1. Introduction
Invented in the late nineteenth century, reinforced concrete (RC) is today the most widespread building material in the world. Unfortunately, during the service life of existing structures (e.g., buildings, bridges, tunnels), the deterioration of RC elements occurs due to many factors including structural loads, quality of materials, environmental conditions, etc. [
1,
2]. Furthermore, the impact of climate change (e.g., global warming) on existing RC structures is considerable, since the corrosion deteriorations induce an increase in repair cost. The elevated carbon dioxide (CO
2) levels related to global warming can increase the potential of carbonation-induced corrosion. Reinforcement corrosion is one of the most dominant deterioration mechanisms. It inflicts damages that lead to a decrease in the performance as well as the safety of existing structures [
3]. Corrosion attacks can be classified into two sources, which are the carbonation of concrete and the penetration of chloride ions. The carbonation of concrete implies a potential risk in the durability of RC structures if the carbonation depth reaches the steel rebars [
4].
Carbon dioxide in gaseous form in the atmosphere penetrates the porous network of concrete. In contact with the pore solution, the CO
2 dissolves and causes a chemical reaction with hydrated cement paste. This reaction transforms the hydration products (e.g., portlandite, calcium silicate hydrates) into calcium carbonate. The carbonation kinetics depend on the concrete mix (e.g., cement type, cement content, water/cement ratio) and exposure conditions (e.g., concrete surface sheltered or not from rain, relative humidity) [
5,
6,
7,
8,
9]. During carbonation, the pore solution pH of concrete should be below 9.0 for a fully carbonated zone [
10]. This process can induce the destabilization of the passive film on the rebar surface. Steel corrosion can then be initiated according to the conditions of humidity and oxygen, which causes disturbances in the mechanical behavior of concrete structures and may lead to cracking [
11,
12].
The carbonation issue has been intensively investigated for decades, mainly in accelerated conditions (high relative pressure of carbon dioxide) due to its slowness in atmospheric conditions. Although the findings of these investigations have brought significant and valuable knowledge, some aspects of the phenomenon remain comprehensively unelucidated. The kinetics and type of hydrated compounds carbonated are drastically different with respect to the exposure conditions. The constant fluctuation of relative humidity and temperature in outdoor conditions is often reduced to an average effect. The diffusion of calcium ions in the pore solution, from the dissolution site to the reactive site, should be taken into account in the carbonation models [
13]. Climate change, the impact of which on concrete structures is expected to increase over the century, ought to be more extensively investigated, in particular the thermo-activation on reactions [
14,
15]. The present paper addresses these aspects from the numerical viewpoint.
Numerous models have been developed to estimate the carbonation depth of concrete, which are classified by either the approach or the targeted objective. Simplified models were established [
16,
17,
18,
19,
20,
21,
22] to provide easy-to-use tools for engineers that estimate the carbonation depth with an acceptable precision using a limited number of input parameters. In general, these models were based on the assumption that the partial pressure of carbon dioxide varies linearly from the exposed surface of the material to the carbonation front. The carbonation depth is thus a square root function of time. Meanwhile, the numerical models of concrete carbonation have been also developed by many researchers [
7,
9,
23,
24,
25,
26,
27,
28,
29]. The numerical models aim to describe the phenomena involved as well as various influencing factors using a more detailed approach than the simplified models. These models are mainly based on solving the mass conservation equations and require implementation in a calculation program.
Among the numerical carbonation models developed in the recent research works, Bary et al.’s model has been the most promising regarding the aspects previously mentioned [
26,
27]. This model couples the transfer of carbon dioxide in the gaseous phase with the transfers of liquid water and calcium ions in an aqueous solution. The chemical model of hydrate leaching is only governed by the concentration of calcium ions. The approach used makes it possible to take into account the carbonation of all hydrated phases consisting of portlandite, C-S-H, ettringite (AFt or trisulfoaluminate), and monosulfoaluminate (AFm). The mass conservation equations in the model of Bary et al. 2004 [
26] have been considered in this study. In addition, specific developments have been achieved to account more realistically for outdoor exposure conditions. The results over a long time of exposure are given through various profiles (hydrated compounds, porosity, saturation degree, calcite, etc.). Results show how the computed carbonation rate is influenced by both the boundary conditions and the usually accepted assumptions.
