Abstract
This paper examines the remediation techniques of cadmium (Cd)-contaminated dredged river sediments after land disposal in a city in East China. Three remediation techniques, including stabilization, soil leaching, and phytoremediation, are compared by analyzing the performance of the techniques for Cd-contaminated soil remediation. The experimental results showed that the stabilization technique reduced the leaching rate of soil Cd from 33.3% to 14.3%, thus effectively reducing the biological toxicity of environmental Cd, but the total amount of Cd in soil did not decrease. Leaching soil with citric acid and oxalic acid achieved Cd removal rates of 90.1% and 92.4%, respectively. Compared with these two remediation techniques, phytoremediation was more efficient and easier to implement and had less secondary pollution, but it took more time, usually several years. In this study, these three remediation techniques were analyzed and discussed from technical, economic, and environmental safety perspectives by comprehensively considering the current status and future plans of the study site. Soil leaching was found to be the best technique for timely treatment of Cd contamination in dredged river sediments after land disposal.
1. Introduction
With recent social and economic developments, an increasing amount of industrial, agricultural, and domestic sewage waste has been regularly discharged into urban rivers, causing continuous river deterioration [1]. Dredging is an effective treatment method for urban rivers with fetid and dark-colored water. Dredged river sediments usually require further treatment, such as ex situ land or landfill disposal. The heavy metal Cd is one of the most common contaminants in river sediments [2,3,4]. Cadmium has high mobility in soil and is highly toxic [5,6,7,8,9]. Even at low concentrations, this contaminant can affect the composition and structure of plant enzymes, thereby slowing plant growth and development, can accumulate in plants, and can even cause plants to be carcinogenic [10,11,12]. For most crops, Cd can also inhibit normal development, leading to substantial crop yield reduction [13]. When Cd enters the human body through the food chain, it accumulates in the kidney and liver, leading to many problems, such as renal dysfunction, lung cancer, and neurological and reproductive diseases [14,15,16,17]. In addition, Cd can also alter the community structure and active biomass of soil microbes, thereby affecting the bioaccessibility and release of nutrients in soil [18,19,20,21,22].
There are two main ideas to remediate Cd-contaminated soil: (1) changing the form of Cd in soil to reduce its mobility and bioavailability, and (2) reducing the Cd concentrations in soil by removing Cd from the soil. The remediation approaches can be divided into two categories: (1) physical and chemical methods, such as stabilization remediation technology [23,24,25], soil leaching [26,27,28], soil exchange method, electric remediation method [29] and thermal desorption technology [30]; (2) biological methods, such as microbial remediation [31], phytoremediation [32,33,34,35], and animal remediation [36]. Although single remediation methods have their own advantages, they often have specific limitations. Therefore, combined remediation technology is also commonly used in the application of soil remediation [37].
Stabilization, soil leaching, and phytoremediation are the most widely used remediation approaches in practical engineering. Stabilization for soil remediation refers to effectively reducing the bioavailability and mobility of Cd in soil with passivators, modifiers, surfactants, and other reagents or materials that react with Cd through complexation, precipitation, adsorption, etc. Soil leaching refers to the removal of Cd from soil by transferring Cd from contaminated soil into a leached solution through the addition of chemical eluents that increase the solubility of Cd in soil via precipitation, adsorption, chelation, etc. Phytoremediation is a decontamination process mediated by the plants, involving various steps including heavy-metal uptake (phytoextraction), accumulation and translocation of heavy metals (phytoaccumulation), their stabilization in the root zone (phytostabilization), and emission to atmosphere (phytovolatilization) [38]. Phytoextraction refers to contaminants are absorbed by the plants along with nutrients and water. The contaminants are then precipitated and accumulated in the shoot or leaves of the plant by the process called phytoaccumulation. Phytostabilization can achieve in-situ immobilization or inactivation of contaminants by absorbing them on the roots of plants [39]. Phytovolatilization refers to plants transport contaminant into the xylem that can further transformed into volatile forms, and finally released in the atmosphere by stomata.
Previous studies have focused on the remediation of Cd contamination in terrestrial soils. However, there are few studies on the remediation of Cd contaminant in river sediments. It is of great significance to select an appropriate remediation method for Cd-contaminated river sediment for improving remediation efficiency and reducing economic cost. A dredged sediment disposal site was used as an example in this paper to investigate the remediation of Cd-contaminated river sediments. The feasibility and applicability of stabilization, soil leaching, and phytoremediation were comparatively analyzed in detail. The results of this study provide a theoretical and reference basis for the further application and development of remediation techniques for Cd-contaminated soil.
2. Materials and Methods
2.1. Basic Description of the Studied Site
The dredged sediment disposal site was located at the urban-rural boundary area, where farmlands and rivers comprise most of the area, with fewer sensitive sites and low population density. The disposal site was mostly wasteland covered with weeds. The layer of dredged river sediments on the disposal site had an average depth of approximately 2.0 m and a volume of approximately 8000 m3. The latest land use plan designates the disposal site as agricultural land. If the dredged river sediments are not decontaminated in a timely manner, the current soil quality might not meet the functional requirements for subsequent agricultural land use, thereby causing environmental pollution incidents. Before dredging, river sediments which consist mainly of fine sand and clay were sampled and measured by inductively coupled plasma atomic emission spectrometry, and the results are shown in Table 1. The pH was measured in suspension of soil and water. The study results showed that only the Cd concentration exceeded the risk screening value.

Table 1.
Heavy metal concentrations (mg/kg) in dredged sediment samples.
