Study of Temporal Variations in Species–Environment Association through an Innovative Multivariate Method: MixSTATICO
Abstract
:1. Introduction
1.1. Contributions of the French School
1.2. Contributions of the Anglo-Saxon School
1.3. Contributions of the Salmantina School
2. Materials and Methods
2.1. Materials
2.1.1. Hydrography and Sampling
2.1.2. Determination of Inorganic Nutrients and Ratios
2.1.3. Phytoplankton Analysis
2.2. Methodological Description of the MixSTATICO Method
- Binary variables must be coded with 0 and 1;
- Ordinal variables must be coded for each scale in ascending numerical order;
- Nominal variables must be transformed to a disjunctive matrix (each category is a new dichotomous variable).
- is the Gower similarity coefficient;
- is the number of continuous quantitative variables;
- is the number of binary variables;
- is the number of qualitative non-binary variables;
- and are the number of matches in binary variables (1,1) and (0,0), respectively;
- is the number of matches in qualitative non-binary variables; and
- is the rank of the h-th quantitative variable.
- is the Gower distance;
- represents the non-nominal variable;
- represents the nominal variable; and
- is the category number of the nominal variable.
2.3. Case Study
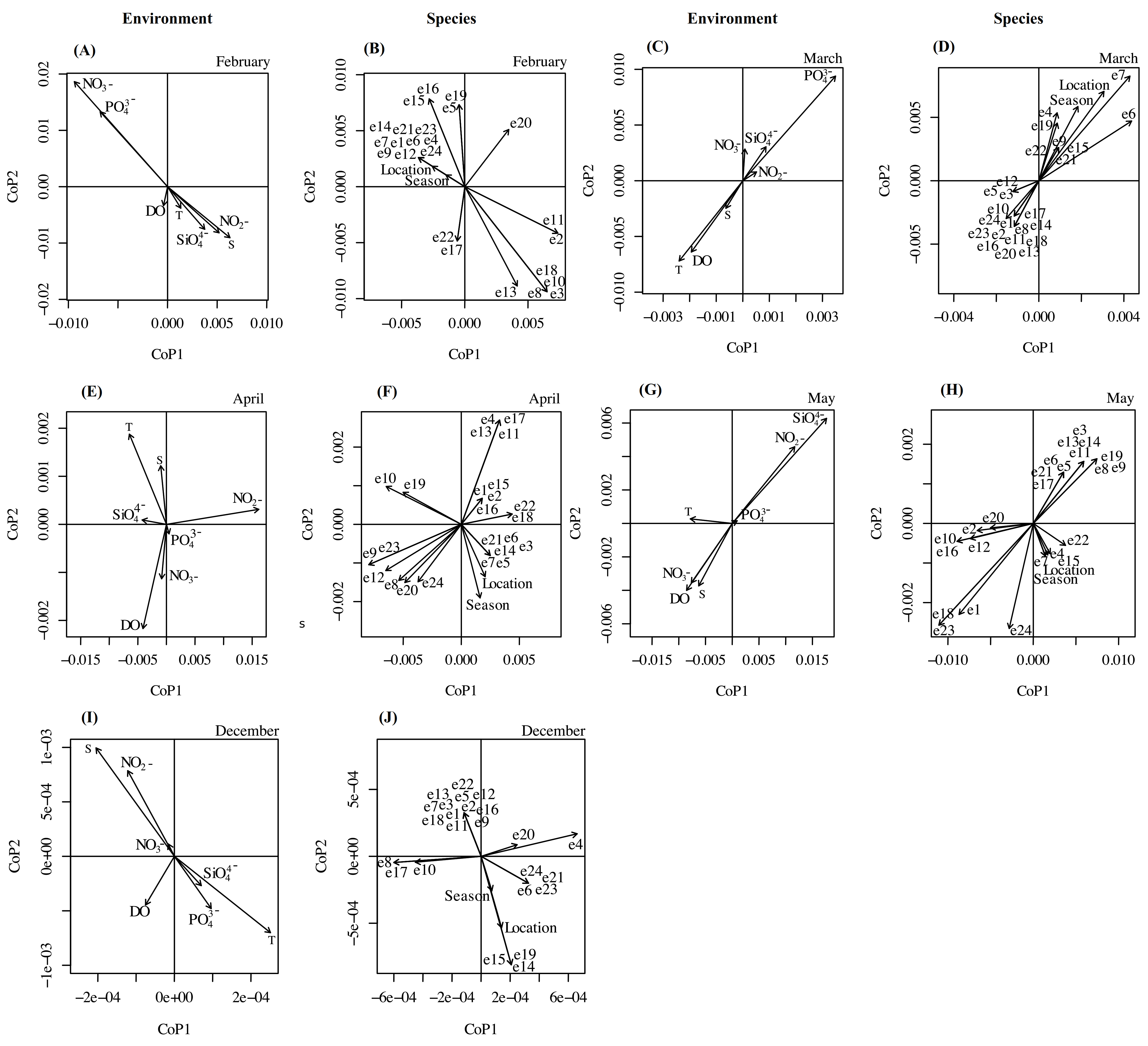
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. MixSTATICO Algorithm
- Input two pre-processed data-cubes and .
- Merge all and matrices.For k = 1 to K
- Compute the Gower distance, considering Equations (6)–(8).For k = 1 to K
- Apply FMA to normalizeFor k = 1 to K
- Vectorize
- Calculate
- Calculate .For k = 1 to K
- SVD to obtain
- Calculate consensus matrix
- SVD ).
- Generate consensus graph using PCoA.
- Generate intra-structure graph using PCoA.
Appendix B. Table Environmental Data
| Space | Time | Temperature (T—°C) | Salinity (S—psu) | Dissolved Oxygen (DO—mg.L−1) | Nitrate (NO3—µg−at.L−1) | Nitrite (NO2—µg−at.L−1) | Phosphate (—µg−at.L−1) | Silicate (—µg−at.L−1) |
|---|---|---|---|---|---|---|---|---|
| Esmeraldas | Feb | 26.74 | 32.65 | 5.29 | 0.77 | 0.08 | 0.71 | 10.42 |
| Manta | Feb | 24.02 | 33.24 | 4.35 | 6.91 | 0.16 | 1.62 | 5.12 |
| La Libertad | Feb | 25.25 | 33.11 | 5.38 | 0.73 | 0.28 | 0.58 | 11.88 |
| Pto. Bolivar | Feb | 22.23 | 33.47 | 4.35 | 8.76 | 0.22 | 1.31 | 7.52 |
| Esmeraldas | Mar | 21.92 | 34.11 | 4.28 | 5.95 | 0.39 | 2.00 | 14.36 |
| Manta | Mar | 23.04 | 33.69 | 4.37 | 2.73 | 0.43 | 1.75 | 13.73 |
| La Libertad | Mar | 23.58 | 34.29 | 4.62 | 3.64 | 0.30 | 1.33 | 5.24 |
| Pto. Bolivar | Mar | 21.17 | 31.56 | 3.35 | 9.36 | 0.29 | 0.97 | 12.87 |
| Esmeraldas | Apr | 27.45 | 33.24 | 4.45 | 0.50 | 0.06 | 0.71 | 11.06 |
| Manta | Apr | 24.47 | 34.15 | 4.36 | 1.55 | 0.28 | 0.38 | 8.07 |
| La Libertad | Apr | 22.79 | 34.47 | 4.46 | 4.96 | 0.09 | 1.26 | 10.08 |
| Pto. Bolivar | Apr | 23.12 | 33.56 | 4.16 | 3.86 | 0.73 | 1.02 | 8.14 |
| Esmeraldas | May | 26.11 | 33.21 | 4.60 | 1.80 | 0.19 | 0.70 | 3.22 |
| Manta | May | 26.07 | 33.40 | 4.35 | 3.85 | 0.15 | 0.75 | 2.10 |
| La Libertad | May | 24.65 | 33.64 | 4.12 | 1.70 | 0.56 | 1.17 | 19.10 |
| Pto. Bolivar | May | 24.95 | 33.81 | 4.53 | 4.92 | 0.02 | 0.51 | 10.66 |
| Esmeraldas | Jun | 26.33 | 33.17 | 4.60 | 2.02 | 0.20 | 0.38 | 10.11 |
| Manta | Jun | 24.31 | 33.95 | 4.32 | 0.65 | 0.34 | 0.31 | 1.63 |
| La Libertad | Jun | 20.45 | 34.41 | 3.20 | 7.75 | 0.41 | 0.95 | 9.55 |
| Pto. Bolivar | Jun | 22.18 | 34.22 | 5.12 | 0.36 | 0.11 | 0.33 | 2.65 |
| Esmeraldas | Jul | 25.50 | 32.91 | 4.73 | 1.13 | 0.03 | 1.02 | 3.66 |
| Manta | Jul | 23.90 | 33.56 | 4.62 | 0.99 | 0.10 | 0.84 | 3.16 |
| La Libertad | Jul | 22.68 | 33.78 | 4.82 | 0.39 | 0.04 | 0.83 | 9.34 |
| Pto. Bolivar | Jul | 22.80 | 33.99 | 5.01 | 1.47 | 0.24 | 1.25 | 8.87 |
| Esmeraldas | Aug | 25.49 | 32.89 | 4.62 | 0.24 | 0.01 | 0.25 | 1.92 |
| Manta | Aug | 22.73 | 33.59 | 4.47 | 2.27 | 0.05 | 0.71 | 6.73 |
| La Libertad | Aug | 19.09 | 34.38 | 3.89 | 4.04 | 0.23 | 1.00 | 12.21 |
| Pto. Bolivar | Aug | 21.60 | 33.93 | 3.90 | 3.19 | 0.04 | 0.56 | 12.46 |
| Esmeraldas | Sep | 26.33 | 32.68 | 4.32 | 0.72 | 0.04 | 0.26 | 1.16 |
| Manta | Sep | 21.35 | 34.20 | 4.53 | 1.09 | 0.01 | 0.38 | 8.84 |
| La Libertad | Sep | 21.85 | 33.96 | 4.00 | 2.43 | 0.09 | 0.58 | 5.01 |
| Pto. Bolivar | Sep | 22.54 | 33.84 | 4.41 | 3.36 | 0.25 | 0.50 | 4.66 |
| Esmeraldas | Oct | 26.09 | 32.20 | 4.23 | 0.58 | 0.18 | 0.10 | 3.73 |
| Manta | Oct | 24.31 | 33.43 | 3.81 | 2.59 | 0.23 | 0.18 | 5.11 |
| La Libertad | Oct | 23.18 | 33.72 | 4.51 | 0.29 | 0.13 | 0.33 | 6.87 |
| Pto. Bolivar | Oct | 22.60 | 33.81 | 4.17 | 1.54 | 0.38 | 0.46 | 11.88 |
| Esmeraldas | Nov | 26.06 | 32.41 | 4.39 | 0.12 | 0.08 | 0.11 | 2.23 |
| Manta | Nov | 24.93 | 32.99 | 4.56 | 0.30 | 0.05 | 0.13 | 2.82 |
| La Libertad | Nov | 20.30 | 34.38 | 3.27 | 5.03 | 0.26 | 0.61 | 9.11 |
| Pto. Bolivar | Nov | 21.21 | 34.24 | 3.58 | 6.08 | 0.31 | 0.63 | 10.89 |