The present study aims to propose an advanced numerical model to simulate the penetration of carbon dioxide and moisture, diffusion of calcium ions, heat transfer, and porosity modification in conventional concrete. The predictions of portlandite profile and carbonation depth are compared with the experimental data. Then, the proposed model has been applied to two concretes having compressive strengths measured on cylindrical specimens that correspond to C25 and C45 classes according to European standard EC2. Furthermore, the actual temporal variabilities of temperature and relative humidity in Toulouse, France, are used as a case study to identify the effect of climatic variations on concrete carbonation.
2. Numerical Model of Concrete Carbonation
The model proposed by Bary and Sellier 2004 [
26] assumes that a volume of concrete is composed of three phases (liquid, solid, and gas), and the chemical reactions of dissolution, solidification, vaporization, and liquefaction occur at the interface between these phases. In this model, the phenomena are considered, such as water permeability of the porous medium, diffusion of CO
2 in the gas phase of the pore solution, migration of calcium ions in the liquid phase, gradual decalcification by the dissolution of hydrated cement paste, formation of calcite by reaction between calcium ions and dissolved CO
2, and variation in porosity due to both decalcification and formation of calcite. The modeling approach proposed is based on the coupling of three equations of mass conservation for water, carbon dioxide, and calcium.
2.1. Mass Conservation Equations
The model is based on three equations of mass conservation that are written for the contents of water, carbon dioxide, and calcium in the concrete medium. These parameters are characterized by the degree of water saturation, the partial pressure of carbon dioxide in the interstitial gas phase, and the concentration of calcium ions in the pore liquid phase [
26,
27]. The mass conservation equations are shown in detail for each parameter in the next paragraphs.
2.1.1. Mass Conservation of Water
The mass variation of water (liquid phase) in a unit volume during a time interval (
dt) is based on the water mass flow (devoted
wl) and the amount of water released (devoted
Sl) with the hydrates dissolution, as expressed in Equation (1) with
Sr being the saturation degree,
ρl (kg/m
3) being the density of water, and
ϕ being the porosity.
The water mass flow
wl is given by Equation (2), with
Kϕ (m
2) being the intrinsic permeability of the material,
η (kg/m.s) being the dynamic viscosity coefficient of water,
krl being the relative permeability that depends on the saturation degree
Sr of concrete [
30],
pcap (Pa) being the capillary pressure, and
hr being the relative humidity.
The water released
Sl through the hydrates dissolution is expressed in Equation (3), with
ksl being the coefficient for the influence of the saturation degree on the reaction process [
31] (due to the variation of the contact surface between water and hydrates),
(kg/mol) is the molar mass of water, the term
nw refers to the concentration of water involved in the solid phase dissolution by unit volume of material, and its value depends on the calcium concentration
Ca (mol/m
3) in the pore solution [
6,
32,
33].
Finally, the equation of water mass conservation is expressed as follows:
2.1.2. Mass Conservation of Carbon Dioxide
The mass variation of carbon dioxide in a volume unit during a time interval (
dt) depends on the carbon dioxide mass flow (devoted
wc) through the volume and on its consumption by the chemical reaction to form calcite (devoted
Sc), as expressed in Equation (5), with
ρc (kg/m
3) being the density of carbon dioxide and
ϕ being the porosity.
The CO
2 mass flow
wc is expressed in Equation (6) with
(m
2/s) being the diffusion coefficient of CO
2 in the air,
being a factor of diffusion resistance,
Mc (kg/mol) being the molar mass of carbon dioxide,
R (m
2·kg/mol·K·s
2) being the ideal gas constant,
T (K) being the temperature, and
pc being the capillary pressure [
26,
34].
The CO
2 mass precipitated
SC from the pore solution per unit of time to form calcite is given by Equation (7), with
ncal being the molar formation rate of calcite.
Thus, the equation of the mass conservation of carbon dioxide can be rewritten as follows:
2.1.3. Mass Conservation of Calcium
The variation of calcium concentration
Ca in the porous solution is expressed in Equation (9) for a volume unit, with
wa being the flux of calcium ions and
Sa being the source term of the production of calcium ions released from the hydrates during the decalcification process [
35].
The flux of calcium ions through the porous solution the calcium flux derives from the concentration gradient is determined by Equation (10), with
being the effective diffusion coefficient of calcium ions in the pore solution that depends on both the saturation degree and the porosity.
The calcium ions production
Sa is partially offset by calcium consumption through the formation of calcite as expressed in Equation (11), with
nCaS (mol/m
3) being the concentration of calcium in the solid phase in a unit volume of a porous material.