2.2. Sampling and Pretreatment
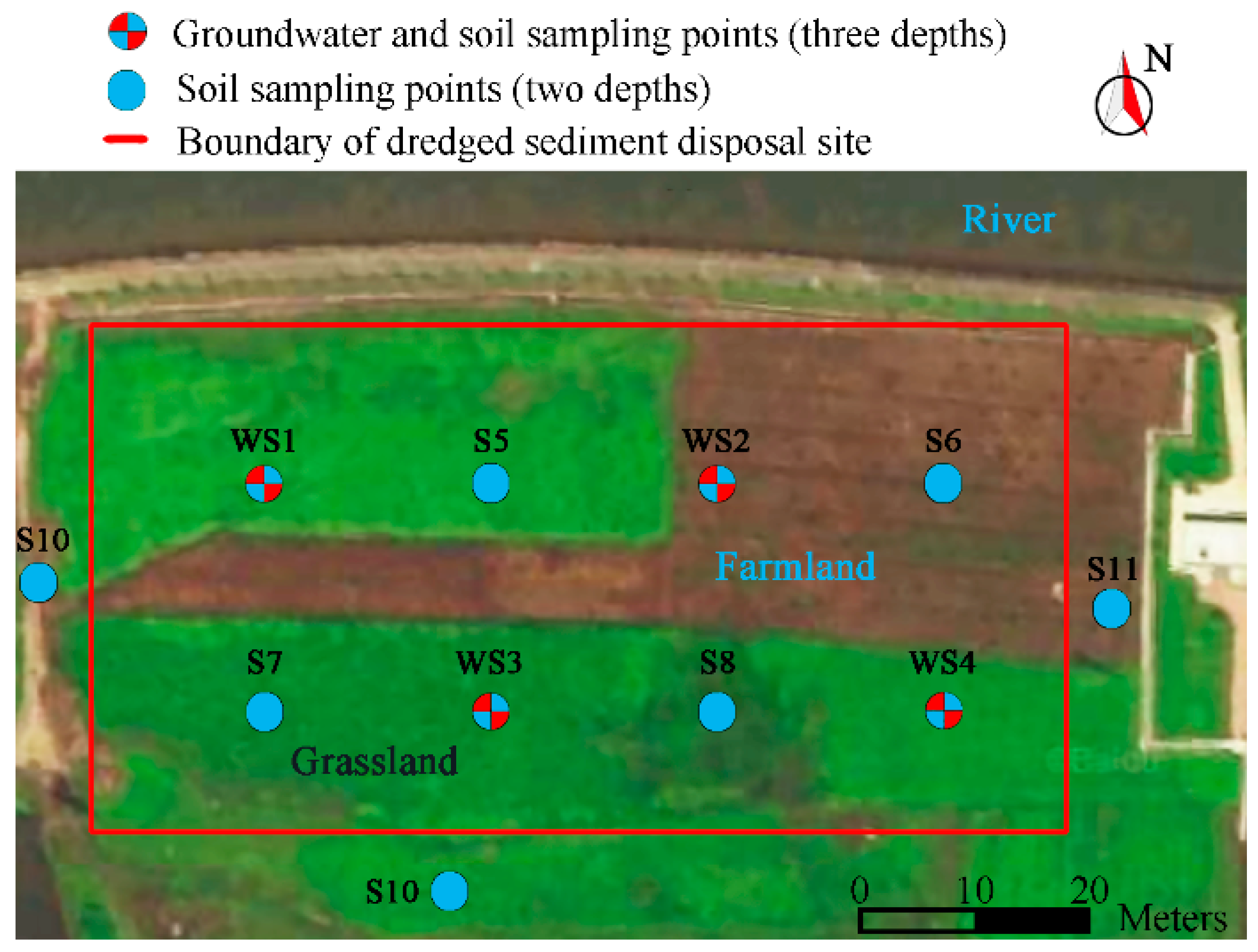
According to the detection results of Cd-contaminated river sediments, a further pollution survey and risk assessment of the disposal site were carried out. The results of the survey and drilling indicated that the dredged sediments were piled in a cuboid shape, with a thickness of approximately 2.0 m, as shown in Figure 1. To determine whether the native soil under the sediment layer was contaminated, stratified sampling at two or three depths was performed at each monitoring site. The depth of deep soil sampling points is more than the thickness of dredged sediments. Meanwhile, four underground water monitoring wells were constructed at each monitoring site to collect groundwater samples for ascertaining whether the soil underlying the sediments was contaminated. In addition, soil sampling sites with two layers were established within 10 m of the east, south, and west boundaries of the disposal site (a river was on the north side of the disposal site) to determine whether the soil surrounding the disposal site was contaminated by dredged sediments. A total of 26 soil samples and four groundwater samples were collected from 11 soil sampling sites and four groundwater sampling sites inside and outside of the study site. The layout of the disposal site and sampling sites is shown in Figure 1.
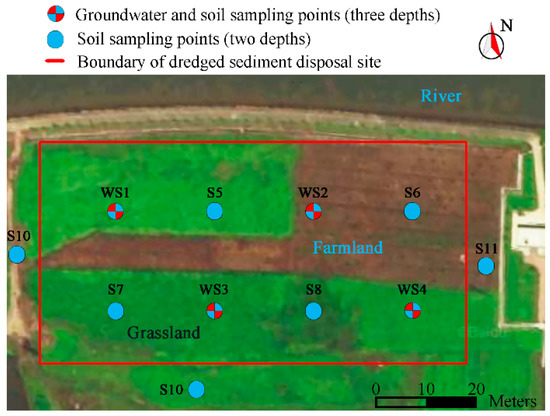
Figure 1.
Distribution of soil and groundwater sampling points.
After removing stones in the soil, the soil samples were reduced to 100 g by quartering method. The soil samples were grinded and pressed with wooden sticks after air drying, and then was screened by 2 mm nylon screen. After evenly mixing the soil samples, grind them with an agate mortar. After passing the 100 mesh nylon screen, it is left to be tested. Groundwater samples were collected in polyethylene plastic bottles. Add nitric acid to the water sample for acidification (pH between 1 and 2) and leave it to be tested.
2.3. Stabilization for Remediation
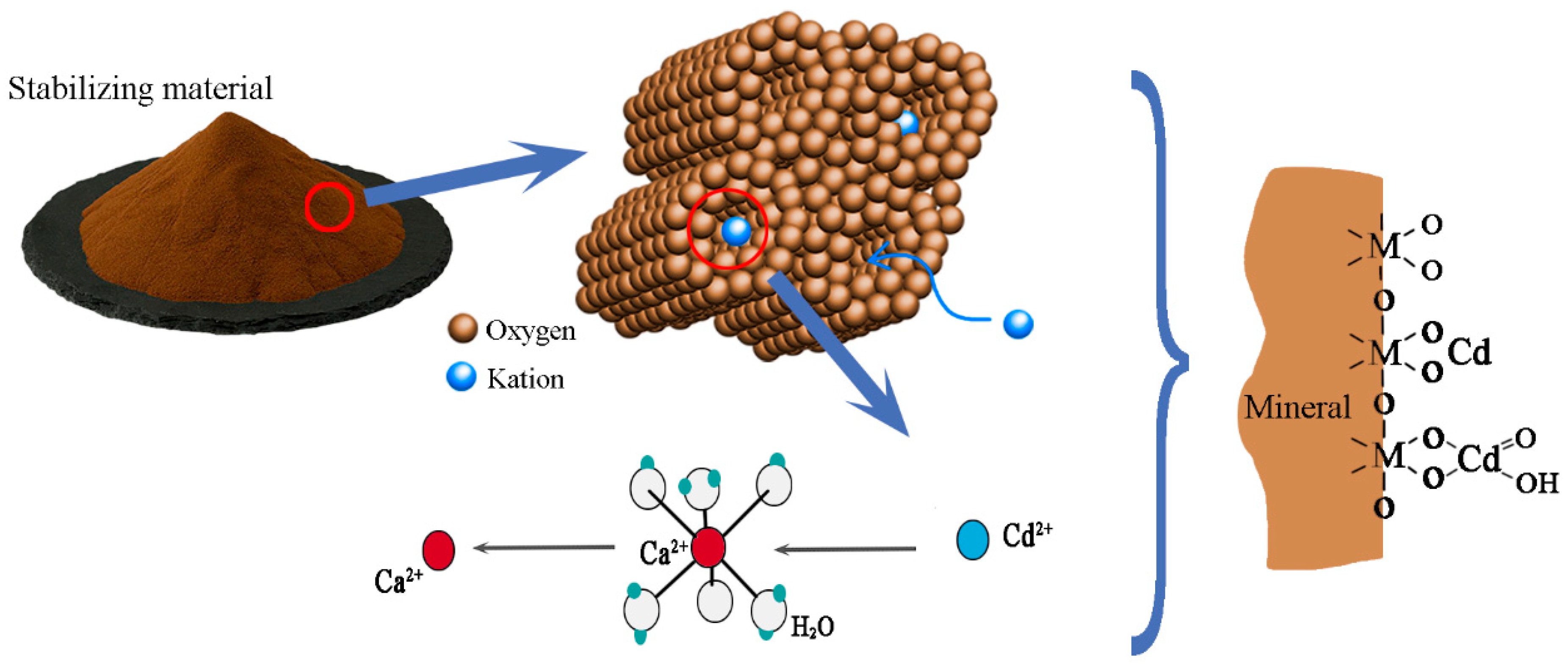
Stabilization for remediation refers to the use of physical and chemical methods that convert contaminants into insoluble, low-mobility, or low-toxicity forms. The key to successful stabilization for remediation is selecting an appropriate stabilizer. We choose a green and efficient stabilizing material with a pH value of about 7.5, the particle size is less than 1 mm, the density is 1.2~1.5 g/cm3, the moisture content is 3~5%. The appearance of the stabilizing material is