, 104593. [Google Scholar] [CrossRef]
- Monballiu, A.; Ghyselbrecht, K.; Pinoy, L.; Meesschaert, B. Phosphorous recovery as calcium phosphate from UASB effluent after nitrification with or without subsequent denitrification. J. Environ. Chem. Eng. 2020, 8. [Google Scholar] [CrossRef]
- Tran, A.T.K.; Zhang, Y.; De Corte, D.; Hannes, J.B.; Ye, W.; Mondal, P.; Jullok, N.; Meesschaert, B.; Pinoy, L.; Van Der Bruggen, B. P-recovery as calcium phosphate from wastewater using an integrated selectrodialysis/crystallization process. J. Clean. Prod. 2014, 77, 140–151. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Kunz, A.; Steinmetz, R.L.R.; Szögi, A.A.; Vanotti, M.B.; Flores, É.M.D.M.; Dressler, V.L. Chemical phosphorus removal: A clean strategy for piggery wastewater management in Brazil. Environ. Technol. 2012, 33, 37–41. [Google Scholar] [CrossRef]
- Viancelli, A.; Kunz, A.; Fongaro, G.; Kich, J.D. Pathogen inactivation and the chemical removal of phosphorus from swine wastewater. Water Air Soil Pollut. 2015, 226, 263. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szögi, A.A.; Hunt, P.G. Extraction of soluble phosphorus from swine wastewater. Trans. ASABE. 2003, 46, 1665–1674. [Google Scholar] [CrossRef]
- Braga, R.R. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent. Mater. 2019, 35, 3–14. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Les orthophosphates de calcium (CaPO4): Occurrence et propriétés. Morphologie 2017, 101, 125–142. [Google Scholar] [CrossRef]
- Suzin, L.; Antes, F.G.; Bedendo, G.C.; Bortoli, M.; Kunz, A. Chemical removal of phosphorus from swine effluent: The impact of previous effluent treatment technologies on process efficiency. Water Air Soil Pollut. 2018, 229, 1–9. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite from farm, municipal and industrial waste: Feedstock suitability, methods and pre-treatments. Waste Manag. 2016, 49, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Di Gesù, R.; Gualandi, C.; Zucchelli, A.; Liguori, A.; Paltrinieri, L.; Focarete, M.L. Biodegradable electrospun fibers enriched with struvite crystal seeds for the recovery of phosphorous and nitrogen. Eur. Polym. J. 2020, 122, 109389. [Google Scholar] [CrossRef]
- Shaddel, S.; Grini, T.; Andreassen, J.P.; Østerhus, S.W.; Ucar, S. Crystallization kinetics and growth of struvite crystals by seawater versus magnesium chloride as magnesium source: Towards enhancing sustainability and economics of struvite crystallization. Chemosphere 2020, 256, 126968. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 142–156. [Google Scholar] [CrossRef]
- Shu, L.; Schneider, P.; Jegatheesan, V.; Johnson, J. An economic evaluation of phosphorus recovery as struvite from digester supernatant. Bioresour. Technol. 2006, 97, 2211–2216. [Google Scholar] [CrossRef]
- Moulessehoul, A.; Gallart-Mateu, D.; Harrache, D.; Djaroud, S.; de la Guardia, M.; Kameche, M. Conductimetric study of struvite crystallization in water as a function of pH. J. Cryst. Growth 2017, 471, 42–52. [Google Scholar] [CrossRef]
- Shih, K.; Yan, H. The Crystallization of Struvite and Its Analog (K-Struvite) From Waste Streams for Nutrient Recycling. In Environmental Materials and Waste: Resource Recovery and Pollution Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 665–686. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef]
- Wang, J.; Ye, X.; Zhang, Z.; Ye, Z.L.; Chen, S. Selection of cost-effective magnesium sources for fluidized struvite crystallization. J. Environ. Sci. 2018, 70, 144–153. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, D.D.; Li, J.; Guo, G.; Tang, S. Phosphate recovery from swine wastewater using plant ash in chemical crystallization. J. Clean. Prod. 2017, 168, 338–345. [Google Scholar] [CrossRef]
- Li, B.; Huang, H.M.; Boiarkina, I.; Yu, W.; Huang, Y.F.; Wang, G.Q.; Young, B.R. Phosphorus recovery through struvite crystallisation: Recent developments in the understanding of operational factors. J. Environ. Manag. 2019, 248, 109254. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Ilhan, F.; Kocak, E.; Akbin, H.M. Feasibility of struvite recovery process for fertilizer industry: A study of financial and economic analysis. J. Clean. Prod. 2017, 152, 88–102. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Li, B.; Zhang, D.; Zhao, N.; Tang, S. Comparison of different K-struvite crystallization processes for simultaneous potassium and phosphate recovery from source-separated urine. Sci. Total Environ. 2019, 651, 787–795. [Google Scholar] [CrossRef]