| Esmeraldas | Dec | 25.45 | 32.65 | 3.96 | 0.26 | 0.03 | 0.21 | 6.24 |
| Manta | Dec | 24.38 | 33.09 | 4.48 | 1.03 | 0.03 | 0.37 | 1.27 |
| La Libertad | Dec | 21.64 | 34.23 | 4.25 | 3.52 | 0.29 | 0.69 | 7.41 |
| Pto. Bolivar | Dec | 21.56 | 34.23 | 3.13 | 3.81 | 0.37 | 0.09 | 8.48 |
Appendix C. Table Species Data
| Space | Time | Phytoplankton Species 1—Abundance Cells L−1 | Localization | Sea-son | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| e1 | e2 | e3 | e4 | e5 | e6 | e7 | e8 | e9 | e10 | e11 | e12 | e13 | e14 | e15 | e16 | e17 | e18 | e19 | e20 | e21 | e22 | e23 | e24 | ||||
| Esmeraldas | Feb | 0.00 | 4.62 | 3.74 | 0.00 | 3.80 | 0.00 | 0.00 | 4.36 | 0.00 | 2.90 | 4.88 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.94 | 4.44 | 3.74 | 0.00 | 0.00 | 0.00 | 0 | North | Rainy |
| Manta | Feb | 0.00 | 3.37 | 3.85 | 0.00 | 0.00 | 0.00 | 0.00 | 3.59 | 0.00 | 4.04 | 3.67 | 0.00 | 3.67 | 0.00 | 0.00 | 0.00 | 0.00 | 2.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0 | Centre North | Rainy |
| Libertad | Feb | 0.00 | 4.46 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.37 | 0.00 | 3.59 | 0.00 | 3.74 | 3.67 | 0.00 | 0.00 | 0.00 | 3.37 | 0.00 | 0.00 | 0.00 | 0 | Centre South | Rainy |
| Pto. Bolivar | Feb | 0.00 | 3.37 | 3.50 | 0.00 | 0.00 | 0.00 | 0.00 | 4.84 | 0.00 | 5.09 | 3.59 | 0.00 | 5.18 | 0.00 | 0.00 | 0.00 | 2.90 | 3.80 | 0.00 | 4.55 | 0.00 | 4.07 | 0.00 | 0 | South | Rainy |
| Esmeraldas | Mar | 4.33 | 4.17 | 4.66 | 0.00 | 2.90 | 0.00 | 0.00 | 4.62 | 0.00 | 4.69 | 4.36 | 3.20 | 3.74 | 3.80 | 0.00 | 3.37 | 3.20 | 3.80 | 0.00 | 3.50 | 0.00 | 0.00 | 4.13 | 3 | North | Rainy |
| Manta | Mar | 4.01 | 4.28 | 4.15 | 3.20 | 3.20 | 0.00 | 0.00 | 5.58 | 2.90 | 4.54 | 3.74 | 4.34 | 4.90 | 4.55 | 3.50 | 4.10 | 3.37 | 4.22 | 4.24 | 3.80 | 2.90 | 2.90 | 4.10 | 3 | Centre North | Rainy |
| Libertad | Mar | 5.35 | 4.52 | 3.80 | 0.00 | 2.90 | 2.90 | 0.00 | 4.52 | 0.00 | 0.00 | 4.28 | 4.41 | 2.90 | 0.00 | 0.00 | 3.20 | 0.00 | 4.22 | 3.50 | 3.67 | 0.00 | 0.00 | 3.50 | 2 | Centre South | Rainy |
| Pto. Bolivar | Mar | 3.80 | 4.26 | 0.00 | 0.00 | 0.00 | 6.30 | 0.00 | 5.06 | 3.20 | 5.45 | 4.56 | 0.00 | 5.55 | 5.03 | 4.04 | 3.67 | 3.80 | 4.46 | 0.00 | 2.90 | 3.20 | 3.37 | 3.74 | 2 | South | Rainy |
| Esmeraldas | Apr | 0.00 | 0.00 | 0.00 | 3.94 | 0.00 | 0.00 | 0.00 | 5.10 | 0.00 | 3.37 | 3.67 | 0.00 | 3.50 | 0.00 | 0.00 | 0.00 | 4.13 | 3.50 | 3.37 | 4.01 | 0.00 | 2.90 | 0.00 | 0 | North | Rainy |
| Manta | Apr | 4.69 | 4.52 | 0.00 | 4.31 | 0.00 | 0.00 | 0.00 | 5.51 | 3.37 | 4.01 | 5.22 | 3.74 | 5.25 | 0.00 | 4.29 | 4.10 | 4.04 | 3.80 | 2.90 | 3.37 | 0.00 | 3.50 | 4.31 | 3 | Centre North | Rainy |
| Libertad | Apr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.49 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.46 | 0.00 | 4.67 | 0.00 | 3.74 | 0.00 | 0 | Centre South | Rainy |
| Pto. Bolivar | Apr | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.17 | 4.24 | 3.97 | 0.00 | 2.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.90 | 3.20 | 0.00 | 0.00 | 3.37 | 2 | South | Rainy |
| Esmeraldas | May | 0.00 | 4.51 | 2.90 | 0.00 | 2.90 | 4.78 | 0.00 | 4.45 | 3.37 | 4.59 | 4.07 | 2.90 | 5.46 | 4.58 | 0.00 | 4.73 | 3.85 | 0.00 | 3.20 | 3.67 | 3.67 | 0.00 | 0.00 | 0 | North | Rainy |
| Manta | May | 4.01 | 4.22 | 3.59 | 0.00 | 0.00 | 0.00 | 0.00 | 3.94 | 4.41 | 0.00 | 2.90 | 0.00 | 4.47 | 3.67 | 0.00 | 0.00 | 0.00 | 0.00 | 2.90 | 3.59 | 0.00 | 2.90 | 0.00 | 0 | Centre North | Rainy |
| Libertad | May | 3.80 | 3.80 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.90 | 0.00 | 3.80 | 0.00 | 0.00 | 0.00 | 2.90 | 0.00 | 2.90 | 0.00 | 2.90 | 0.00 | 0.00 | 3.20 | 1 | Centre South | Rainy |
| Pto. Bolivar | May | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.13 | 0.00 | 0.00 | 0.00 | 2.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.20 | 4.53 | 0.00 | 0.00 | 0.00 | 0 | South | Rainy |
| Esmeraldas | Jun | 4.94 | 5.25 | 4.34 | 4.74 | 3.74 | 4.72 | 0.00 | 3.74 | 0.00 | 4.15 | 4.87 | 0.00 | 4.34 | 0.00 | 0.00 | 5.88 | 3.50 | 0.00 | 0.00 | 3.37 | 3.20 | 0.00 | 4.04 | 3 | North | Dry |
| Manta | Jun | 4.76 | 5.03 | 5.12 | 3.90 | 4.89 | 0.00 | 0.00 | 4.47 | 2.90 | 0.00 | 4.53 | 3.20 | 4.73 | 0.00 | 0.00 | 4.75 | 3.37 | 2.90 | 0.00 | 3.20 | 0.00 | 0.00 | 0.00 | 0 | Centre North | Dry |
| Libertad | Jun | 4.67 | 5.22 | 4.41 | 0.00 | 4.62 | 0.00 | 0.00 | 4.61 | 2.90 | 0.00 | 4.40 | 4.01 | 4.58 | 0.00 | 0.00 | 4.93 | 3.94 | 3.80 | 0.00 | 2.90 | 3.74 | 0.00 | 4.01 | 3 | Centre South | Dry |
| Pto. Bolivar | Jun | 5.01 | 6.16 | 4.01 | 5.06 | 4.99 | 0.00 | 4.34 | 4.61 | 3.59 | 4.15 | 4.95 | 3.20 | 4.10 | 0.00 | 0.00 | 5.87 | 0.00 | 3.67 | 0.00 | 4.01 | 3.20 | 0.00 | 4.10 | 3 | South | Dry |
| Esmeraldas | Jul | 4.86 | 5.14 | 4.99 | 4.49 | 4.41 | 4.28 | 3.67 | 4.40 | 3.74 | 3.74 | 4.68 | 0.00 | 4.49 | 5.27 | 0.00 | 4.44 | 0.00 | 3.90 | 0.00 | 0.00 | 3.37 | 0.00 | 3.20 | 1 | North | Dry |