light brown powder, which is mainly made of natural mineral crystals of zeolite, mixed with a small amount of calcium and magnesium compounds, iron and manganese salts and clay. Zeolite has a large specific surface area and strong electrostatic filed, which performs significant ion exchange capacity and adsorption characteristics [40]. Mineral crystals have excess negative charge, and the crystal structure can facilitate the adsorption of Cd ions in contaminated soil and exchange with compensating cations (Ca2+, Mg2+, Mn2+ and Fe2+) to form more stable compounds. According to Appelo and Postma [41], the order of adsorption affinity of divalent cations is: Cd2+ > Ca2+ > Mg2+ > Mn2+ > Fe2+. This means that Cd2+ has a stronger adsorption affinity than Ca2+, Mg2+, Mn2+ and Fe2+, thus converting Cd into a less soluble form through cation exchange, as shown in Figure 2. The related ion exchange reaction equations are shown below:
where ( )-X2 denotes the cation exchanger or an exchange position. Stabilizing material can reduce the chemical effectiveness of pollutants, weaken their migration and diffusion ability, and thus achieve the purpose of preventing their transformation and endangering human health. The stabilized material has the following main characteristics: (a) the cation exchange capacity is up to 140 meq/100 g, (b) no biological toxicity, (c) low cost and quick results.
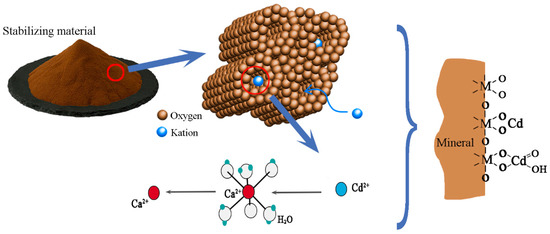
Figure 2.
Transformation mechanisms of stabilization materials.
The dose-dependence of the stabilizing effect of stabilizing material on the soil Cd concentration was experimentally investigated. The detection index was the leaching rate of Cd from stabilized soil samples. Soil samples were collected from site and were dried and passed through a 2-mm sieve. A total of ten samples (200 g each) were accurately weighed and placed in ten 1000-mL beakers. Stabilizing materials were added at mass fractions of 0, 2.0%, 2.5%, 3.0%, 3.5%, 4.0%, 4.5%, 5.0%, 5.5%, and 6.0%, respectively. Deionized water was added to each sample at a solid-to-liquid ratio of 1:0.5, mixed well, sealed, and aged at room temperature (15 °C) for seven days. Samples were then dried and subjected to leaching and detection. The above experiment was repeated three times to ensure the reliability of the results.
2.4. Soil Leaching
Soil leaching removes Cd from soil by adding chemical eluents to contaminated soil through desorption, chelation, dissolution, etc. This method typically involves three steps: mixing the eluent with soil, collecting the leached solution, and treating and recovering the leached solution [42]. Commonly used eluents mainly include inorganic solvents, chelating agents, and surfactants.