Through this mass balance equation, the concentration of calcium ions in the porous solution, as well as the concentration of calcium in the solid phase of the porous material, is calculated depending on the water flow, the calcium ions flow, and the calcite formation and hydrates dissolution rates.
2.2. Additional Equations
In order to assess detailed evolutions in the porous material, the additional equations are also used for the porosity variation, the content of portlandite, other hydrates, and calcite.
2.2.1. Equation of Porosity Variation
The porosity variation is due to a difference in molar volume between the initial products (hydrates) and final products (calcite, vaterite, aragonite) of the carbonation process. The dissolution of one mole of portlandite releases one mole of calcium that precipitates in one mole of calcite. The ratio is somehow different for other hydrates (including C-S-H, aluminates, ettringite, etc.). The difference between the molar volumes of these components induces a decrease in the material porosity. This variation is modeled by Equation (12), in which
VdS (m
3/mol) is the volume fraction of dissolved hydrates in a unit volume of the hydrated material,
Ca (mol/m
3) is the concentration of calcium ion in the aqueous phase, and
(37 × 10
−6 m
3/mol) is the molar volume of calcite [
17].
2.2.2. Equation of Hydrates Dissolution Rate
The hydrates of cement paste are dissolved during the carbonation process, and the kinetic of the solubilization depends on the dissolved carbon dioxide and the saturation degree. Different kinds of kinetics for portlandite and other hydrates have been considered here, due to the high dependency of the global carbonation kinetics on the dissolution of portlandite.
● Portlandite Dissolution Rate
The kinetics of portlandite dissolution is highly important to assess the carbonation degradation, because this hydrate is the most sensitive to any chemical disorder and is responsible for the basic pH of concrete. Therefore, carbonation depth estimation is based on the portlandite dissolution. In this study, a different kinetic is proposed for portlandite and other hydrates, as in Equation (13) proposed by Thiery 2005 [
7] and Bary and Mügler 2006 [
27].
where
kH (mol·m
−3·Pa
−1) is Henry’s constant for carbon dioxide [
33,
35] and
kCH is the kinetic coefficient for portlandite dissolution [
7,
27], depending on the current volume fraction of portlandite (
Vp). This coefficient expresses the formation and growth of a calcite layer around spherical portlandite crystals during the carbonation process. Thus, the kinetic coefficient decreases as the calcite layer grows:
where
Vp (m
3/mol) is the current volume fraction of portlandite;
Rp0 (m) is the equivalent radius of the initial portlandite crystals;
and
(m
3/mol) are portlandite and calcite molar volumes;
λ stands for the equivalent thickness of the calcite layer.
The degradation index
kp (m) is determined following Equation (15):
where:
nCH,0 and
nCH (mol/m
3) are the initial and remaining amount of portlandite per unit volume, respectively.
● Other Hydrates Dissolution Rate
All other hydrates are assimilated to C-S-H. The dissolution kinetic is assumed to depend linearly on the carbon dioxide pressure.
where
kCSH is the kinetic coefficient of hydrates dissolution that is calculated for C-S-H, aluminates, and ettringite as Equation (17) [
7,
27].
where
αCSH is a model’s coefficient;
nCSH,0 and
nCSH (mol/m
3) are the initial and current amounts of other hydrates assimilated to C-S-H, respectively.
2.2.3. Equation of Calcite Formation Rate
The molar velocity of calcite formation is expressed by the rate of dissolution of molar portlandite and other hydrates (C-S-H mainly), introducing
αHYD a coefficient of equivalent stoichiometry for calcium in hydrates (but portlandite) and calcite.
2.2.4. Carbonation Depth
The carbonation depth is estimated through the amount of remaining portlandite in the material. The damaged area is assimilated to the part of the material where the portlandite concentration is less than 10% of the initial amount for the accelerated carbonation [
17,
27] and less than 50% for the atmospheric carbonation.
3. Validation of Numerical Model
Two-dimensional simulation is performed using COMSOL Multiphysics software for modeling the carbonation process into a concrete structure of 10 cm length × 10 cm width × 1 cm height, with a regular spacing of 1.0 mm between nodes. The direction of carbonation is estimated according to the two sections 1-1 and 2-2, as shown in
Figure 1. The mesh at the corner of the model is illustrated in
Figure 2.