| Manta | Jul | 2.90 | 0.00 | 4.29 | 4.78 | 0.00 | 0.00 | 0.00 | 4.07 | 3.37 | 3.20 | 4.34 | 3.59 | 4.20 | 0.00 | 0.00 | 0.00 | 3.20 | 4.04 | 0.00 | 3.20 | 2.90 | 2.90 | 4.13 | 3 | Centre North | Dry |
| La Libertad | Jul | 4.53 | 5.33 | 4.71 | 5.79 | 3.20 | 3.59 | 0.00 | 3.67 | 2.90 | 0.00 | 3.50 | 4.24 | 4.41 | 0.00 | 0.00 | 0.00 | 0.00 | 3.59 | 0.00 | 2.90 | 0.00 | 0.00 | 3.67 | 2 | Centre South | Dry |
| Pto. Bolivar | Jul | 4.34 | 5.16 | 4.01 | 5.15 | 0.00 | 4.26 | 4.52 | 3.80 | 3.74 | 5.39 | 4.53 | 4.26 | 4.26 | 4.53 | 0.00 | 0.00 | 4.41 | 3.20 | 0.00 | 3.20 | 3.37 | 3.20 | 0.00 | 0 | South | Dry |
| Esmeraldas | Aug | 4.39 | 5.04 | 4.66 | 4.37 | 4.13 | 3.94 | 0.00 | 4.28 | 3.97 | 4.46 | 4.51 | 3.50 | 4.56 | 3.94 | 0.00 | 3.67 | 3.67 | 4.01 | 0.00 | 3.20 | 2.90 | 0.00 | 3.67 | 2 | North | Dry |
| Manta | Aug | 4.86 | 5.22 | 5.84 | 5.57 | 4.24 | 4.40 | 0.00 | 4.64 | 3.85 | 4.39 | 5.18 | 3.80 | 4.67 | 3.80 | 0.00 | 4.45 | 4.36 | 3.97 | 0.00 | 0.00 | 0.00 | 3.50 | 4.45 | 3 | Centre North | Dry |
| La Libertad | Aug | 4.07 | 4.94 | 5.83 | 5.71 | 4.71 | 0.00 | 4.13 | 3.94 | 3.20 | 3.67 | 5.01 | 4.51 | 4.31 | 0.00 | 0.00 | 4.68 | 4.65 | 4.37 | 3.20 | 3.80 | 0.00 | 2.90 | 0.00 | 0 | Centre South | Dry |
| Pto. Bolivar | Aug | 0.00 | 3.50 | 4.40 | 0.00 | 0.00 | 0.00 | 0.00 | 3.85 | 3.20 | 0.00 | 3.59 | 3.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.04 | 0.00 | 4.22 | 0.00 | 0.00 | 0.00 | 0 | South | Dry |
| Esmeraldas | Sep | 3.37 | 4.93 | 4.66 | 3.50 | 4.26 | 0.00 | 0.00 | 4.07 | 3.50 | 0.00 | 4.39 | 0.00 | 4.44 | 0.00 | 0.00 | 3.20 | 3.20 | 4.04 | 0.00 | 3.37 | 0.00 | 3.90 | 3.80 | 2 | North | Dry |
| Manta | Sep | 4.53 | 5.02 | 4.95 | 3.94 | 3.94 | 0.00 | 0.00 | 4.92 | 3.94 | 3.74 | 5.12 | 0.00 | 4.41 | 0.00 | 0.00 | 3.37 | 0.00 | 3.90 | 0.00 | 3.90 | 2.90 | 3.59 | 3.67 | 2 | Centre North | Dry |
| La Libertad | Sep | 0.00 | 4.33 | 4.82 | 2.90 | 0.00 | 0.00 | 0.00 | 4.41 | 3.20 | 4.10 | 4.34 | 0.00 | 4.01 | 0.00 | 0.00 | 0.00 | 0.00 | 4.17 | 0.00 | 3.37 | 0.00 | 2.90 | 0.00 | 0 | Centre South | Dry |
| Pto. Bolivar | Sep | 0.00 | 3.37 | 3.20 | 0.00 | 0.00 | 0.00 | 0.00 | 4.59 | 3.67 | 0.00 | 0.00 | 2.90 | 3.80 | 0.00 | 0.00 | 0.00 | 3.37 | 4.46 | 0.00 | 3.90 | 0.00 | 0.00 | 4.40 | 3 | South | Dry |
| Esmeraldas | Oct | 0.00 | 3.85 | 3.97 | 0.00 | 3.20 | 0.00 | 0.00 | 3.37 | 3.59 | 0.00 | 3.37 | 3.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.04 | 0.00 | 3.59 | 0.00 | 3.59 | 0.00 | 0 | North | Dry |
| Manta | Oct | 4.10 | 4.69 | 3.80 | 4.10 | 3.59 | 4.29 | 0.00 | 3.74 | 3.85 | 4.22 | 4.39 | 3.59 | 4.40 | 0.00 | 0.00 | 3.67 | 3.50 | 4.51 | 0.00 | 3.74 | 0.00 | 3.20 | 3.50 | 2 | Centre North | Dry |
| La Libertad | Oct | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.13 | 3.85 | 3.37 | 0.00 | 4.01 | 0.00 | 0.00 | 0.00 | 3.67 | 3.50 | 4.44 | 0.00 | 3.50 | 0.00 | 3.80 | 0.00 | 0 | Centre South | Dry |
| Pto. Bolivar | Oct | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.85 | 2.90 | 0.00 | 0.00 | 3.67 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.51 | 0.00 | 3.37 | 0.00 | 2.90 | 0.00 | 0 | South | Dry |
| Esmeraldas | Nov | 4.46 | 5.03 | 4.20 | 4.55 | 4.50 | 0.00 | 0.00 | 3.80 | 3.97 | 4.45 | 4.62 | 4.07 | 3.80 | 0.00 | 0.00 | 4.43 | 3.90 | 4.33 | 0.00 | 3.90 | 0.00 | 4.36 | 4.31 | 3 | North | Dry |
| Manta | Nov | 4.22 | 5.08 | 4.07 | 3.80 | 4.40 | 5.94 | 0.00 | 4.67 | 4.39 | 4.07 | 5.62 | 3.97 | 5.09 | 3.80 | 0.00 | 4.69 | 4.40 | 4.15 | 0.00 | 3.74 | 0.00 | 3.90 | 3.85 | 2 | Centre North | Dry |
| La Libertad | Nov | 4.45 | 5.31 | 6.14 | 4.07 | 3.50 | 0.00 | 4.37 | 5.12 | 4.24 | 4.15 | 5.12 | 3.50 | 4.57 | 0.00 | 0.00 | 3.80 | 4.26 | 4.31 | 0.00 | 3.94 | 0.00 | 3.37 | 0.00 | 0 | Centre South | Dry |
| Pto. Bolivar | Nov | 3.80 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.87 | 3.85 | 3.20 | 4.13 | 3.50 | 3.74 | 0.00 | 0.00 | 3.20 | 4.31 | 3.37 | 0.00 | 3.20 | 0.00 | 2.90 | 0.00 | 0 | South | Dry |
| Esmeraldas | Dec | 4.24 | 4.64 | 3.20 | 4.75 | 4.20 | 3.97 | 3.20 | 0.00 | 3.85 | 3.37 | 4.78 | 3.94 | 4.26 | 0.00 | 0.00 | 3.85 | 0.00 | 3.97 | 0.00 | 3.85 | 2.90 | 3.80 | 4.26 | 3 | North | Rainy |
| Manta | Dec | 4.65 | 5.07 | 4.26 | 4.68 | 3.80 | 0.00 | 4.44 | 4.15 | 4.50 | 0.00 | 4.59 | 3.74 | 4.57 | 0.00 | 0.00 | 4.33 | 3.97 | 4.13 | 0.00 | 3.59 | 0.00 | 4.15 | 0.00 | 0 | Centre North | Rainy |
| La Libertad | Dec | 4.91 | 5.09 | 5.21 | 4.28 | 4.24 | 4.52 | 4.43 | 5.35 | 5.35 | 4.31 | 4.75 | 4.26 | 4.59 | 0.00 | 0.00 | 4.77 | 3.74 | 3.74 | 0.00 | 0.00 | 3.20 | 4.13 | 3.85 | 2 | Centre South | Rainy |
| Pto. Bolivar | Dec | 4.40 | 5.43 | 4.61 | 0.00 | 4.22 | 0.00 | 4.01 | 5.25 | 4.29 | 3.74 | 4.75 | 4.01 | 4.15 | 0.00 | 0.00 | 3.74 | 3.85 | 3.94 | 0.00 | 3.90 | 0.00 | 4.49 | 0.00 | 0 | South | Rainy |
References
- United Nations Agua. Available online: https://www.un.org/es/sections/issues-depth/water/index.html (accessed on 10 March 2021).