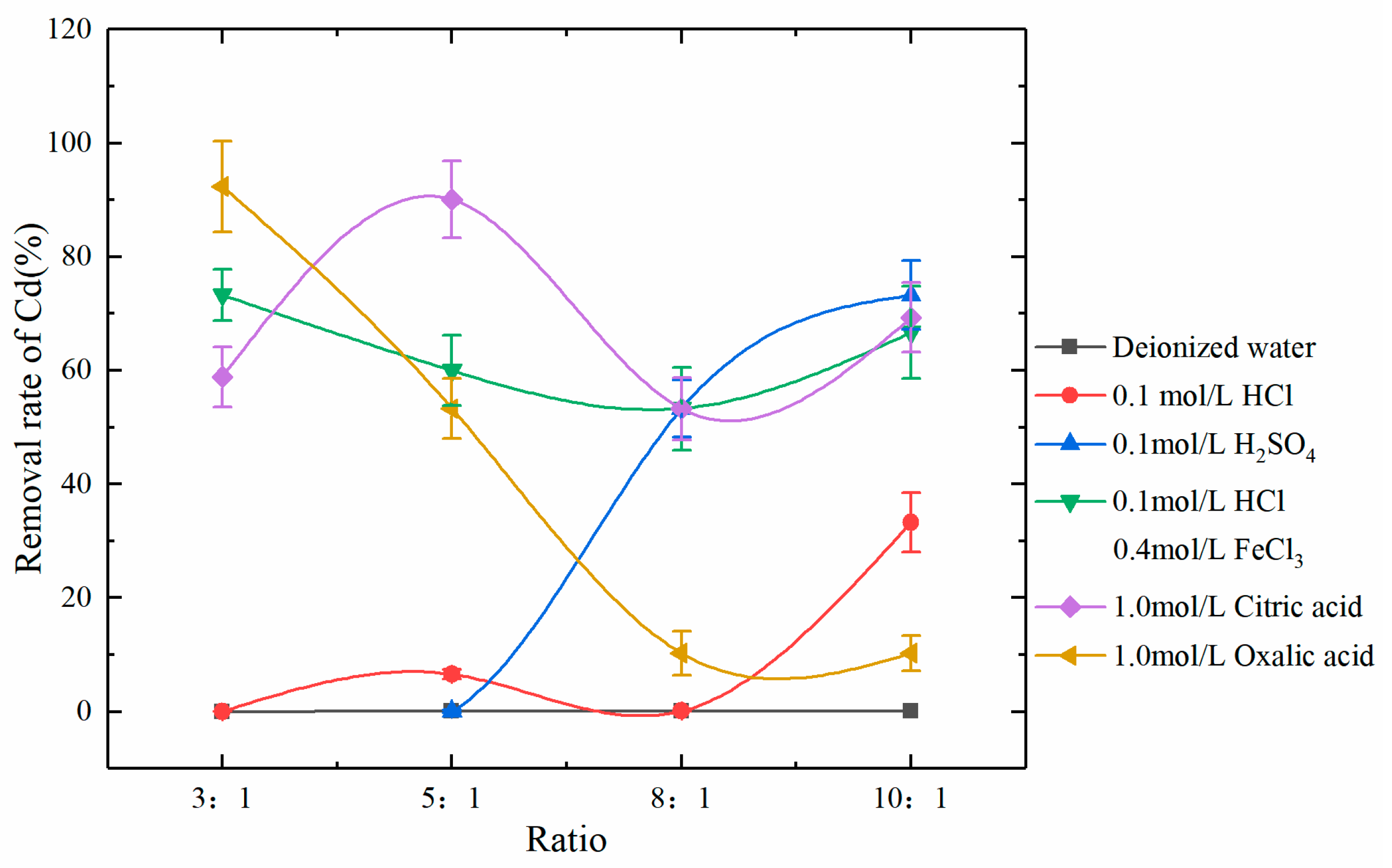
The present study investigated the influences of eluent type and the eluent-to-soil ratio on Cd leaching from soil. The effectiveness of leaching was evaluated with the Cd concentrations detected in the soil samples after leaching. The eluents used in this study included (1) deionized water, (2) 0.1 mol/L hydrochloric acid (HCl), (3) 0.1 mol/L sulfuric acid (H2SO4), (4) 0.1 mol/L HCl + 0.4 mol/L ferric chloride (FeCl3), (5) 1.0 mol/L citric acid (C6H8O7), and (6) 1.0 mol/L oxalic acid (H2C2O4). Three control experiments were designed for each eluent. The related ion exchange reaction equations are shown below:
3. Result
3.1. Determination of Cd
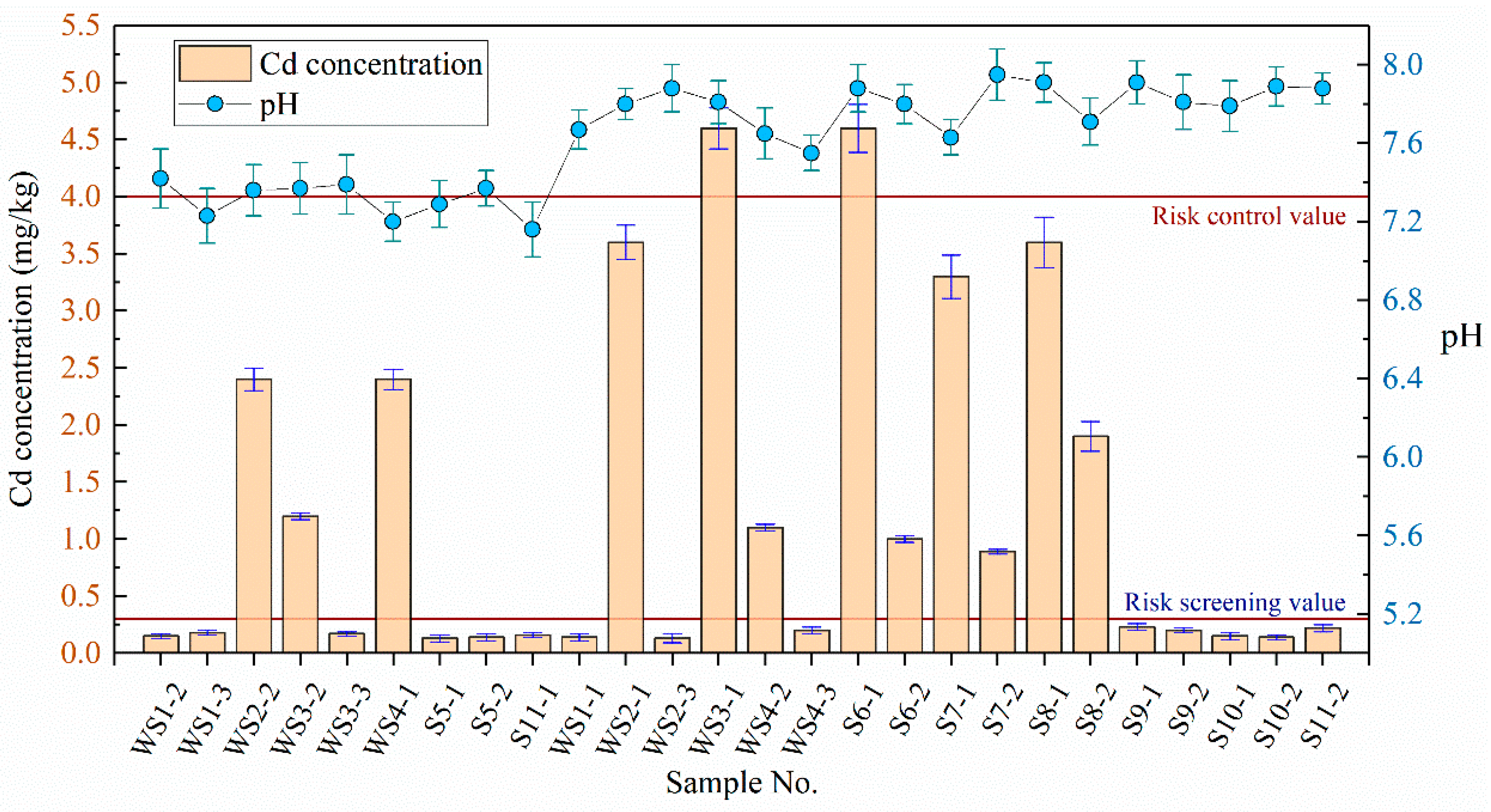
The concentration of Cd in soil and groundwater was determined by referring to the “Soil quality–Determination of lead, cadmium–Graphite furnace atomic absorption spectrophotometry” (GB/T 17141-1997) and “Water quality–Determination of copper, zinc, lead and cadmium–Atomic absorption spectrometry” (GB 7475-87) in China. Cd concentration in 26 soil samples was detected three times by AAS9000 atomic absorption spectrometer. The results were compared with the corresponding standard limits, as shown in Figure 3. Risk screening value (0.3 mg/kg) and risk control value (4.0 mg/kg) refer to the “Soil Environment Quality—Risk control standard for soil contamination of agriculture land” (GB 15618-2018) in China. Figure 3 shows that the Cd in disposal site mainly exists in the river sediment and does not spread to the surrounding area (S9 and S11 are lower than the risk screening value). The pH value of the sediments at the disposal site ranged from 7.20–7.95. Cd was detected in all river sediment samples. The Cd concentrations in 12 soil samples were 0.5 to 7.0 times the risk screening value, with an over-limit ratio of 60%. The Cd concentrations in two samples (WS3-1 and S6-1) exceeded the risk control value. The pH values of soil samples outside the disposal site ranged from 7.16 to 7.91, with no Cd concentrations exceeding the risk screening value. In addition, the pH values of the four groundwater samples ranged from 7.20 to 7.29, with Cd detected in one of the samples at a concentration lower than the Class III limit of the “standard for groundwater quality” (GB/T14848-2017) in China, that is, the groundwater of the disposal site was not polluted.