The first case is studied for the concrete carbonation under atmospheric conditions. The material is totally saturated and then exposed on one face to the outdoor relative humidity of 65% and temperature of 20 °C. Material is broadly equivalent to the concrete that is made of cement CEM I with a water–cement ratio of 0.42. The input parameters of the model can be found in the study conducted by Bary and Mügler 2006 [
27]. The predicted carbonation depth over 230 days using the proposed model is compared to that of the existing models [
27,
28], which are based on the same equations under constant exposure conditions.
Figure 3 presents the evolution of predicted carbonation depth in time by the proposed model and two existing models. The obtained results in
Figure 3 show that the carbonation depth predictions are greatly fitting between these models.
The second case is employed to study accelerated carbonation. The comparison between the predicted results and the experimental results from the study of Thiery 2005 [
7] illustrated in
Figure 4 does not show a good agreement for portlandite profile at 14–day exposure. Nevertheless, it must be kept in mind that predictions should be regarded in the context of possible uncertainties of many input parameters (obtained from the experimental process), which would lead to estimate a confidence interval of outputs in place of a single result. Such an approach is beyond the scope of the present paper.
5. Application of Proposed Numerical Model in the Case Study
A comprehensive finite element modeling of concrete carbonation has been proposed in the previous sections. It encompasses the carbon dioxide transfer in the gaseous phase; the transfer of calcium ions provided by the dissolution of the main hydrated compounds of the cement paste includes interdependent and nonlinear source and sink terms of mass conservation equations, the heat transfer, and the chemical and physical heat activation and accounts for variable boundary conditions. In the present section, its utilization will highlight some still questionable aspects of carbonation modeling.
Although some previous modeling works have integrated non-constant boundary conditions in terms of outer moisture and temperature, very few have investigated in detail the effect of the variability of these conditions on the long-term carbonation rate. Bakker 1993 [
18] attempted to assess the influence of wetting–drying cycles in the framework of a simplified approach where carbonation was stopped under wetting. More recently, Thiery et al., 2012 [
43] added to Bakker’s model [
18], simplified modeling of moisture transfer. Nevertheless, how far accounting for realistic outdoor conditions can change the predicted carbonation rate remained has not been experienced until now.
The calcium ion concentration in the pore solution has been demonstrated to present a significant effect on the carbonation phenomenon. However, it has not been discovered whether the motion of ions plays a significant role in carbonation kinetics. The extended modeling proposed above has enabled addressing such analyses.
5.1. Concrete Material
The concrete cover of existing structures constitutes the main barrier to prevent the initiation corrosion of steel reinforcement by carbonation due to carbon dioxide penetration. The concrete structure considered herein is only the concrete cover, with 1D ingress of carbon dioxide. The amount and type of hydrated compounds of the cement paste, as well as the porosity mainly depending on the concrete mix, are essential parameters influencing the carbonation rate. In this paper, two concretes having compressive strengths corresponding to C25 and C45 classes are studied, respectively. The characteristics of the two concretes are synthesized in
Table 1.
5.2. Case Study: Outdoor Environmental Variables in Toulouse, France
Hourly average values of relative humidity and temperature have been computed over the period from 1970 to 2020 using the data recorded by a meteorological station in Toulouse, Southern France, in order to identify the evolution of outdoor environmental conditions. The boundary conditions of the numerical problem that are the outside temperature and the relative humidity are simulated using average values during each time step in the numerical simulation. The shorter this time step is, the more precise the simulation is, but computation time will also increase.
Figure 7 and
Figure 8 illustrate the chronicle of environmental conditions (temperature, relative humidity) used in the simulation with a temporal discretization ranging from 5 to 90 days that is assumed to be repeated every year.