- Xu, K.; Wang, C.; Liu, H.; Qian, Y. Simultaneous removal of phosphorus and potassium from synthetic urine through the precipitation of magnesium potassium phosphate hexahydrate. Chemosphere 2011, 84, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Tarragó, E.; Ruscalleda, M.; Colprim, J.; Balaguer, M.D.; Puig, S. Towards a methodology for recovering K-struvite from manure. J. Chem. Technol. Biotechnol. 2018, 93, 1558–1562. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, C.; Li, J.; Cheng, X.; Wang, C. Removal and recovery of N, P and K from urine via ammonia stripping and precipitations of struvite and struvite-K. Water Sci. Technol. 2017, 75, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Wilsenach, J.A.; Schuurbiers, C.A.H.; van Loosdrecht, M.C.M. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szögi, A.A.; Dub, P.J. Systems and Methods for Recovering Ammonium and Phosphorus from Liquid Effluents. U.S. Patent 9,926,213 B2, 27 March 2018. [Google Scholar]
- Vanotti, M.B.; Dube, P.J.; Szögi, A.A.; García-González, M.C. Recovery of ammonia and phosphate minerals from swine wastewater using gas-permeable membranes. Water Res. 2017, 112, 137–146. [Google Scholar] [CrossRef]
- Antonini, S.; Nguyen, P.T.; Arnold, U.; Eichert, T.; Clemens, J. Solar thermal evaporation of human urine for nitrogen and phosphorus recovery in Vietnam. Sci. Total Environ. 2012, 414, 592–599. [Google Scholar] [CrossRef]
- Arnout, S.; Nagels, E. Modelling thermal phosphorus recovery from sewage sludge ash. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 55, 26–31. [Google Scholar] [CrossRef]
- Chaharsooghi, S.K.; Honarvar, M.; Modarres, M. A multi-stage stochastic programming model for dynamic pricing and lead time decisions in multi-class make-to-order firm. Sci. Iran. 2011, 18, 711–721. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Q.; Xu, J.; Gao, H.; Yan, J. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 2019, 1, 99–115. [Google Scholar] [CrossRef]
- Leng, L.; Bogush, A.A.; Roy, A.; Stegemann, J.A. Characterisation of ashes from waste biomass power plants and phosphorus recovery. Sci. Total Environ. 2019, 690, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Bergfeldt, B.; Morgano, M.T.; Leibold, H.; Richter, F.; Stapf, D. Recovery of phosphorus and other nutrients during pyrolysis of chicken manure. Agriculture 2018, 8, 187. [Google Scholar] [CrossRef]
- Więckol-ryk, A.; Białecka, B.; Cempa, M.; Adamczyk, Z. Optimization of chicken manure combustion parameters in the aspect of phosphorus recovery. Int. J. Recycl. Org. Waste Agric. 2020, 273–285. [Google Scholar] [CrossRef]
- Lang, Q.; Zhang, B.; Liu, Z.; Jiao, W.; Xia, Y.; Chen, Z.; Li, D.; Ma, J.; Gai, C. Properties of hydrochars derived from swine manure by CaO assisted hydrothermal carbonization. J. Environ. Manag. 2019, 233, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shan, S.; Müller, K.; Wu, S.; Niazi, N.K.; Xu, S.; Shen, Y.; Rinklebe, J.; Liu, D.; Wang, H. Characterization of pig manure-derived hydrochars for their potential application as fertilizer. Environ. Sci. Pollut. Res. 2018, 25, 25772–25779. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zheng, H.; Shan, S.; Wu, S.; Wang, H.; Christie, P. Low-Temperature Hydrothermal Carbonization of Fresh Pig Manure: Effects of Temperature on Characteristics of Hydrochars. J. Environ. Eng. 2019, 145, 4019029. [Google Scholar] [CrossRef]
- Novak, J.M.; Johnson, M.G.; Spokas, K.A. Concentration and release of phosphorus and potassium from lignocellulosic and manure—Based biochars for fertilizer reuse. Front. Sustain. Food Syst. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Xu, X.; Harris, W. Phosphorus release from dairy manure, the manure—Derived biochar, and their amended soil: Effects of phosphorus nature and soil property. J. Environ. Qual. 2014, 43, 1504–1509. [Google Scholar] [CrossRef]
- Adam, C.; Peplinski, B.; Michaelis, M.; Kley, G.; Simon, F.G. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 2009, 29, 1122–1128. [Google Scholar] [CrossRef]