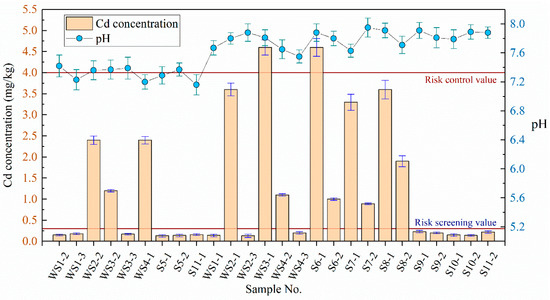
Figure 3.
Detection results of Cd and pH in soil samples.
3.2. Stabilization for Remediation
Soil sample WS3-1 (the Cd concentration in the sample exceeded the risk control value) was selected to investigate the dose-dependence of stabilizing effect of stabilizing material on the soil Cd concentration. The detection results are shown in Figure 4.
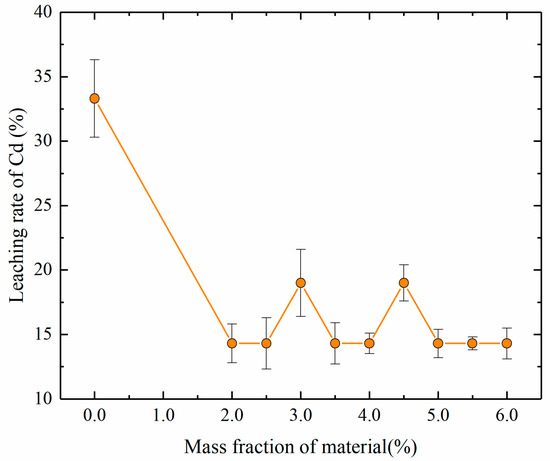
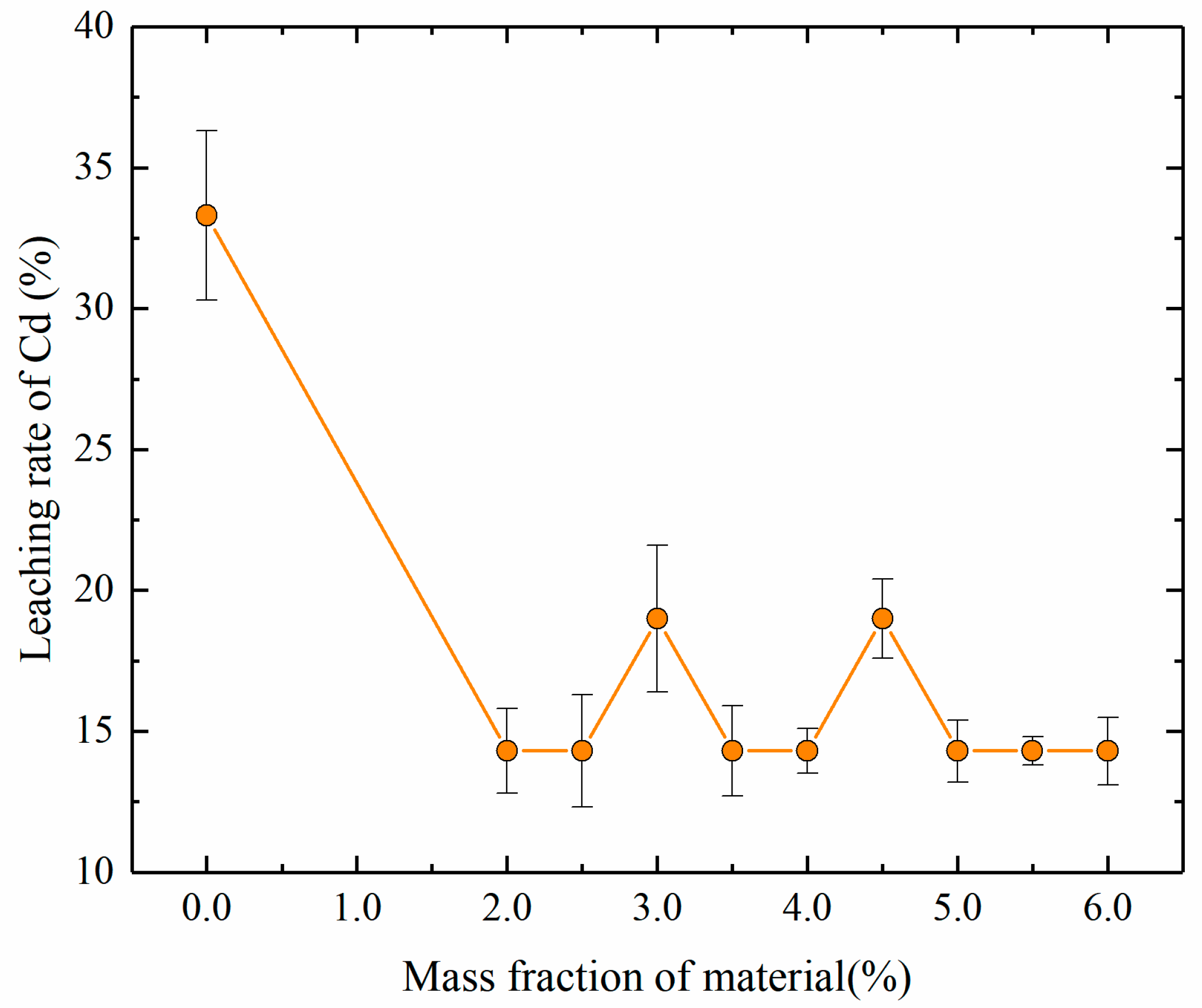
Figure 4.
Dependence of the leaching rate of Cd on the mass fractions of stabilizing material.