5.3. Results and Discussion
5.3.1. Effect of Time Granularity on the Carbonation Depth
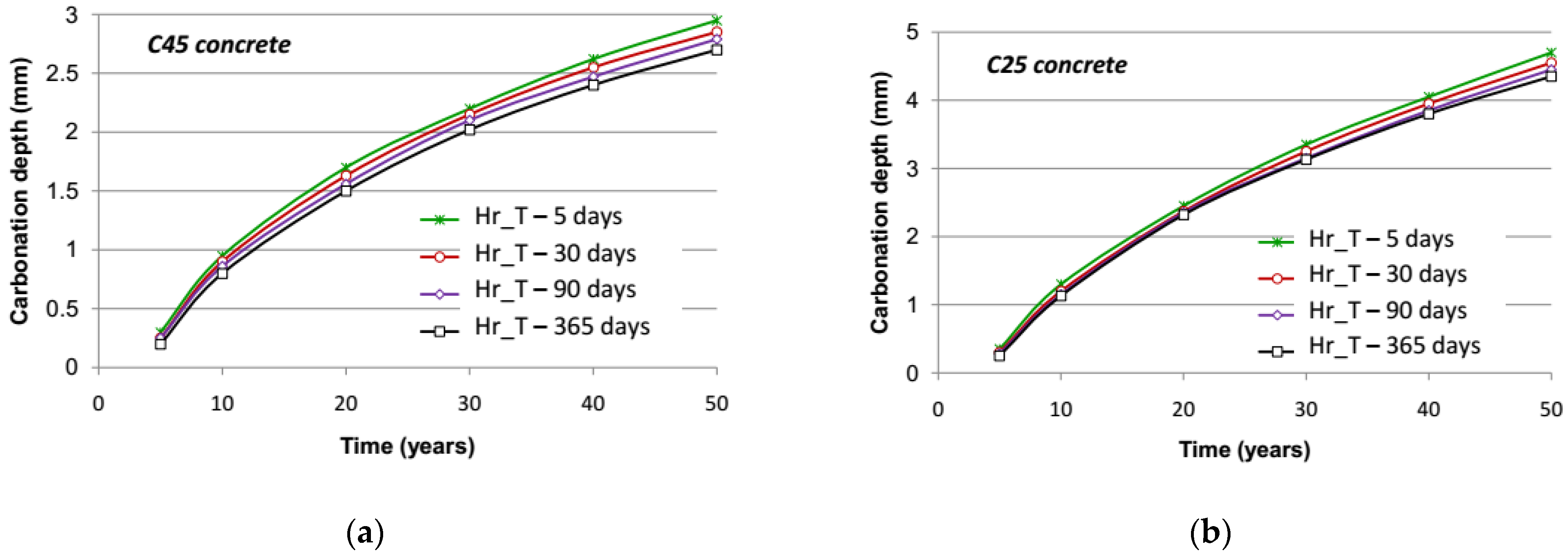
The results of carbonation simulations for C45 concrete and C25 concrete under the different spatial discretization hypotheses are shown in
Figure 9, respectively. These results are summarized in
Table 2, in which the relative difference of the simulation results appears by taking the carbonation depth at 50 years under the lowest time step as a reference value. It is noted that the impact of an increase in the time step is relatively limited: the difference is about 10% between a time step of five days and 365 days (annual average value) after 50 years of exposure for C45 concrete and C25 concrete.
It is worth noting that the larger the time step is, the lower the carbonation depth becomes. When increasing the time step, the variation of relative humidity taken into account with regard to its average value is reduced. If the average value is fairly elevated (higher than 75%), eliminating low values of relative humidity (slightly less than 65%) by reduction of its extent, which are favorable to carbonation compared to the average value, leads to a decrease in the long-term carbonation rate. When only annual average moisture is accounted for, the resulting carbonation depth is far smaller than that obtained with a 30-day step. Comparing computational run times with respect to the time step, it can be seen that a 30-day step constitutes a good compromise between accuracy and efficiency.
The calculation time for a numerical simulation is a direct function of the time step retained to model the external conditions and of the calculation step of the simulation itself.
Table 3 and
Table 4 present the lengthening of the duration of the atmospheric carbonation simulations to 50 years and accelerated carbonation to 180 days, respectively, and the estimated depth of carbonation, according to the size of the time step for the description of the climatic chronicles and the step of the numerical simulations. These results on atmospheric and accelerated carbonation show that the calculation step only affects the calculation time and not the depth of carbonation. The obtained results presented above make it possible to consider a step of calculation and discretization of the environmental chronicles of 30 days for atmospheric carbonation and three days for accelerated carbonation.
Due to the fact that both warm temperature and low moisture of the material are favorable factors of concrete carbonation, a matter arises regarding the influence of the season when the concrete surface is first exposed to carbonation on the future of the phenomenon. The carbonation is more favorable in summer under high temperature and low moisture than in winter under favorable moisture. Results reported in
Figure 10 and
Figure 11 show that a noteworthy difference is visible at first with respect to the season at the onset of exposure and that this difference decreases after several months, yet it remains. It should be noted anyway that this difference is mainly significant for an accelerated process. It can be explained by the global decreasing kinetic of the carbonation process, which allows greater importance to the first months of the degradation. If the onset is in spring, for instance, the first six months of the degradation are under the warmest months of the year; therefore, the degradation is accelerated in comparison to an onset in autumn and six cold first months.