- Gorazda, K.; Tarko, B.; Werle, S.; Wzorek, Z. Sewage sludge as a fuel and raw material for phosphorus recovery: Combined process of gasification and P extraction. Waste Manag. 2018, 73, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Exploring technological alternatives of nutrient recovery from digestate as a secondary resource. Renew. Sustain. Energy Rev. 2020, 134, 110379. [Google Scholar] [CrossRef]
- Meng, X.; Huang, Q.; Gao, H.; Tay, K.; Yan, J. Improved utilization of phosphorous from sewage sludge (as Fertilizer) after treatment by Low-Temperature combustion. Waste Manag. 2018, 80, 349–358. [Google Scholar] [CrossRef]
- Salman, C.A.; Schwede, S.; Thorin, E.; Li, H.; Yan, J. Identification of thermochemical pathways for the energy and nutrient recovery from digested sludge in wastewater treatment plants. Energy Procedia 2019, 158, 1317–1322. [Google Scholar] [CrossRef]
- Ischia, G.; Fiori, L. Hydrothermal Carbonization of Organic Waste and Biomass: A Review on Process, Reactor, and Plant Modeling. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Couto, N.; Guedes, P.; Mateus, E.P.; Santos, C.; Ribau Teixeira, M.; Nunes, L.M.; Hansen, H.K.; Gutierrez, C.; Ottosen, L.M.; Ribeiro, A.B. Phosphorus recovery from a water reservoir-potential of nanofiltration coupled to electrodialytic process. Waste Biomass Valorization 2013, 4, 675–681. [Google Scholar] [CrossRef][Green Version]
- Schütte, T.; Niewersch, C.; Wintgens, T.; Yüce, S. Phosphorus recovery from sewage sludge by nanofiltration in diafiltration mode. J. Memb. Sci. 2015, 480, 74–82. [Google Scholar] [CrossRef]
- Blöcher, C.; Niewersch, C.; Melin, T. Phosphorus recovery from sewage sludge with a hybrid process of low pressure wet oxidation and nanofiltration. Water Res. 2012, 46, 2009–2019. [Google Scholar] [CrossRef]
- Xu, R.; Qin, W.; Zhang, B.; Wang, X.; Li, T.; Zhang, Y.; Wen, X. Nanofiltration in pilot scale for wastewater reclamation: Long-term performance and membrane biofouling characteristics. Chem. Eng. J. 2020, 395, 125087. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Hong, S.U.; Ouyang, L.; Bruening, M.L. Recovery of phosphate using multilayer polyelectrolyte nanofiltration membranes. J. Memb. Sci. 2009, 327, 2–5. [Google Scholar] [CrossRef]
- Niewersch, C.; Battaglia Bloch, A.L.; Yüce, S.; Melin, T.; Wessling, M. Nanofiltration for the recovery of phosphorus—Development of a mass transport model. Desalination 2014, 346, 70–78. [Google Scholar] [CrossRef]
- Remmen, K.; Müller, B.; Köser, J.; Wessling, M.; Wintgens, T. Phosphorus recovery in an acidic Environment using layer-by-layer modified membranes. J. Memb. Sci. 2019, 582, 254–263. [Google Scholar] [CrossRef]
- Zhang, X.; Scott, J.; Sharma, B.K.; Rajagopalan, N. Fouling mitigation and carbon recovery in nanofiltration processing of hydrothermal liquefaction aqueous waste stream. J. Memb. Sci. 2020, 614, 118558. [Google Scholar] [CrossRef]
- Yue, C.; Dong, H.; Zhang, W.; Zhu, Z.; Yin, F.; Wang, S. Effects of membrane concentration processes on flux, nutrient recovery, and antibiotic isolation for anaerobically digested slurry from swine manure. Trans. ASABE 2020, 63, 1639–1647. [Google Scholar] [CrossRef]
- Adam, G.; Mottet, A.; Lemaigre, S.; Tsachidou, B.; Trouvé, E.; Delfosse, P. Fractionation of anaerobic digestates by dynamic nanofiltration and reverse osmosis: An industrial pilot case evaluation for nutrient recovery. J. Environ. Chem. Eng. 2018, 6, 6723–6732. [Google Scholar] [CrossRef]
- Juntarasakul, O.; Yonezu, K.; Kawamoto, D.; Ohashi, H.; Kobayashi, Y.; Sugiyama, T.; Watanabe, K.; Yokoyama, T. Chemical state of Fe3+ in a Fe3+ type cation exchange resin for the removal and recovery of phosphate ions and the adsorption mechanism of phosphate ion to the resin. Colloids Surf. A Phys. Eng. Asp. 2020, 605, 125314. [Google Scholar] [CrossRef]
- Xia, W.J.; Guo, L.X.; Yu, L.Q.; Zhang, Q.; Xiong, J.R.; Zhu, X.Y.; Wang, X.C.; Huang, B.C.; Jin, R.C. Phosphorus removal from diluted wastewaters using a La/C nanocomposite-doped membrane with adsorption-filtration dual functions. Chem. Eng. J. 2021, 405, 126924. [Google Scholar] [CrossRef]
- Lian, F.; Gao, S.; Fu, Q.; Wu, Y.; Wang, J.; Huang, Q.; Hu, S. A comprehensive study of phosphorus removal and recovery with a Fe-loaded sulfoaluminate cement (FSC) adsorbent. J. Water Process Eng. 2021, 39, 101744. [Google Scholar] [CrossRef]