- Checa Artos, M.; Castillo, D.; Ruiz-Barzola, O.; Barcos-Arias, M. Presence of pharmaceutical products in water and its impact on the environment. Bionatura 2021, 6, 1618–1627. [Google Scholar] [CrossRef]
- Sperotto, A.; Molina, J.L.; Torresan, S.; Critto, A.; Pulido-Velazquez, M.; Marcomini, A. Water quality sustainability evaluation under uncertainty: A multi-scenario analysis based on bayesian networks. Sustainability 2019, 11, 4764. [Google Scholar] [CrossRef] [Green Version]
- Adger, W.N. Vulnerability. Glob. Environ. Chang. 2006, 16, 268–281. [Google Scholar] [CrossRef]
- Ding, Q.; Shi, X.; Zhuang, D.; Wang, Y. Temporal and spatial distributions of ecological vulnerability under the Influence of natural and anthropogenic factors in an eco-province under construction in China. Sustainability 2018, 10, 3087. [Google Scholar] [CrossRef] [Green Version]
- Gyamfi, C.; Ndambuki, J.M.; Salim, R.W. Simulation of sediment Yield in a semi-arid river Basin under changing land Uuse: An integrated approach of hydrologic modelling and Principal Component Analysis. Sustainability 2016, 8, 1133. [Google Scholar] [CrossRef] [Green Version]
- Molina, J.L.; Zazo, S.; Martín-Casado, A.M.; Patino-Alonso, M.C. Rivers’ temporal sustainability through the evaluation of predictive runoff methods. Sustainability 2020, 12, 1720. [Google Scholar] [CrossRef] [Green Version]
- Lake, Q.; Gu, Q.; Zhang, Y.; Ma, L.; Li, J.; Wang, K.; Zheng, K.; Zhang, X. Assessment of reservoir water quality using multivariate statistical techniques: A case study. Sustainability 2016, 8, 243. [Google Scholar] [CrossRef] [Green Version]
- Pearson, K. On lines and planes of closest fit to systems of points in space. Philos. Mag. 1901, 2, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Hotelling, H. Analysis of complex statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Hotelling, H. Simplified calculation of principal components. Psychometrika 1936, 1, 27–35. [Google Scholar] [CrossRef]
- Spearman, C. The proof and measurement of association between two things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Thurstone, L.L. Multiple Factor Analysis; University of Chicago Press: Chicago, IL, USA, 1947. [Google Scholar]
- Benzécri, J.P. L’analyse des données: L’analyse des correspondances. In L’Analyse des Données: Leçons sur L’analyse Factorielle et la Reconnaissance des Formes et Travaux, 22nd ed.; Benzécri, J.P., Ed.; Dunod: Paris, France, 1973; ISBN 9782040072254. [Google Scholar]
- Hill, M.O. Reciprocal averaging: An eigenvector method of ordination. J. Ecol. 1973, 61, 237–249. [Google Scholar] [CrossRef]
- Tenenhaus, M.; Young, F.W. An analysis and synthesis of multiple correspondence analysis, optimal scaling, dual scaling, homogeneity analysis and other methods for quantifying categorical multivariate data. Psychometrika 1985, 50, 91–119. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Gradient Anal. Author Cajo J. F. Ter Braak Source Ecol. Ecol. 1986, 67, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780444538680. [Google Scholar]
- Escofier, B.; Pagès, J. L’analyse factorielle multiple. Cah. Bur. Univ. Rech. Opérationnelle Série Rech. 1984, 42, 3–68. [Google Scholar]
- Escofier, B.; Pagès, J. Analyses factorielles simples et multiples: Objectifs. Méthodes Interpret. 1990, 1, 284. [Google Scholar]
- Escofier, B.; Pagès, J. Multiple factor analysis (AFMULT package). Comput. Stat. Data Anal. 1994, 18, 121–140. [Google Scholar] [CrossRef]
- L’Hermier Des Plantes, H. Structuration des Tableaux a Trois Indices de la Statistique: Theorie et Application d’une Methode D’analyse Conjointe; Université des Sciences et Techniques du Languedoc: Montpellier, France, 1976. [Google Scholar]
- Lavit, C.; Escoufier, Y.; Sabatier, R.; Traissac, P. The ACT (STATIS method). Comput. Stat. Data Anal. 1994, 18, 97–119. [Google Scholar] [CrossRef]
- Jaffrenou, P.A. Sur L’analyse des Familles Finies de Variables Vectorielles: Bases Algébriques et Application a la Description Statistique. Ph.D Thesis, Université Lyon 1, Villeurbanne, France, 1978. [Google Scholar]
- Gaertner, J.C.; Chessel, D.; Bertrand, J. Stability of spatial structures of demersal assemblages: A multitable approach. Aquat. Living Resour. 1998, 11, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Simier, M.; Blanc, L.; Pellegrin, F.; Nandris, D. Approche simultanée de K couples de tableaux: Application à l’étude des relations pathologie végétale–environnement. Rev. Stat. Appliquée 1999, 47, 31–46. [Google Scholar]
- Thioulouse, J.; Simier, M.; Chessel, D. Simultaneous analysis of a sequence of paired ecological tables. Ecology 2004, 85, 272–283. [Google Scholar] [CrossRef]
- Vivien, M.; Sabatier, R. A generalization of STATIS-ACT strategy: DO-ACT for two multiblocks tables. Comput. Stat. Data Anal. 2004, 46, 155–171. [Google Scholar] [CrossRef]
- Sauzay, L.; Hanafi, M.; Qannari, E.M.; Schlich, P. Analyse de K+ 1 Tableauxa L’aide De La Méthode STATIS: Application en Évaluation Sensorielle; 9ieme Journées Européennes Agro-Industrie et Méthodes Statistiques; Société Française de Statistique (SFdS): Montpellier, France, 2006. [Google Scholar]
- Abdi, H.; Valentin, D.; Chollet, S.; Chrea, C. Analyzing assessors and products in sorting tasks: DISTATIS, theory and applications. Food Qual. Prefer. 2007, 18, 627–640. [Google Scholar] [CrossRef]
- Sabatier, R.; Vivien, M. A new linear method for analyzing four-way multiblocks tables: STATIS-4. J. Chemom. 2008, 22, 399–407. [Google Scholar] [CrossRef]
- Marcondes Filho, D.; Fogliatto, F.S.; de Oliveira, L.P.L. Multivariate control charts for monitoring non-linear batch processes. Producao 2011, 21, 132–148. [Google Scholar] [CrossRef] [Green Version]
- Thioulouse, J. Simultaneous analysis of a sequence of paired ecological tables: A comparison of several methods. Ann. Appl. Stat. 2011, 5, 2300–2325. [Google Scholar] [CrossRef] [Green Version]
- Bénasséni, J.; Bennani Dosse, M. Analyzing multiset data by the Power STATIS-ACT method. Adv. Data Anal. Classif. 2012, 6, 49–65. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J.; Valentin, D.; Bennani-Dosse, M. STATIS and DISTATIS: Optimum multitable principal component analysis and three way metric multidimensional scaling. Wiley Interdiscip. Rev. Comput. Stat. 2012, 4, 124–167. [Google Scholar] [CrossRef]
- Sabatier, R.; Vivien, M.; Reynès, C.; Myrtille, V.; Christelle, R. Une nouvelle proposition, l’Analyse Discriminante Multitableaux: STATIS-LDA. J. Soc. Fr. Stat. Rev. Stat. Appliquée 2013, 154, 31–43. [Google Scholar]