5.3.2. Effect of Heat Transfer on the Carbonation Depth
A temperature gradient always exists between surface and bulk concrete due to the fact that external temperature is not constant. Within the concrete, there is hence a variation of temperature that potentially impacts the carbonation phenomenon, therefore justifying the modeling of the heat transfer. The latter prominently depends on the concrete thermal conductivity, which may strongly vary among various concretes according to the nature of the aggregates and slightly more with respect to the moisture and temperature of the concrete.
In the present section, it is assumed that the thermal conductivity may vary by a ratio of 1 to 1000. Nonetheless, the evolution of the carbonation depth remains almost similar whatever the value of the thermal conductivity, as illustrated in
Figure 12. It could then be suggested to consider that the temperature over the cover depth is constant and equal to the external one, if the time step accounted for in the modeling of outdoor conditions is stated at least at 30 days, as proposed in the former section. This conclusion derives from the kinetics of the heat transfer, which are significantly higher than those of the diffusion process.
5.3.3. Effect of Diffusion of Calcium on the Carbonation Depth
The proposed model enables the diffusion of calcium ions in the pore solution to be considered. These ions are in equilibrium with the hydrated products in a sound concrete at a concentration of 22 mol/m
3. According to the decalcification process, the solubility of hydrated compounds decreases, and the concentration of calcium ions drops at a value ranging between 0 and 0.4 mol/m
3 in the fully carbonated area [
30]. The calcium ions are hence prone to migrating from the sound area to the carbonated one. The transfer of these ions depends on the saturation degree and porosity that both affect the diffusion coefficient for calcium. It can be seen in
Figure 13 that the effect of the motion of calcium ions is very limited even for a high value of diffusion coefficient under reference conditions. This results from the fact that the gaseous diffusion kinetics of carbon dioxide remain much greater than those of calcium ions in the liquid phase for the outdoor climatic conditions retained in the study.
However, when the concrete surface is exposed to rain for a long time, the saturated concrete extent may reach the sound concrete and subsequently connect the sound region to the carbonated one in a continuously saturated way. In this case, a more significant transfer of calcium ions is expected to occur, because the ingress of carbon dioxide is blocked by the liquid phase, and the formation of calcite is not possible anymore. Extreme situations have been simulated by the change of the yearly average relative humidity in the C25 concrete to 95% and 65% in place of 78%. A porosity of 0.18 is used to increase the transfer of calcium ions. Longer periods with almost complete saturation of concrete cover then appear. Taking into account various diffusion coefficients of calcium ions shows in
Figure 14 that the faster the motion of ions, the lower the long-term carbonation rate as well. In this case, indeed, calcite is formed close to the concrete surface because calcium ions are available, even if the region is already carbonated.
6. Conclusions
This study proposed a FE model for modeling concrete carbonation by using a simplified approach that is based on simple numerical models and detailed chemo-physical processes. Each numerical model used aims to assess the initiation corrosion time in reinforced concrete structures when the carbonation front reaches the reinforcing bar. The model was used with acceptable accuracy for atmospheric and accelerated carbonation. It is noted that predictions should be related to the possibility of the uncertainty of several input parameters, which lead to a range of output values instead of a single result. Some tests have been achieved on 2D simulation for atmospheric carbonation under outdoor environmental conditions in Toulouse, France, as a case study.
It appears that for modeling the concrete carbonation process under atmospheric conditions, it is relevant to take into account the variation of the environmental conditions (e.g., temperature, relative humidity) with each time step being 30 days. It is shown that the season for the onset of the degradation has a slight influence on the kinetics of the process during the first year. It has also been discovered that, provided the time step for the numerical simulation is long enough, it is not necessary to model the heat transfer within the material, and the temperature could be considered equal to the external one.
This study also focuses on the process of calcium ion diffusion, whose kinetics are much slower than for the diffusion processes for water and carbon dioxide. Therefore, unless the material is fully saturated all along with the degradation duration, it appears that a local chemical equilibrium hypothesis for calcium can be satisfactorily formulated. Therefore, modeling the carbonation process requires two coupled mass conservation equations for water and carbon dioxide instead of three conservation equations (with calcium ions).