- Liu, X.; Fu, J.; Tang, Y.; Smith, R.L.; Qi, X. Mg-coordinated self-assembly of MgO-doped ordered mesoporous carbons for selective recovery of phosphorus from aqueous solutions. Chem. Eng. J. 2021, 406, 126748. [Google Scholar] [CrossRef]
- Dong, H.; Wei, L.; Tarpeh, W.A. Electro-assisted regeneration of pH-sensitive ion exchangers for sustainable phosphate removal and recovery. Water Res. 2020, 184, 116167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, H.; Li, H.; He, X.; Shi, Y.; Kruse, A. Microwave digestion-assisted HFO/biochar adsorption to recover phosphorus from swine manure. Sci. Total Environ. 2018, 621, 1512–1526. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Zhang, Q.; Liu, H. Overview of recent developments of resource recovery from wastewater via electrochemistry-based technologies. Sci. Total Environ. 2021, 757, 143901. [Google Scholar] [CrossRef] [PubMed]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Hu, B. The effects of electrocoagulation on phosphorus removal and particle settling capability in swine manure. Sep. Purif. Technol. 2018, 200, 112–119. [Google Scholar] [CrossRef]
- Emerick, T.; Vieira, J.L.; Silveira, M.H.L.; Joaõ, J.J. Ultrasound-assisted electrocoagulation process applied to the treatment and reuse of swine slaughterhouse wastewater. J. Environ. Chem. Eng. 2020, 8, 104308. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Scaria, J.; Babu, D.S.; Kumar, M.S. An overview on combined electrocoagulation-degradation processes for the effective treatment of water and wastewater. Chemosphere 2021, 263, 127907. [Google Scholar] [CrossRef]
- Omwene, P.I.; Kobya, M.; Can, O.T. Phosphorus removal from domestic wastewater in electrocoagulation reactor using aluminium and iron plate hybrid anodes. Ecol. Eng. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Ngo, H.H.; Guo, W.; Nguyen, T.T.; Chang, S.W.; Jang, A.; Yoon, Y.S. Can electrocoagulation process be an appropriate technology for phosphorus removal from municipal wastewater? Sci. Total Environ. 2016, 563–564, 549–556. [Google Scholar] [CrossRef]
- Mores, R.; de Mello, P.A.; Zakrzevski, C.A.; Treichel, H.; Kunz, A.; Steffens, J.; Dallago, R.M. Reduction of soluble organic carbon and removal of total phosphorus and metals from swine wastewater by electrocoagulation. Braz. J. Chem. Eng. 2018, 35, 1231–1240. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, W.; Hu, J.; Liu, B.; Wang, D.; Zhu, Q.; Yang, J.; Hou, H. Integration of electrochemical and calcium hypochlorite oxidation for simultaneous sludge deep dewatering, stabilization and phosphorus fixation. Sci. Total Environ. 2021, 750, 141408. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Hong, X.; Wu, K.; Hui, K.S.; Du, Y.; Hui, K.N. Simultaneous removal of ammonia and phosphate by electro-oxidation and electrocoagulation using Ru2–IrO2/Ti and microscale zero-valent iron composite electrode. Water Res. 2020, 169, 115239. [Google Scholar] [CrossRef] [PubMed]
- Mahvi, A.H.; din Ebrahimi, S.J.A.; Mesdaghinia, A.; Gharibi, H.; Sowlat, M.H. Performance evaluation of a continuous bipolar electrocoagulation/electrooxidation-electroflotation (ECEO-EF) reactor designed for simultaneous removal of ammonia and phosphate from wastewater effluent. J. Hazard. Mater. 2011, 192, 1267–1274. [Google Scholar] [CrossRef]
- Chakchouk, I.; Elloumi, N.; Belaid, C.; Mseddi, S.; Chaari, L.; Kallel, M. A combined electrocoagulation-electrooxidation treatment for dairy wastewater. Braz. J. Chem. Eng. 2017, 34, 109–117. [Google Scholar] [CrossRef]
- Chia, W.Y.; Chew, K.W.; Le, C.F.; Lam, S.S.; Chee, C.S.C.; Ooi, M.S.L.; Show, P.L. Sustainable utilization of biowaste compost for renewable energy and soil amendments. Environ. Pollut. 2020, 267, 115662. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Zhang, C.; Zhang, D.; Yao, C.; Meng, Q.; Zhao, R.; Wei, Z. Speciation, toxicity mechanism and remediation ways of heavy metals during composting: A novel theoretical microbial remediation method is proposed. J. Environ. Manag. 2020, 272, 111109. [Google Scholar] [CrossRef]
- Qin, R.; Su, C.; Mo, T.; Liao, L.; Zhu, F.; Chen, Y.; Chen, M. Effect of excess sludge and food waste feeding ratio on the nutrient fractions, and bacterial and fungal community during aerobic co-composting. Bioresour. Technol. 2021, 320, 124339. [Google Scholar] [CrossRef]