- Rodríguez, O.; Corrales, D. Interstatis: The STATIS method for interval valued data. Rev. Mat. Teory Appl. 2014, 21, 73–83. [Google Scholar]
- Sautron, V.; Chavent, M.; Viguerie, N.; Villa-Vialaneix, N. Multiway-SIR for Longitudinal Multi-Table Data Integration. In Proceedings of the 22nd International Conference on Computational Statistics (COMPSTAT), Oviedo, Spain, 23–26 August 2016; p. 1. [Google Scholar]
- Kriegsman, M.A. Discriminant DiSTATIS: A Multi-Way Discriminant Analysis for Distance Matrices, Illustrations with the Sorting Task. Ph.D. Thesis, University of Texas at Dallas, Richardson, TX, USA, 2018. [Google Scholar]
- Llobell, F.; Cariou, V.; Vigneau, E.; Labenne, A.; Qannari, E.M. A new approach for the analysis of data and the clustering of subjects in a CATA experiment. Food Qual. Prefer. 2019, 72, 31–39. [Google Scholar] [CrossRef]
- Mérigot, B.; Gaertner, J.C.; Brind’amour, A.; Carbonara, P.; Esteban, A.; Garcia-Ruiz, C.; Gristina, M.C.; Imzilen, T.; Jadaud, A.; Joksimovic, A.; et al. Stability of the relationships among demersal fish assemblages and environmental-trawling drivers at large spatio-temporal scales in the northern mediterranean sea. Sci. Mar. 2019, 83, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Llobell, F.; Cariou, V.; Vigneau, E.; Labenne, A.; Qannari, E.M. Analysis and clustering of multiblock datasets by means of the STATIS and CLUSTATIS methods. Application to sensometrics. Food Qual. Prefer. 2020, 79, 1–9. [Google Scholar] [CrossRef]
- Tucker, L.R. Some mathematical notes on three-mode factor analysis. Psychometrika 1966, 31, 279–311. [Google Scholar] [CrossRef]
- Tucker, L.R. Relations between multidimensional scaling and three-mode factor analysis. Psychometrika 1972, 37, 3–27. [Google Scholar] [CrossRef]
- Tucker, L.R. Three-mode Factor Analysis Applied to Multidimensional Scaling. In Proceedings of the US-Japan Conference on Multidimensional Scaling, La Jolla, CA, USA, August 1975. [Google Scholar]
- Israelsson, A. Three-way (or second order) component analysis. Nonlinear Iterative Partial Least-Sq. Estim. Proced. Bull. Int. Stat. Inst. 1969, 43, 29–51. [Google Scholar]
- Kroonenberg, P.M.; De Leeuw, J. Principal component analysis of three-mode data by means of alternating least squares algorithms. Psychometrika 1980, 45, 69–97. [Google Scholar] [CrossRef]
- Harshman, R.A. Foundations of the PARAFAC procedure: Models and conditions for an “explanatory” multimodal factor analysis. UCLA Work. Pap. Phon. 1970, 16, 1–84. [Google Scholar]
- Carroll, J.D.; Chang, J.J. Analysis of individual differences in multidimensional scaling via an n-way generalization of “Eckart-Young” decomposition. Psychometrika 1970, 35, 283–319. [Google Scholar] [CrossRef]
- Carroll, J.D. IDIOSCAL (Individual Difference In Orientation SCALing): A generalization of INDSCAL allowing IDIOsyncratic reference systems as well as analytic approximation to INDSCAL. In Proceedings of the Paper Presented at Meeting of Psychometric Society, Princeton, NJ, USA, 20 August 1972. [Google Scholar]
- Harshman, R.A. Models for analysis of Asymmetrical Relationships Among N Objects or Stimuli. In Proceedings of the First Joint Meeting of the Psychometric Society and the Society of Mathematical Psychology, Hamilton, ON, Canada, 30 March 1978. [Google Scholar]
- Carroll, J.D.; Pruzansky, S.; Kruskal, J.B. CANDELINC: A general approach to multidimensional analysis of many-way arrays with linear constraints on parameters. Psychometrika 1980, 45, 3–24. [Google Scholar] [CrossRef]
- Harshman, R.A.; Lundy, M.E. Uniqueness proof for a family of models sharing features of Tucker’s three-mode factor analysis and PARAFAC/CANDECOMP. Psychometrika 1996, 61, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Bahram, M.; Bro, R. A novel strategy for solving matrix effect in three-way data using parallel profiles with linear dependencies. Anal. Chim. Acta 2007, 584, 397–402. [Google Scholar] [CrossRef]
- de Almeida, A.L.F.; Favier, G.; Mota, J.C.M. A constrained factor decomposition with application to MIMO antenna systems. IEEE Trans. Signal Process. 2008, 56, 2429–2442. [Google Scholar] [CrossRef]
- Giordani, P.; Rocci, R. Candecomp/Parafac with ridge regularization. Chemom. Intell. Lab. Syst. 2013, 129, 3–9. [Google Scholar] [CrossRef]
- Giordani, P.; Rocci, R. Constrained CP via the Lasso. Psychometrica 2013, 78, 669–684. [Google Scholar] [CrossRef]
- Martín-Rodríguez, J. Contribuciones a la Integración de Subespacios Desde una Perspectiva Biplot. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 1996. [Google Scholar]
- Martín-Rodríguez, J.; Galindo-Villardón, M.P.; Vicente-Villardón, J.L. Comparison and integration of subspaces from a biplot perspective. J. Stat. Plan. Inference 2002, 102, 411–423. [Google Scholar] [CrossRef]
- Baccalá, N. Contribuciones al Análisis de Matrices de Datos Multivía: Tipología de las Variables. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2004. [Google Scholar]
- Vallejo-Arboleda, A.; Vicente-Villardón, J.L.; Galindo-Villardón, M.P. Canonical STATIS: Biplot analysis of multi-table group structured data based on STATIS-ACT methodology. Comput. Stat. Data Anal. 2007, 51, 4193–4205. [Google Scholar] [CrossRef]
- Cortés Saud, Á. Contribuciones al Análisis de Tablas de Tres Vías Restringido. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2005. [Google Scholar]
- Basso, L.C. Análisis Conjunto de Varias Matrices de Datos: Contribuciones a la Tipología de los Individuos. Ph.D. Thesis, Universidad Salamanca, Salamanca, Spain, 2006. [Google Scholar]
- Vallejo, A.; Vicente, J.L.; Galindo, P.; Fernández, M.; Fernández, C.; Bécares, E. Análisis de la evolución en el tiempo para datos con estructura de grupos: STATIS dual canónico y modelo de medidas repetidas doblemente multivariantes. Rev. Colomb. Estad. 2008, 31, 321–340. [Google Scholar]
- Pinzón Sarmiento, L.M. Biplot Consenso para Análisis de Tablas Múltiples. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2011. [Google Scholar]
- Mendes, S.; Fernández-Gómez, M.J.; Marques, S.C.; Pardal, M.Â.; Azeiteiro, U.M.; Galindo-Villardón, M.P. CO-tucker: A new method for the simultaneous analysis of a sequence of paired tables. J. Appl. Stat. 2017, 44, 2729–2755. [Google Scholar] [CrossRef]
- Mendes, S. Métodos Multivariantes para Evaluar Patrones de Estabilidad y Cambio Desde Una Perspectiva Biplot. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2011. [Google Scholar]
- Frutos Bernal, E. Análisis de Datos Acoplados: Modelo T3-PCA. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2014. [Google Scholar]