The leaching rate of Cd decreased from 33.3% to 14.3% as the mass fraction of stabilizing material increased from 0 to 2%, and further increases of the mass fraction of stabilizing material did not significantly affect the leaching rate of Cd. The results indicate that although stabilizing material can stabilize Cd, it did not significantly affect the leaching rate of Cd within the scope of this study. This finding may have occurred because the rich soluble organic matter in soil samples from river sediments binds to Cd and precipitates together during the leaching process. This result also indicates that stabilization for remediation can significantly reduce the toxicity of Cd-contaminated soil.
3.3. Soil Leaching
Experimental results of soil leaching showed that the leaching effect of deionized water on Cd was the worst, followed by the inorganic eluents HCl and H2SO4, while the mixed inorganic eluent HCl + FeCl3 and the organic eluents, citric acid and oxalic acid, had good leaching effects of Cd in the soil, with the highest removal rates of 73.3%, 90.1%, and 92.4%, respectively (Figure 5).
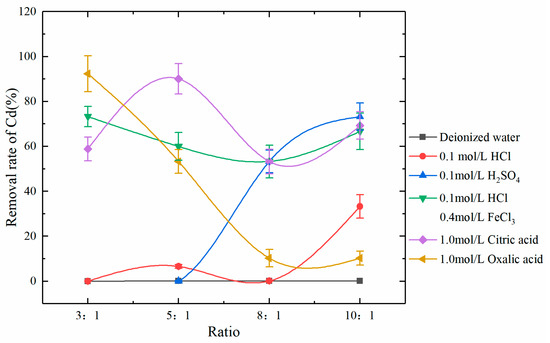
Figure 5.
Removal rates of Cd by various eluents at different eluent-to-soil ratios.
After leaching with deionized water, there was almost no removal of Cd, indicating that there was very little water-soluble Cd in the soil samples. Theoretically, all three types of inorganic eluents can provide H+ to replace carbonate-bound Cd in soil samples so that Cd can be dissolved into water. However, the ability of H+ to replace Cd is weak [43], resulting in the poor leaching effect of Cd by the eluents HCl and H2SO4 that mainly rely on H+. The mixed eluent HCl + FeCl3 not only provides H+ and Fe3+ to replace Cd in soil but also provides a large amount of H+ due to hydrolysis of Fe3+ during leaching. In addition, the excellent complexation ability of highly soluble Cl- for Cd can significantly increase the extraction of Cd from soil [44,45]. It is worth noting that the removal capacity of the mixed eluent for Cd did not increase with the eluent-to-soil ratio, because H+ only had a significant removal effect on the carbonate-bound Cd. The excess H+ would lose its value when the carbonate-bound Cd was removed. Unlike inorganic eluents, which are more targeted on removing carbonate-bound Cd, the organic eluents, citric acid, and oxalic acid can also chelate exchangeable Cd and thus enhance the removal of exchangeable Cd in soil. In this study, the leaching effects of citric acid and oxalic acid on Cd were superior to those of inorganic acids, which may be due to the large proportion of exchangeable Cd. The deprotonated –OH group and –COOH group formed coordination compounds with exchangeable Cd ions, which improved their mobility in the soil and significantly increased the leaching rate of Cd [46,47]. In addition, the results showed that the removal rate of citric acid and oxalic acid on Cd be restricted by the eluent-to-soil ratio. The optimal ratio was 3:1 and 5:1 respectively in the research range. Thus, the removal rate of Cd can be significantly improved through optimization measures, such as choosing a proper eluent-to-soil ratio, performing leaching multiple times, and prolonging the leaching duration.
3.4. Phytoremediation
Phytoremediation is an in situ remediation technique mainly using hyperaccumulators to absorb heavy metal ions. Heavy metal ions are retained and accumulate in plants through complexation and localization by glutathione, phytochelatins, metallothioneins, and organic acids in the plants, thereby reducing the toxicity of and environmental pollution caused by heavy metals [48]. Due to the variety of hyperaccumulators and long growth cycle, it is usually difficult to carry out relevant experiments in soil remediation. Previous studies usually have excellent reference significance. The known Cd hyperaccumulators and corresponding study cases are shown in Table 2.

Table 2.
Cd hyperaccumulators and application cases.
It is worth noting that the common unit of Cd background value is mg/kg or μmol/L due to different experimental conditions. The former is usually used to characterize background value in contaminated soil, while the latter is mostly used in artificially prepared solutions in the laboratory. Previous studies have shown that both plant species and Cd concentration in the soil have an impact on the remediation effect. The efficiency of various hyperaccumulators is different under the same concentration, for example, Viola inconspicua and Viola baoshanensis. Furthermore, the background value of contaminants in soil has a significant effect on the accumulation efficiency of plants. Li et al. (2020) found that the remediation ability of the hyperaccumulator was inhibited when the Cd concentration in the soil was too high [50]. As shown in Table 2, Festuca arundinacea, Celosia argentea, and Bidens pilosa have relatively significant effects in the remediation of contaminated soil with low Cd concentration, while Brassica napus, Indian mustard, and Sedum plumbizincicola are often used in the remediation of contaminated soil with high Cd concentration. Although Viola baoshanensis, Viola inconspicua, and Ricinus communis also show outstanding accumulation capacity for Cd, they have not been widely used due to the limitations of survival rate, soil environment, and climate conditions. In general, phytoremediation is low cost, efficient, simple, effective, and pollution free. However, this technique has some limitations: (1) the slow growth rate of plants can significantly extend the remediation time to several years. (2) Many phytoremediation studies are still in the experimental stage, with few known plant species that only possess tolerance to single factors. Enhancing phytoremediation ability and resource utilization after phytoremediation are feasible ways to improve the practical application of phytoremediation [63,64].
Considering that various hyperaccumulators have different application ranges of Cd concentration, Festuca arundinacea that is most similar to that of the disposal site (Figure 3) is an appropriate choice for the remediation of the study site. Qin et al. (2021) conducted a series of experiments to investigate the impact of planting density on the phytoremediation efficiency of Festuca arundinacea in Cd-contaminated soil [49], they founded that Festuca arundinacea can remove 1.78–2.66 g Cd/m3 from the soil at a planting density of 1.0–1.4 kg/m2, if the whole plant was harvested at the end of the treatment. Assuming that the remediation efficiency of Qin et al. (2021) is also applicable to the disposal site, in first growth cycle, the Cd remove rate is 23.9~36.0% in areas exceeding the risk control value, and 48.6~72.7% in areas exceeding the risk screening value. Cd removal rate can be further improved by repeated planting of Festuca arundinacea, but it significantly increases the duration of remediation.
4. Discussion
According to the experimental results of the three remediation techniques and related case studies, all three remediation techniques can remove Cd from soil samples collected at the study site. Under the premise that the remediation effect meets the standards, three remediation techniques were compared in this paper from various aspects, and the evaluation indexes of each remediation technique are shown in Table 3.

Table 3.
Comparative analysis of three remediation techniques.