- Mieldažys, R.; Jotautienė, E.; Jasinskas, A.; Pekarskas, J.; Zinkevičienė, R. Investigation of physical-mechanical properties and impact on soil of granulated manure compost fertilizers. J. Environ. Eng. Landsc. Manag. 2019, 27, 153–162. [Google Scholar] [CrossRef]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N.L. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Xiao, L.; Young, E.B.; Berges, J.A.; He, Z. Integrated photo-bioelectrochemical system for contaminants removal and bioenergy production. Environ. Sci. Technol. 2012, 46, 11459–11466. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; de Nys, R.; Paul, N.A. Biorecovery of nutrient waste as protein in freshwater macroalgae. Algal Res. 2015, 7, 58–65. [Google Scholar] [CrossRef]
- Li, R.; Duan, N.; Zhang, Y.; Liu, Z.; Li, B.; Zhang, D.; Lu, H.; Dong, T. Co-digestion of chicken manure and microalgae Chlorella 1067 grown in the recycled digestate: Nutrients reuse and biogas enhancement. Waste Manag. 2017, 70, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Shi, J. Assessing nutrient removal kinetics in flushed manure using Chlorella vulgaris biomass production. Front. Bioeng. Biotechnol. 2017, 5. [Google Scholar] [CrossRef]
- Juárez, J.M.; Vladic, J.; Rodríguez, S.B.; Vidovic, S. Sequential valorisation of microalgae biomass grown in pig manure treatment photobioreactors. Algal Res. 2020, 50, 101972. [Google Scholar] [CrossRef]
- Sobhi, M.; Guo, J.; Cui, X.; Sun, H.; Li, B.; Aboagye, D.; Shah, G.M.; Dong, R. A promising strategy for nutrient recovery using heterotrophic indigenous microflora from liquid biogas digestate. Sci. Total Environ. 2019, 690, 492–501. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, W.; Tao, T.; Li, Y. A novel AAO-SBSPR process based on phosphorus mass balance for nutrient removal and phosphorus recovery from municipal wastewater. Water Res. 2018, 144, 763–773. [Google Scholar] [CrossRef]
- Sayedin, F.; Kermanshahi-pour, A.; He, Q.S.; Tibbetts, S.M.; Lalonde, C.G.E.; Brar, S.K. Microalgae cultivation in thin stillage anaerobic digestate for nutrient recovery and bioproduct production. Algal Res. 2020, 47, 101867. [Google Scholar] [CrossRef]
- Hongyang, S.; Yalei, Z.; Chunmin, Z.; Xuefei, Z.; Jinpeng, L. Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour. Technol. 2011, 102, 9884–9890. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Abou-Shanab, R.A.I.; Hwang, J.-H.; Timmes, T.C.; Kim, H.-C.; Oh, Y.-K.; Jeon, B.-H. Removal of Nitrogen and Phosphorus from Piggery Wastewater Effluent Using the Green Microalga Scenedesmus obliquus. J. Environ. Eng. 2013, 139, 1198–1205. [Google Scholar] [CrossRef]
- Khanzada, Z.T. Phosphorus removal from landfill leachate by microalgae. Biotechnol. Rep. 2020, 25, e00419. [Google Scholar] [CrossRef]
- Lavrinovičs, A.; Mežule, L.; Juhna, T. Microalgae starvation for enhanced phosphorus uptake from municipal wastewater. Algal Res. 2020, 52, 102090. [Google Scholar] [CrossRef]
- Li, X.; Yang, C.; Zeng, G.; Wu, S.; Lin, Y.; Zhou, Q.; Lou, W.; Du, C.; Nie, L.; Zhong, Y. Nutrient removal from swine wastewater with growing microalgae at various zinc concentrations. Algal Res. 2020, 46, 101804. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Kumar, S.M. Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour. Technol. 2019, 275, 109–122. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Silva-Núñez, A.; García-Perez, J.S.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Castillo-Zacarías, C.; Afewerki, S.; Barceló, D.; Iqbal, H.N.M.; Parra-Saldívar, R. Phyco-remediation of swine wastewater as a sustainable model based on circular economy. J. Environ. Manag. 2021, 278, 111534. [Google Scholar] [CrossRef]
- Wang, R.; Yang, C.; Hu, H.; Yang, Q.; Du, B. The impact of the varying nutrient concentrations on the enhanced biological phosphorus removal performance and functional phosphorus-accumulating and denitrifying genes in an anaerobic–aerobic–anoxic sequencing batch reactor. Environ. Technol. Innov. 2021, 21, 101256. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Eldyasti, A. Understanding microbial shift of Enhanced Biological Phosphorus Removal process (EBPR) under different Dissolved Oxygen (DO) concentrations and Hydraulic Retention Time (HRTs). Biochem. Eng. J. 2020, 166, 107833. [Google Scholar] [CrossRef]