- Egido, J.; Galindo, P. Dynamic Biplot. Evolution of the Economic Freedom in the European Union. Br. J. Appl. Sci. Technol. 2015, 11, 1–13. [Google Scholar] [CrossRef]
- Egido Miguélez, J.F. Biplot Dinámico. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2015. [Google Scholar]
- Rodríguez Rosa, M. Contribuciones al Análisis de la Sostenibilidad Internacional, Desde Una Perspectiva Algebraica Multivariante Comparada. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2016. [Google Scholar]
- Rodriguez-Rosa, M.; Gallego-Álvarez, I.; Galindo-Villardón, M.P. Spatio-temporal analysis of economic, social, and environmental issues in the framework of sustainable development in worldwide countries. Sustain. Dev. 2019, 27, 429–447. [Google Scholar] [CrossRef]
- González García, N. Análisis Sparse de Tensores Multidimensionales. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2019. [Google Scholar]
- Lavit, C.; Pernin, M.-O. Multivariate and longitudinal data on growing children: Solution using STATIS. In Data Analysis. The Ins and Outs of Solving Real Problems; Janssen, J., Marcotorchino, F., Proth, J.M., Eds.; Plenum: New York, NY, USA, 1987; pp. 13–29. [Google Scholar]
- Arcidiacono, C.; Sarnacchiaro, P.; Velleman, R. Testing fidelity to a new psychological intervention for family members of substance misusers during implementation in Italy. J. Subst. Use 2008, 13, 361–381. [Google Scholar] [CrossRef]
- Bono, F.; Giacomarra, M. The photovoltaic growth in the European Union requires stronger RES support. J. Policy Model. 2016, 38, 324–339. [Google Scholar] [CrossRef]
- Ramos-Barberán, M.; Hinojosa-Ramos, M.V.; Ascencio-Moreno, J.; Vera, F.; Ruiz-Barzola, O.; Galindo-Villardón, M.P. Batch process control and monitoring: A Dual STATIS and Parallel Coordinates (DS-PC) approach. Prod. Manuf. Res. 2018, 6, 470–493. [Google Scholar] [CrossRef] [Green Version]
- Niang, N.; Fogliatto, F.S.; Saporta, G. Batch process monitoring by three-way data analysis approach. In Proceedings of the XIIIth International Conference Applied Stochastic Models and Data Analysis ASMDA, Vilnius, Lithuania, 30 June–3 July 2009. [Google Scholar]
- Niang, N.; Fogliatto, F.; Saporta, G. Non parametric on-line control of batch processes based on STATIS and clustering. J. Soc. Fr. Stat. Stat. 2013, 154, 124–142. [Google Scholar]
- Scepi, G. Parametric and non parametric multivariate quality control charts. In Multivariate Total Quality Control; Lauro, C., Antoch, J., Esposito Vinzi, V., Sporta, G., Eds.; Physica-Verlag HD: New York, NY, USA, 2002; pp. 163–189. ISBN 9788578110796. [Google Scholar]
- Génard, M.; Souty, M.; Holmes, S.; Reich, M.; Breuils, L. Correlations among quality parameters of peach fruit. J. Sci. Food Agric. 1994, 66, 241–245. [Google Scholar] [CrossRef]
- Meyners, M.; Kunert, J.; Qannari, E.M. Comparing generalized procrustes analysis and statis. Food Qual. Prefer. 2000, 11, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Chaya, C.; Perez-Hugalde, C.; Judez, L.; Wee, C.S.; Guinard, J.X. Use of the STATIS method to analyze time-intensity profiling data. Food Qual. Prefer. 2003, 15, 3–12. [Google Scholar] [CrossRef]
- Lavit, C.; Perez Hugalde, C. Application de la méthode STATIS à des données économiques: Évolution des secteurs agricoles et non agricoles des provinces espagnoles de 1960 à 1979. Quatr. J. Int. Anal. des Données Inform. 1985, 9, 11. [Google Scholar]
- Lavit, C. Application de la méthode STATIS. Stat. Anal. Données 1985, 10, 103–116. [Google Scholar]
- Sandoval Solís, L.; Linares Fleites, G.; Quiroz Clemente, A.; Romero Váquez, M.G. Implementacion del Método Statis en R y su aplicación al estudio de satisfacción de usuarios del sistema bibliotecario de la BUAP. Rev. Investig. Oper. 2012, 32, 56–66. [Google Scholar]
- Ubertalli, B.; Pernin, M.-O. Utilisation d’ une méthode multi-tableaux en sciences sociales. Une étude longitudinale de carrières: Les 12 premières promotions de l ’ école d ’ infirmières de Roanne. Population 1990, 45, 1092–1100. [Google Scholar] [CrossRef]
- Volkova, M. Russian and European Population’s Quality of Life Analysis with the Instruments of Common Principal Components (CPC). Ekon. Mat. Metod. 2019, 55, 34–46. [Google Scholar] [CrossRef]
- Raymond, O.; Fiasson, J.L.; Jay, M. Synthetic taxonomy of Rosa races using ACT-STATIS. Z. Naturforsch. C. 2000, 55, 399–409. [Google Scholar] [CrossRef]
- Grossi, C.; Raymond, O.; Sanlaville-Boisson, C.; Jay, M. Rosa taxonomy and hierarchy of markers defined by ACT STATIS. Z. Naturforsch. C 1999, 54, 25–34. [Google Scholar] [CrossRef]
- Coquet, R.; Troxler, L.; Wipff, G. The STATIS method: Characterization of conformational states of flexible molecules from molecular dynamics simulations in solution. J. Mol. Graph. 1996, 14, 206–212. [Google Scholar] [CrossRef]
- L’Hermier Des Plantes, H.; Thiébaut, B. Étude de la pluviosité au moyen de la méthode STATIS. Rev. Stat. Appliquée 1977, 25, 57–81. [Google Scholar]
- Márquez, J.L.; Díaz de Pascual, A.; Defives, G. Aplicación del método Statis: Factores físico-químicos del agua del embalse Uribante. Economía 1992, 17, 35–58. [Google Scholar]
- Stanimirova, I.; Walczak, B.; Massart, D.L.; Simeonov, V.; Saby, C.A.; Di Crescenzo, E. STATIS, a three-way method for data analysis. Application to environmental data. Chemom. Intell. Lab. Syst. 2004, 73, 219–233. [Google Scholar] [CrossRef]
- Mendes, S.; Marques, S.C.; Azeiteiro, U.M.; Fernández-Gómez, M.J.; Galindo-Villardon, M.P.; Maranhão, P.; Morgado, F.; Leandro, S.M. Zooplankton Distribution in a Marine Protected Area: The Berlengas Natural Reserve (Western Coast of Portugal). Fresenius Environ. Bull. 2011, 20, 496–505. [Google Scholar]
- Mendes, S.; Fernández-Gómez, M.J.; Pereira, M.J.; Azeiteiro, U.M.; Galindo-Villardón, M.P. An empirical comparison of Canonical Correspondence Analysis and STATICO in the identification of spatio-temporal ecological relationships. J. Appl. Stat. 2012, 39, 979–994. [Google Scholar] [CrossRef]
- Mendes, S.; Fernández-Gómez, M.J.; Resende, P.; Jorge Pereira, M.; Galindo-Villardón, M.P.; Azeiteiro, U.M. Spatio-temporal structure of diatom assemblages in a temperate estuary. A STATICO analysis. Estuar. Coast. Shelf Sci. 2009, 84, 637–644. [Google Scholar] [CrossRef]
- Marques, S.C.; Primo, A.L.; Falcão, J.; Martinho, F.; Mendes, S.; Azeiteiro, U.M.; Pardal, M.A. The Impact of Conspicuous Environmental Changes on the Spatial and Temporal Dynamics of Acartia Tonsa and Acartia Clausi: A decadal Study in a Temperate Estuary (Mondego, Portugal). In Trends in Copepod Studies: Distribution, Biology and Ecology; Uttieri, M., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2018; pp. 145–171. ISBN 978-1-53612-593-1. [Google Scholar]