As shown in Table 3, stabilization technology and leaching technology both can be used for remediation of various heavy metal contaminated soils, while phytoremediation is often suitable for contaminated soil with single heavy metal due to the single tolerance characteristics of most hyperaccumulators. The stabilization technology reduced the leaching rate of Cd from 33.3% to 14.3%, which is inferior to the soil leaching technology with Cd removal rate of 92.4%. The effectiveness of phytoremediation can be continuously improved through repeated planting of hyperaccumulator. Take Festuca arundinacea as an example, the estimated Cd removal rate is about 30% in areas exceeding the risk control value in first growth cycle. Previous studies have shown that the Cd removal rates of more than 80% can be expected with sufficient remediation cycles [49]. It is worth noting that the effectiveness of a single remediation cycle (a growth cycle of hyperaccumulator) usually decrease continuously as the concentration of Cd in the soil decreases with the process of phytoremediation. Moreover, phytoremediation has greater uncertainty in terms of the control factors of remediation effectiveness, so it is necessary to select plants according to local conditions [60,66]. The remediation time of phytoremediation is about seven times that of stabilization technology and 13 times that of leaching technology, which means that phytoremediation is not suitable for emergency remediation. The cost of removing or stabilizing 1% of Cd was estimated to compare the cost-effective of the three methods. Phytoremediation is the best cost-effectiveness, followed by soil leaching and stabilization. In other words, Phytoremediation will be the first choice when remediation time is sufficient. Nutrient loss is a highly concerned evaluation index for contaminated sites planned for agricultural used in the future. Stabilization and phytoremediation can usually preserve native soil nutrients, while soil leaching will cause considerable nutrient loss. However, some scholars believe that soil nutrients can be effectively replenished by introducing microbial community and building level ditches or rills, and thus nutrient loss is not a particularly serious problem [67,68,69]. In addition, with the development and improvement of technology, the probability of secondary pollution caused by the three remediation methods is relatively low.
5. Conclusions
The selection of remediation method requires comprehensive consideration of technology effectiveness, economy and environment safety. In the remediation engineering of the river sediment disposal sites, both stabilization and soil leaching meet the timeliness requirements of the remediation project. It is worth noting that since the city plans to use the study site as agricultural land, the reference standard mainly requires the maximum total Cd concentration. Therefore, stabilization for remediation cannot meet the remediation target. Soil leaching is the better choice, with an estimated remediation efficiency of about 90%. Phytoremediation can be used as an auxiliary means of remediation under the condition of sufficient remediation time. According to the degree of pollution, this site can be divided into two regions, a region with an over-limit risk control value and a region with an over-limit risk screening value but below-limit risk control value. Soil leaching can be adopted for the former region, and phytoremediation can be adopted for the latter area. This divided-region approach can maintain the remediation efficiency at 80%~90% and reduce the economic cost by 74%.
Author Contributions
Conceptualization, C.Z. and X.Z. (Xueke Zang); methodology, C.Z.; formal analysis, Z.M. and X.Z.; investigation, C.Z. and Z.M.; resources, C.Z.; data curation, X.Z.; writing—original draft preparation, Z.M.; writing—review and editing, Z.D. and X.Z.; supervision, X.Z.; project administration, C.Z. and Z.D.; funding acquisition, C.Z. and Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly supported by the Natural Science Foundation of Shanghai City of China (Gky201908) and National Natural Science Foundation of China (No: 41772253).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available and explained in this article; readers can access the data supporting the conclusions of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silkina, A.; Ginnever, N.E.; Fernandes, F.; Fuentes-Grunewald, C. Large-Scale Waste Bio-Remediation Using Microalgae Cultivation as a Platform. Energies 2019, 12, 2772. [Google Scholar] [CrossRef]
- Nino-Savala, A.G.; Zhuang, Z.; Ma, X.; Fangmeier, A.; Li, H.; Tang, A.; Liu, X. Cadmium pollution from phosphate fertilizers in arable soils and crops: An overview. Front. Agric. Sci. Eng. 2019, 6, 419–430. [Google Scholar] [CrossRef]
- Song, H.; Peng, L.; Li, Z.; Deng, X.; Shao, J.; Gu, J. Metal distribution and biological diversity of crusts in paddy fields polluted with different levels of cadmium. Ecotox. Environ. Saf. 2019, 184, 109620. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, B.; He, B.; Li, X.; Wang, L. Effect of the pyrolysis duration and the addition of zeolite powder on the leaching toxicity of copper and cadmium in biochar produced from four different aquatic plants. Ecotox. Environ. Saf. 2019, 183, 109517. [Google Scholar] [CrossRef]
- Tian, H.; Kong, L.; Megharaj, M.; He, W. Contribution of attendant anions on cadmium toxicity to soil enzymes. Chemosphere 2017, 187, 19–26. [Google Scholar] [CrossRef]
- Tan, X.; Wang, Z.; Lu, G.; He, W.; Wei, G.; Huang, F.; Xu, X.; Shen, W. Kinetics of soil dehydrogenase in response to exogenous Cd toxicity. J. Hazard. Mater. 2017, 329, 299–309. [Google Scholar] [CrossRef]
- Aamer, M.; Muhammad, U.H.; Li, Z.; Abid, A.; Su, Q.; Liu, Y.; Adnan, R.; Muhammad, A.U.K.; Tahir, A.K.; Huang, G. Foliar application of glycinebetaine (GB) alleviates the cadmium (Cd) toxicity in spinach through reducing Cd uptake and improving the activity of anti-oxidant system. Appl. Ecol. Environ. Res. 2018, 16, 7575–7583. [Google Scholar] [CrossRef]
- Toth, T.; Zsiros, O.; Kis, M.; Garab, G.; Kovacs, L. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC6803. Plant Cell Environ. 2012, 35, 2075–2086. [Google Scholar] [CrossRef]
- Li, S.S.; Wang, M.; Zhao, Z.Q.; Ma, C.B.; Chen, S.B. Adsorption and desorption of Cd by soil amendment: Mechanisms and environmental implications in field-soil remediation. Sustainability 2018, 10, 2337. [Google Scholar] [CrossRef]
- Song, W.; Chen, S.; Liu, J.; Chen, L.; Song, N.; Li, N.; Liu, B. Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J. Integr. Agric. 2015, 14, 1845–1854. [Google Scholar] [CrossRef]