- Mohamed, A.Y.A.; Welles, L.; Siggins, A.; Healy, M.G.; Brdjanovic, D.; Rada-Ariza, A.M.; Lopez-Vazquez, C.M. Effects of substrate stress and light intensity on enhanced biological phosphorus removal in a photo-activated sludge system. Water Res. 2021, 189, 116606. [Google Scholar] [CrossRef]
- Nielsen, P.H.; McIlroy, S.J.; Albertsen, M.; Nierychlo, M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr. Opin. Biotechnol. 2019, 57, 111–118. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, B.; Peng, Y.; Li, X.; Zhang, Q. Simultaneous enhanced biological phosphorus removal and semi-nitritation (EBPR-SN) followed by anammox process treating municipal wastewater at seasonal temperatures: From summer to winter. Sci. Total Environ. 2020, 757, 144048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, L.; Shao, H.; Zhang, S.; Gu, P.; Peng, Y. Enhanced nutrients removal from municipal wastewater through biological phosphorus removal followed by partial nitritation/anammox. Front. Environ. Sci. Eng. 2017, 11, 1–6. [Google Scholar] [CrossRef]
- Liu, Z.; Pruden, A.; Ogejo, J.A.; Knowlton, K.F. Polyphosphate and glycogen accumulating organisms in one EBPR system for liquid dairy manure. Water Environ. Res. 2014, 86, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Barat, R.; Montoya, T.; Seco, A.; Ferrer, J. The role of potassium, magnesium and calcium in the enhanced biological phosphorus removal treatment plants. Environ. Technol. 2005, 26, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: Technological challenges, advancements, innovations, and future perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Dinnebier, H.C.F.; Matthiensen, A.; Michelon, W.; Tápparo, D.C.; Fonseca, T.G.; Favretto, R.; Steinmetz, R.L.R.; Treichel, H.; Antes, F.G.; Kunz, A. Phycoremediation and biomass production from high strong swine wastewater for biogas generation improvement: An integrated bioprocess. Bioresour. Technol. 2021, 332, 125111. [Google Scholar] [CrossRef]
- Mores, R.; Treichel, H.; Zakrzevski, C.A.; Kunz, A.; Steffens, J.; Dallago, R.M. Remove of phosphorous and turbidity of swine wastewater using electrocoagulation under continuous flow. Sep. Purif. Technol. 2016, 171, 112–117. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van Der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Puchongkawarin, C.; Gomez-Mont, C.; Stuckey, D.C.; Chachuat, B. Optimization-based methodology for the development of wastewater facilities for energy and nutrient recovery. Chemosphere 2015, 140, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Struhs, E.; Mirkouei, A.; You, Y.; Mohajeri, A. Techno-economic and environmental assessments for nutrient-rich biochar production from cattle manure: A case study in Idaho, USA. Appl. Energy 2020, 279, 115782. [Google Scholar] [CrossRef]
- Havukainen, J.; Väisänen, S.; Rantala, T.; Saunila, M.; Ukko, J. Environmental impacts of manure management based on life cycle assessment approach. J. Clean. Prod. 2020, 264, 121576. [Google Scholar] [CrossRef]
- Lijó, L.; Frison, N.; Fatone, F.; González-García, S.; Feijoo, G.; Moreira, M.T. Environmental and sustainability evaluation of livestock waste management practices in Cyprus. Sci. Total Environ. 2018, 634, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.S.; Liu, Y.Q.; Zhang, C. Upgrading a large and centralised municipal wastewater treatment plant with sequencing batch reactor technology for integrated nutrient removal and phosphorus recovery: Environmental and economic life cycle performance. Sci. Total Environ. 2020, 749, 141465. [Google Scholar] [CrossRef]
- Peters, G.M.; Rowley, H. V Environmental comparison of biosolids management systems using life cycle assessment. Environ. Sci. Technol. 2009, 43, 2674–2679. [Google Scholar] [CrossRef]
- Fang, L.L.; Valverde-Pérez, B.; Damgaard, A.; Plósz, B.G.; Rygaard, M. Life cycle assessment as development and decision support tool for wastewater resource recovery technology. Water Res. 2016, 88, 538–549. [Google Scholar] [CrossRef]
- Nakakubo, T.; Tokai, A.; Ohno, K. Comparative assessment of technological systems for recycling sludge and food waste aimed at greenhouse gas emissions reduction and phosphorus recovery. J. Clean. Prod. 2012, 32, 157–172. [Google Scholar] [CrossRef]