- Slimani, N.; Guilbert, E.; Ayni, F.; El Jrad, A.; Boumaiza, M.; Thioulouse, J. The use of STATICO and COSTATIS, two exploratory three-ways analysis methods: An application to the ecology of aquatic heteroptera in the Medjerda watershed (Tunisia). Environ. Ecol. Stat. 2017, 24, 269–295. [Google Scholar] [CrossRef]
- Feki-Sahnoun, W.; Hamza, A.; Béjaoui, B.; Mahfoudi, M.; Rebai, A.; Bel Hassen, M. Multi-table approach to assess the biogeography of phytoplankton: Ecological and management implications. Hydrobiologia 2018, 815, 229–251. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, C.C.; García-Sánchez, I.M.; Vicente-Galindo, P.; Galindo-Villardón, P. Exploring relationships between environmental performance, E-Government and corruption: A multivariate perspective. Sustainability 2019, 11, 6497. [Google Scholar] [CrossRef] [Green Version]
- Amor-Esteban, V.; Galindo-Villardón, M.P.; García-Sánchez, I.M. Industry mimetic isomorphism and sustainable development based on the X-STATIS and Hj-biplot methods. Environ. Sci. Pollut. Res. 2018, 25, 26192–26208. [Google Scholar] [CrossRef]
- Martínez-Córdoba, P.J.; Amor-Esteban, V.; Benito, B.; García-Sánchez, I.M. The commitment of spanish local governments to sustainable development goal 11 from a multivariate perspective. Sustainability 2021, 13, 1222. [Google Scholar] [CrossRef]
- Gudmundsson, L.; Tallaksen, L.M.; Stahl, K. Spatial cross-correlation patterns of European low, mean and high flows. Hydrol. Process. 2011, 25, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Fournier, M.; Motelay-Massei, A.; Massei, N.; Aubert, M.; Bakalowicz, M.; Dupont, J.P. Investigation of transport processes inside karst aquifer by means of STATIS. Ground Water 2009, 47, 391–400. [Google Scholar] [CrossRef]
- González-Narváez, M. Distribución Espacio-Temporal Del Fitoplancton en el Pacífico Ecuatorial Oriental, Zona de la Región Niño 1 + 2, Aplicación del Método STATICO. Master’s Thesis, Universidad de Salamanca, Salamanca, Spain, 2016. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Supply and Services Canada: Ottawa, ON, Canada, 1972; Volume 167. [Google Scholar]
- Utermöhl, H. Zur Vervollkommnung der Quantitativen Phytoplankton-Methodik; Schweizerbart: Stuttgart, Germany, 1958. [Google Scholar]
- Reguera, B.; Alonso, R.; Moreira, Á.; Méndez, S. Guía para el diseño y puesta en marcha de un plan de seguimiento de microalgas productoras de toxinas. Com. Ocean. Intergub. 2011, 59, 1–65. [Google Scholar]
- Torres, G. Evaluación del fitoplancton Como un Mecanismo Preventivo a la Ocurrencia de Bloom Algal Frente a las Costas de Esmeraldas, Manta, La Libertad y Puerto Bolívar en Ecuador 2013–2015. Ph.D. Thesis, Universidad Nacional Mayor de San Marcos, Perú, Lima, 2017. [Google Scholar]
- Jiménez, R. Diatomeas y silicoflagelados del fitoplancton del Golfo de Guayaquil II Edición. Acta Ocean. Pacífico 1983, 2, 193–281. [Google Scholar]
- Pesantes, F. Los dinoflagelados como indicadores de El Niño en el mar ecuatoriano. Acta Ocean. Pacífico 1983, 2, 1–117. [Google Scholar]
- Balech, E. Los dinoflagelados del Atlántico Sudoccidental; Ministerio de Agricultura Pesca y Alimentación; Secretaría General Técnica: Madrid, Spain, 1988; ISBN 84-7479-711-X. [Google Scholar]
- Tomas, C.R. Marine Phytoplankton: A Guide to Naked Flagellates and Coccolithophorids; Academic Press: Cambridge, MA, USA, 1993; ISBN 978-0-12-693010-8. [Google Scholar]
- Taylor, F.J.R.; Fukuyo, Y.; Larsen, J. Taxonomy of Harmful Dinoflagellates in Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; IOC Manuals and Guides No 33; UNESCO: Paris, France, 1995; ISBN 2831708265. [Google Scholar]
- Hasle, G.R.; Syvertsen, E.E.; Steidinger, K.A.; Tangen, K.; Tomas, C.R. Identifying Marine Diatoms and Dinoflagellates; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1996; ISBN 978-0-12-693015-3. [Google Scholar]
- Tomas, C.R. Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA, 1997; ISBN 9780080534428. [Google Scholar]
- Young, J.; Geisen, M.; Cros, L.; Kleijne, A.; Sprengel, C.; Probert, I.; Østergaard, J. A guide to extant coccolithophore taxonomy. J. Nannoplankt. Res. Spec. 2003, 1, 1–132. [Google Scholar]
- Jiménez, R. Diatomeas y silicoflagelados del fitoplancton del Golfo de Guayaquil III Edición. Acta Ocean. Pacífico 2014, 19, 1–89. [Google Scholar]
- Kroonenberg, P.M. Applied Multiway Data Analysis; Wiley-Interscience: Hoboken, NJ, USA, 2008; ISBN 9780470164976. [Google Scholar]
- Legendre, L.; Legendre, P. Le traitement multiple des données écologiques. Ecol. Numer. Tom 1 1979, 66, 775–776. [Google Scholar] [CrossRef]
- Rao, C.R. The use and interpretation of Principal Component Analysis in applied research stable. Indian J. Stat. 1964, 26, 329–358. [Google Scholar]
- Dolédec, S.; Chessel, D. Co-inertia analysis: An alternative method for studying species-environment relationships. Freshw. Biol. 1994, 31, 277–294. [Google Scholar] [CrossRef]
- Gower, J.C. A general coefficient of similarity and some of its properties. Biometrics 1971, 27, 857–871. [Google Scholar] [CrossRef]
- Escofier, B.; Pagès, J. Analyses Factorielles Simples et Múltiples; Dunod: Paris, France, 1998. [Google Scholar]
- Escoufier, Y. Le Traitement des Variables Vectorielles. Biometrics 1973, 29, 751–760. [Google Scholar] [CrossRef]
- Gower, J.C. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 1966, 53, 325–338. [Google Scholar] [CrossRef]
- INOCAR Capítulo I: Información general de la República del Ecuador Inocar 2012. Available online: https://www.inocar.mil.ec/docs/derrotero/derrotero_cap_I.pdf (accessed on 15 September 2019).
- Santos, J.L. The impact of El Niño—Southern Oscillation Events on South America. Adv. Geosci. 2006, 6, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Schlitzer, R. Ocean Data View 2020. Available online: https://odv.awi.de (accessed on 23 May 2021).
- Bastidas-Arteaga, E. Reliability of reinforced concrete structures subjected to corrosion-fatigue and climate change. Int. J. Concr. Struct. Mater. 2018, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Mackey, K.R.M. Phytoplankton as key mediators of the biological carbon pump: Their responses to a changing climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- Zacchei, E.; Molina, J.L. Reviewing arch-dams’ building risk reduction through a sustainability-safety management approach. Sustainability 2020, 12, 392. [Google Scholar] [CrossRef] [Green Version]
- Anzecc, A. Australian and New Zealand Guidelines for Fresh and Marine Water Quality; Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand: Canberra, Australia, 2000; pp. 1–103. [Google Scholar]