- Temizel-Sekeryan, S.; Wu, F.; Hicks, A.L. Life cycle assessment of struvite precipitation from anaerobically digested dairy manure: A Wisconsin perspective. Integr. Environ. Assess. Manag. 2020, 17, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Stichnothe, H.; Schuchardt, F.; Li, G.; Huaitalla, R.M.; Xu, W. Life cycle assessment of manure management and nutrient recycling from a Chinese pig farm. Waste Manag. Res. 2014, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Bora, R.R.; Lei, M.; Tester, J.W.; Lehmann, J.; You, F. Life cycle assessment and technoeconomic analysis of thermochemical conversion technologies applied to poultry litter with energy and nutrient recovery. ACS Sustain. Chem. Eng. 2020, 8, 8436–8447. [Google Scholar] [CrossRef]
- Sena, M.; Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- Pedizzi, C.; Noya, I.; Sarli, J.; González-García, S.; Lema, J.M.; Moreira, M.T.; Carballa, M. Environmental assessment of alternative treatment schemes for energy and nutrient recovery from livestock manure. Waste Manag. 2018, 77, 276–286. [Google Scholar] [CrossRef]
- Haase, M.; Rösch, C.; Ulrici, O. Feasibility study on the processing of surplus livestock manure into an organic fertilizer by thermal concentration—The case study of Les Plenesses in Wallonia. J. Clean. Prod. 2017, 161, 896–907. [Google Scholar] [CrossRef]
- De Vrieze, J.; Colica, G.; Pintucci, C.; Sarli, J.; Pedizzi, C.; Willeghems, G.; Bral, A.; Varga, S.; Prat, D.; Peng, L.; et al. Resource recovery from pig manure via an integrated approach: A technical and economic assessment for full-scale applications. Bioresour. Technol. 2019, 272, 582–593. [Google Scholar] [CrossRef]
- Tonini, D.; Saveyn, H.G.M.; Huygens, D. Environmental and health co-benefits for advanced phosphorus recovery. Nat. Sustain. 2019, 2, 1051–1061. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, L.-C.; Chang, J.-S. Environmental life cycle comparisons of pig farming integrated with anaerobic digestion and algae-based wastewater treatment. J. Environ. Manag. 2020, 264, 110512. [Google Scholar] [CrossRef]
- Golroudbary, S.R.; El Wali, M.; Kraslawski, A. Environmental sustainability of phosphorus recycling from wastewater, manure and solid wastes. Sci. Total Environ. 2019, 672, 515–524. [Google Scholar] [CrossRef]
- Withers, P.J.A. Closing the phosphorus cycle. Nat. Sustain. 2019, 2, 1001–1002. [Google Scholar] [CrossRef]
- Huygens, D.; Saveyn, H.G.M.; Tonini, D.; Eder, P.; Delgado, L.S. Technical Proposals for Selected New Fertilising Materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009); European Union: Luxembourg, 2019; pp. 1–466. [Google Scholar] [CrossRef]
- Porterfield, K.K.; Joblin, R.; Neher, D.A.; Curtis, M.; Dvorak, S.; Rizzo, D.M.; Faulkner, J.W.; Roy, E.D. Upcycling phosphorus recovered from anaerobically digested dairy manure to support production of vegetables and flowers. Sustainability 2020, 12, 1139. [Google Scholar] [CrossRef]
- Christiansen, N.H.; Sørensen, P.; Labouriau, R.; Christensen, B.T.; Rubæk, G.H. Characterizing phosphorus availability in waste products by chemical extractions and plant uptake. J. Plant Nutr. Soil Sci. 2020, 183, 416–428. [Google Scholar] [CrossRef]
- Achat, D.L.; Sperandio, M.; Daumer, M.-L.; Santellani, A.-C.; Prud’Homme, L.; Akhtar, M.; Morel, C. Plant-availability of phosphorus recycled from pig manures and dairy effluents as assessed by isotopic labeling techniques. Geoderma 2014, 232–234, 24–33. [Google Scholar] [CrossRef]
- Möller, K.; Oberson, A.; Bünemann, E.K.; Cooper, J.; Friedel, J.K.; Glæsner, N.; Hörtenhuber, S.; Løes, A.-K.; Mäder, P.; Meyer, G.; et al. Improved phosphorus recycling in organic farming: Navigating between constraints. Adv. Agron. 2018, 147, 159–237. [Google Scholar] [CrossRef]

| Reference | Animal Waste | TP (g kg−1) | DM (%) | Organic C (g kg−1) | Total N (g kg−1) | C/N Ratio |
|---|---|---|---|---|---|---|
| Handin and Eriksson [25] | Horse manure | 1.5 | 23.5 | 7.9 | 0.34 | 23.2 |
| Szögi et al. [26] | Fresh swine manure | 18.2 | 21.3 | - | 37.8 | - |
| Lemming et al. [27] | Cattle manure | 1.7 | 26 | 432 | 21 | 21 |
| Li et al. [28] | Chicken manure | 3.0 | 24.26 | - | 12 | - |
| Li et al. [28] | Dairy manure | 0.6 | 17.91 | - | 3.6 | - |
| Grigatti et al. [29] | Bovine slurry | 8.0 | 4.25 | 488.1 | 32.6 | 15 |
| Grigatti et al. [29] | Swine slurry | 33.0 | 5.16 | 394 | 46.5 | 8.5 |
| Grigatti et al. [29] | Anaerobic digestate from bovine slurry and energy crops | 6.2 | 5.05 | 515 | 43 | 12 |
| Kunz et al. [30] | Swine manure | 0.46 a | - | 14.59 a | 1.52 a | 9.6 |
| Reference | Residue | Thermal Process | Temperature (°C) | Product | N | P | K |
|---|---|---|---|---|---|---|---|
| g kg−1 | |||||||
| Leng et al. [100] | Poultry litter | Combustion | 850 | Ash | - | 97.8 | 148 |
| Bergfeldt et al. [101] | Poultry litter | Combustion | 815 | Ash | - | 73.0 | 89.2 |
| Więckol-Ryk et al. [102] | Chicken manure | Combustion | 500–900 | Ash | - | 125.6 | 130.0 |
| Lang et al. [103] | Swine manure | Hydrothermal carbonization | 180–220 | Hydrochar | 20.2 | 20.7 | - |
| Song et al. [104] | Swine manure | Hydrothermal carbonization | 160–240 | Hydrochars | 28.5 | 26.0 | 8.0 |
| Song et al. [105] | Swine manure | Hydrothermal carbonization | 140–220 | Hydrochars | 44.8 | 18.8 | 1.7 |
| Nest et al. [22] | Swine manure | Pyrolysis | 500 | Biochar | 17.0 | 1.2 | 67.1 |
| Novak et al. [106] | Poultry litter | Pyrolysis | 500 | Biochar | 40 | 31.5 | 69.4 |
| Liang et al. [107] | Dairy manure | Pyrolysis | 450 | Biochar | - | 25.2 | - |
| Reference | Residue | Microalgae | Biomass Productivity (mg L−1 d−1) | Biomass Concentration | |
|---|---|---|---|---|---|
| TP (g kg−1) | TN (g kg−1) | ||||
| Cole et al. [152] | Fish farm wastewater | Oedogonium sp. | 24–36 a | 3.4 | - |
| Li et al. [153] | Digestate Chicken manure | Chlorella 1067 | 251 | 18.75 ± 2.78 b | 85.11 ± 3.52 b |
| Pandey et al. [154] | Dairy wastewater | Chlorella vulgaris | 19.6 | 66.3 c | 156.4 c |
| Juárez et al [155] | Digestate swine manure | Scenedesmus obliquus (39%), Scenedesmus lagerheimii (33%), Scenedesmus opoliensis (13%), Scenedesmus magnus (4%) | - | 3.8 a | 63.8 a |
| Sobhi et al. [156] | Digestate chicken manure | Indigenous microflora “Heterotrophic” | 410–3690 | 32–42 a | - |
| Reference | Waste | Recovery Way | Evaluation Methodology | Functional Unit | Impact Category | |
|---|---|---|---|---|---|---|
| GWP (kg CO2 eq) | EP (kg PO4 eq) | |||||
| Lijó et al. [184] | Livestock waste | Struvite | ReCiPe Midpoint H | 1 t of treated manure | −22 | −0.001 |
| Rashid et al. [185] | Municipal wastewater | Struvite | CML-IA | 1 m3 of treated wastewater | 7.47 × 10−15 | 1.62 × 10−14 |
| Peters and Rowley [186] | Sludge of municipal wastewater treatment | Biosolids | Carbon Footprint | 2 dry tonnes per day of sludge | −490 | - |
| Fang et al. [187] | Municipal wastewater | Algal biomass | ILCD 2011 | 1 m3 of treated wastewater | 1.2 × 10−2 mPE * | −9.2 × 10−2 mPE * |
| Nakakubo et al. [188] | Food waste and sewage sludge | Dry granulation | Carbon Footprint | Unit as the processing capacity to provide disposal services for 100,000 people | 1000 | - |
| Temizel-Sekeryan et al. [189] | Dairy Manure | Struvite | TRACI 2.1 | 1 kg of struvite produced | 7.02 | 8.0 × 10−2 a |
| Luo et al. [190] | Swine manure | Compost | CML 2 | Annual production of a typical pig farm in Beijing area (1956 LU annually) | 5.611 × 106 | 3.41 × 104 |
| Struhs et al. [182] | Cattle manure | Biochar | CML-IA | 50 metric tons of manure | 8642 | 0.28 |
| Bora et al. [191] | Poultry Litter | Biochar | IMPACT 2002+ | 1000 kg of fresh or wet poultry litter | 657 | −4.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollas, C.E.; Bolsan, A.C.; Venturin, B.; Bonassa, G.; Tápparo, D.C.; Cândido, D.; Antes, F.G.; Vanotti, M.B.; Szögi, A.A.; Kunz, A. Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains. Sustainability 2021, 13, 5919. https://doi.org/10.3390/su13115919
Hollas CE, Bolsan AC, Venturin B, Bonassa G, Tápparo DC, Cândido D, Antes FG, Vanotti MB, Szögi AA, Kunz A. Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains. Sustainability. 2021; 13(11):5919. https://doi.org/10.3390/su13115919
Chicago/Turabian StyleHollas, Camila Ester, Alice Chiapetti Bolsan, Bruno Venturin, Gabriela Bonassa, Deisi Cristina Tápparo, Daniela Cândido, Fabiane Goldschmidt Antes, Matias B. Vanotti, Ariel A. Szögi, and Airton Kunz. 2021. "Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains" Sustainability 13, no. 11: 5919. https://doi.org/10.3390/su13115919
APA StyleHollas, C. E., Bolsan, A. C., Venturin, B., Bonassa, G., Tápparo, D. C., Cândido, D., Antes, F. G., Vanotti, M. B., Szögi, A. A., & Kunz, A. (2021). Second-Generation Phosphorus: Recovery from Wastes towards the Sustainability of Production Chains. Sustainability, 13(11), 5919. https://doi.org/10.3390/su13115919