- Wolanski, E.; Boorman, L.A.; Chícharo, L.; Langlois-Saliou, E.; Lara, R.; Plater, A.J.; Uncles, R.J.; Zalewski, M. Ecohydrology as a new tool for sustainable management of estuaries and coastal waters. Wetl. Ecol. Manag. 2004, 12, 235–276. [Google Scholar] [CrossRef]
- Conde, A.; Hurtado, M.; Prado, M. Phytoplankton response to a weak El Niño event. Ecol. Indic. 2018, 95, 394–404. [Google Scholar] [CrossRef]
- Torres, G.; Recalde, S.; Narea, R.; Renteria, W.; Troccoli, L. Variabilidad espacio-temporal del fitoplancton y variables oceanográficas en el Golfo de Guayaquil durante el 2013-15. Rev. Inst. Investig. FIGMMG-UNMSM 2017, 20, 70–79. [Google Scholar]
- Torres, G.; Carnicer, O.; Canepa, A.; De La Fuente, P.; Recalde, S.; Narea, R.; Pinto, E.; Borbor-Córdova, M.J. Spatio-Temporal pattern of dinoflagellates along the Tropical Eastern Pacific coast (Ecuador). Front. Mar. Sci. 2019, 6, 1–20. [Google Scholar] [CrossRef]
- Torres, G. Importancia ecológica del fitoplancton durante El Niño 1991-1993, en el Pacífico Ecuatorial (Ecuador). Acta Ocean. Pacífico 2005, 13, 35–49. [Google Scholar]
- Torres, G.; Tapia, M.E. Distribución del fitoplancton en la región costera del mar ecuatoriano, durante diciembre 2000. Acta Ocean. Pacífico 2002, 11, 62–72. [Google Scholar]





| Location (Both Seasons) | Season (Entire Coastal Profile) | Total | |||||
|---|---|---|---|---|---|---|---|
| North | Centre North | Centre South | South | Rainy | Dry | ||
| Temperature (T—°C) | 25.77 ± 1.34 | 23.96 ± 1.18 | 22.31 ± 1.79 | 22.36 ± 1.03 | 24.03 ± 1.79 | 23.24 ± 2.04 | 23.6 ± 1.97 |
| Salinity (S—psu) | 32.92 ± 0.49 | 33.57 ± 0.38 | 34.03 ± 0.41 | 33.7 ± 0.72 | 33.49 ± 0.68 | 33.61 ± 0.63 | 33.56 ± 0.66 |
| Dissolved Oxygen (DO—mg.L−1) | 4.5 ± 0.33 | 4.38 ± 0.2 | 4.23 ± 0.61 | 4.16 ± 0.6 | 4.34 ± 0.49 | 4.3 ± 0.48 | 4.32 ± 0.49 |
| Nitrate (—µg-at.L−1) | 1.28 ± 1.59 | 2.18 ± 1.81 | 3.13 ± 2.2 | 4.25 ± 2.74 | 3.53 ± 2.6 | 2.03 ± 1.97 | 2.71 ± 2.4 |
| Nitrite (—µg-at.L−1) | 0.12 ± 0.11 | 0.17 ± 0.13 | 0.24 ± 0.15 | 0.27 ± 0.19 | 0.25 ± 0.18 | 0.16 ± 0.12 | 0.2 ± 0.16 |
| Phosphate (—µg-at.L−1) | 0.59 ± 0.53 | 0.67 ± 0.52 | 0.85 ± 0.31 | 0.69 ± 0.37 | 0.91 ± 0.51 | 0.53 ± 0.31 | 0.7 ± 0.45 |
| Silicate (—µg-at.L−1) | 6.19 ± 4.31 | 5.33 ± 3.62 | 9.62 ± 3.76 | 9.01 ± 3.07 | 8.85 ± 4.3 | 6.44 ± 3.65 | 7.54 ± 4.14 |
| Group | Species Phytoplankton Abundance Cells L−1 | Location (Both Seasons) | Season (Entire Coastal Profile) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| North | Centre North | Centre South | South | Rainy | Dry | |||
| CD | Dactyliosolen fragilissimus (e1) | 2.78 ± 2.14 | 3.88 ± 1.34 | 2.89 ± 2.22 | 1.94 ± 2.15 | 2.41 ± 2.21 | 3.26 ± 1.94 | 2.87 ± 2.11 |
| CD | Guinardia striata (e2) | 4.29 ± 1.42 | 4.23 ± 1.43 | 3.91 ± 1.9 | 2.84 ± 2.3 | 3.52 ± 1.82 | 4.07 ± 1.91 | 3.82 ± 1.89 |
| CD | Rhizosolenia imbricata (e3) | 3.76 ± 1.34 | 3.99 ± 1.41 | 3.17 ± 2.47 | 2.16 ± 2 | 2.37 ± 2 | 4.02 ± 1.65 | 3.27 ± 1.99 |
| CD | Dactyliosolen antarcticus (e4) | 2.76 ± 2.11 | 3.48 ± 1.74 | 2.07 ± 2.38 | 0.93 ± 1.97 | 1.26 ± 1.94 | 3.18 ± 2.15 | 2.31 ± 2.27 |
| CD | Proboscia alata (e5) | 3.46 ± 1.22 | 2.55 ± 1.97 | 2.11 ± 1.99 | 0.84 ± 1.78 | 1.61 ± 1.82 | 2.76 ± 2 | 2.24 ± 2.01 |
| CD | Skeletonema costatum (e6) | 1.97 ± 2.17 | 1.33 ± 2.21 | 1 ± 1.67 | 0.96 ± 2.08 | 1.12 ± 2.02 | 1.48 ± 2.12 | 1.32 ± 2.08 |
| CD | Lauderia borealis (e7) | 0.62 ± 1.33 | 0.4 ± 1.28 | 1.17 ± 1.92 | 1.17 ± 1.91 | 0.8 ± 1.62 | 0.88 ± 1.71 | 0.84 ± 1.67 |
| PD | Nitzschia longissima (e8) | 3.84 ± 1.29 | 4.48 ± 0.64 | 3.75 ± 1.83 | 4.46 ± 0.5 | 3.96 ± 1.74 | 4.28 ± 0.49 | 4.13 ± 1.24 |
| PD | Nitzschia sp. (e9) | 2.36 ± 1.8 | 3.41 ± 1.2 | 2.33 ± 1.88 | 2.97 ± 1.46 | 1.97 ± 2.04 | 3.43 ± 0.83 | 2.77 ± 1.67 |
| PD | Pseudo-nitzschia pungens (e10) | 3.25 ± 1.63 | 2.93 ± 1.82 | 2.05 ± 1.9 | 2.82 ± 2.23 | 2.85 ± 1.97 | 2.69 ± 1.95 | 2.76 ± 1.96 |
| CD | Leptocylindrus danicus (e11) | 4.38 ± 0.47 | 4.48 ± 0.77 | 3.25 ± 2.03 | 2.74 ± 2.11 | 3.41 ± 1.78 | 3.97 ± 1.59 | 3.71 ± 1.7 |
| CD | Thalassiosira sp. (e12) | 1.89 ± 1.75 | 2.73 ± 1.69 | 2.98 ± 1.84 | 2.77 ± 1.38 | 2.21 ± 1.85 | 2.91 ± 1.54 | 2.59 ± 1.73 |
| CD | Chaetoceros affinis (e13) | 3.51 ± 1.72 | 4.58 ± 0.41 | 3 ± 1.9 | 2.8 ± 2.18 | 3.29 ± 2.01 | 3.62 ± 1.65 | 3.47 ± 1.83 |
| CD | Chaetoceros curvisetus (e14) | 1.6 ± 2.14 | 1.44 ± 1.91 | 0 ± 0 | 0.87 ± 1.85 | 1.08 ± 1.89 | 0.89 ± 1.75 | 0.98 ± 1.82 |
| CD | Chaetoceros didymus (e15) | 0 ± 0 | 0.71 ± 1.51 | 0.34 ± 1.08 | 0.37 ± 1.16 | 0.78 ± 1.56 | 0 ± 0 | 0.35 ± 1.12 |
| CD | Hemiaulus sinensis (e16) | 3.05 ± 1.99 | 3.04 ± 1.9 | 2.87 ± 1.86 | 1.5 ± 2.08 | 2.32 ± 1.94 | 2.86 ± 2.13 | 2.62 ± 2.07 |
| PD | Thalassionema nitzschioides (e17) | 2.31 ± 1.77 | 2.75 ± 1.72 | 1.83 ± 2.02 | 2.06 ± 1.92 | 1.84 ± 1.86 | 2.56 ± 1.85 | 2.24 ± 1.89 |
| D | Gymnodinium sp. (e18) | 3.23 ± 1.53 | 3.5 ± 1.21 | 3.64 ± 1.23 | 3.22 ± 1.57 | 2.89 ± 1.71 | 3.82 ± 0.89 | 3.4 ± 1.41 |
| D | Gyrodinium sp. (e19) | 1 ± 1.66 | 0.91 ± 1.53 | 0.61 ± 1.29 | 0.55 ± 1.18 | 1.53 ± 1.73 | 0.13 ± 0.64 | 0.77 ± 1.44 |
| C | Mesodinium rubrum (e20) | 3.29 ± 1.07 | 2.92 ± 1.39 | 3.18 ± 1.13 | 3.72 ± 0.55 | 3.34 ± 1.21 | 3.23 ± 1.03 | 3.28 ± 1.12 |
| CD | Ditylum brightwellii (e21) | 1.46 ± 1.61 | 0.79 ± 1.29 | 0.63 ± 1.34 | 0.89 ± 1.45 | 0.79 ± 1.38 | 1.07 ± 1.52 | 0.94 ± 1.46 |
| PD | Navicula sp. (e22) | 1.69 ± 1.87 | 2.77 ± 1.36 | 1.89 ± 1.76 | 1.9 ± 1.79 | 2 ± 1.85 | 2.12 ± 1.68 | 2.06 ± 1.76 |
| PD | Stauroneis membranacea (e23) | 2.49 ± 1.91 | 2.55 ± 1.94 | 1.66 ± 1.82 | 1.42 ± 1.89 | 1.72 ± 1.92 | 2.28 ± 1.95 | 2.03 ± 1.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Narváez, M.; Fernández-Gómez, M.J.; Mendes, S.; Molina, J.-L.; Ruiz-Barzola, O.; Galindo-Villardón, P. Study of Temporal Variations in Species–Environment Association through an Innovative Multivariate Method: MixSTATICO. Sustainability 2021, 13, 5924. https://doi.org/10.3390/su13115924
González-Narváez M, Fernández-Gómez MJ, Mendes S, Molina J-L, Ruiz-Barzola O, Galindo-Villardón P. Study of Temporal Variations in Species–Environment Association through an Innovative Multivariate Method: MixSTATICO. Sustainability. 2021; 13(11):5924. https://doi.org/10.3390/su13115924
Chicago/Turabian StyleGonzález-Narváez, Mariela, María José Fernández-Gómez, Susana Mendes, José-Luis Molina, Omar Ruiz-Barzola, and Purificación Galindo-Villardón. 2021. "Study of Temporal Variations in Species–Environment Association through an Innovative Multivariate Method: MixSTATICO" Sustainability 13, no. 11: 5924. https://doi.org/10.3390/su13115924