- Varalakshmi, L.R.; Ganeshamurthy, A.N. Phytotoxicity of cadmium in radish and its effects on growth, yield, and cadmium uptake. Commun. Soil Sci. Plant Anal. 2013, 44, 1444–1456. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.M.; Zia-Ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Li, Y.; Zhang, H.; Mi, X.; Huang, H. Cadmium Addition Effects on Anaerobic Digestion with Elevated Temperatures. Energies 2019, 12, 2367. [Google Scholar] [CrossRef]
- Xue, S.; Shi, L.; Wu, C.; Wu, H.; Qin, Y.; Pan, W.; Hartley, W.; Cui, M. Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environ. Res. 2017, 156, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Mcdonald, L.M. Metal uptake in plants and health risk assessments in metal—Contaminated smelter soils. Land Degrad. Dev. 2015, 26, 785–792. [Google Scholar] [CrossRef]
- Cai, K.; Li, C.; Song, Z.F.; Gao, X.; Wu, M.X. Pollution and health risk assessment of carcinogenic elements As, Cd, and Cr in multiple media-a case of a sustainable farming area in China. Sustainability 2019, 11, 5208. [Google Scholar] [CrossRef]
- Mactovic, V.; Buha, A.; Dukic-Cosic, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Han, J.; Wang, S.; Fan, D.; Guo, Y.; Liu, C.; Zhu, Y. Time-dependent hormetic response of soil alkaline phosphatase induced by Cd and the association with bacterial community composition. Microb. Ecol. 2019, 78, 961–973. [Google Scholar] [CrossRef]
- Kuang, J.; Huang, L.; He, Z.; Chen, L.; Hua, Z.; Jia, P.; Li, S.; Liu, J.; Li, J.; Zhou, J.; et al. Predicting taxonomic and functional structure of microbial communities in acid mine drainage. ISME J. 2016, 10, 1527–1539. [Google Scholar] [CrossRef]
- Li, K.; Yin, G.C.; Xu, Q.Y.; Yan, J.H.; Hseu, Z.Y.; Zhu, L.W.; Lin, Q.T. Influence of aged biochar modified by Cd(2+)on soil properties and microbial community. Sustainability 2020, 12, 4868. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Zhao, Y.; Sun, S.; Liu, Z. Effects of growing seasons and genotypes on the accumulation of cadmium and mineral nutrients in rice grown in cadmium contaminated soil. Sci. Total Environ. 2017, 579, 1282–1288. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Xu, Y.; Liang, X.; Wang, L. In situ stabilization remediation of cadmium (Cd) and lead (Pb) co-contaminated paddy soil using bentonite. Appl. Clay Sci. 2015, 105, 200–206. [Google Scholar] [CrossRef]
- Appel, C.; Ma, L. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J. Environ. Qual. 2002, 3l, 581–589. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; O’Connor, D.; Hou, D. Solidification/stabilization for soil remediation: An old technology with new vitality. Environ. Sci. Technol. 2019, 53, 11615–11617. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.; Harter, R.D. Effect of different organic ligands on cadmium sorption by and extractability from soils. Soil Sci. Soc. Am. J. 1998, 62, 644–650. [Google Scholar] [CrossRef]
- Li, S.M.; Wu, M.D.; Lu, L.H.; Zhu, J.B. Removal of Cd(II) from water by HPEI modified humin. Sustainability 2020, 12, 7931. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Li, B.; Lai, Y.; Zang, L.; Tang, X. Process design and validation of a new mixed eluent for leaching Cd, Cr, Pb, Cu, Ni, and Zn from heavy metal-polluted soil. Anal. Methods 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, J.; Peng, D.; Wu, Z.; Li, Q.; Huang, T. Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies 2019, 12, 387. [Google Scholar] [CrossRef]
- Sun, Z.C.; Wu, B.; Guo, P.H.; Wang, S.; Guo, S.H. Enhanced electrokinetic remediation and simulation of cadmium-contaminated soil by superimposed electric field. Chemosphere 2019, 233, 17–24. [Google Scholar] [CrossRef]
- Zhao, C.; Dong, Y.; Feng, Y.P.; Li, Y.Z.; Dong, Y. Thermal desorption for remediation of contaminated soil: A review. Chemosphere 2019, 221, 841–855. [Google Scholar] [CrossRef]
- Mahadevan, G.D.; Zhao, F. A concise review on microbial remediation cells (MRCs) in soil and groundwater radionuclides remediation. J. Radioanal. Nucl. Chem. 2017, 314, 1477–1485. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, S.; Huang, R.; Wang, M.; Cao, H.; Li, Z. Accumulation of Cd by three forage mulberry (Morus atropurpurea Roxb.) cultivars in heavy metal–polluted farmland: A field experiment. Environ. Sci. Pollut. Res. 2020, 28, 3354–3360. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jiang, P.; Fu, X.; Liu, J.; Sunahara, G.I.; Chen, Z.; Xiao, H.; Lin, F.; Wang, X. Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn.: A long-term field study. Environ. Pollut. 2020, 266, 115408. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, C.; Wang, G.; Xiong, B.J.; Zhou, W.M.; Xue, F.L.; Qi, W.L.; Qiu, C.S.; Liu, Z.B. Mechanism underlying earthworm on the remediation of cadmium—Contaminated soil. Sci. Total Environ. 2020, 728, 138904. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Zhang, H.H.; Zhang, X.F.; Li, Q.; Chenh, C.K.; Shen, H.; Zhang, Z.Z. Bioelectrochemical remediation of Cr(VI)/Cd(II)-contaminated soil in bipolar membrane microbial fuel cells. Environ. Res. 2020, 186, 109582. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, A.; Grobelak, A.; Almås, A.R.; Singh, B.R. Effect of biowastes on soil remediation, plant productivity and soil organic carbon sequestration: A review. Energies 2020, 13, 5813. [Google Scholar] [CrossRef]
- Grzegórska, A.; Rybarczyk, P.; Rogala, A.; Zabrocki, D. Phytoremediation—From environment cleaning to energy generation—Current status and future perspectives. Energies 2020, 13, 2905. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, M.; Wang, J.; Liu, Y.; Liao, Y.; Liu, Y. Assessment of zeolite, biochar, and their combination for stabilization of multimetal-contaminated soil. ASC Omega 2020, 5, 27374–27382. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; Balkema: Rotterdam, The Netherlands, 1993; p. 519. [Google Scholar]
- Xie, X.; Yang, S.; Liu, H.; Pi, K.; Wang, Y. The behavior of cadmium leaching from contaminated soil by nitrilotriacetic acid: Implication for Cd-contaminated soil remediation. Water Air Soil Pollut. 2020, 231, 166. [Google Scholar] [CrossRef]
- Otrembska, P.; Gega, J. Separation of nickel (II) and cadmium (II) ions with ion-exchange and membrane processes. Sep. Sci. Technol. 2016, 51, 2675–2680. [Google Scholar] [CrossRef]
- Makino, T.; Sugahara, K.; Sakurai, Y.; Takano, H.; Kamiya, T.; Sasaki, K.; Itou, T.; Sekiya, N. Remediation of cadmium contamination in paddy soils by washing with chemicals: Selection of washing chemicals. Environ. Pollut. 2006, 144, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Gao, Y.; Yi, S.; Jiang, J.; Aihemaiti, A.; Li, D.; Yang, M. Multi-step column leaching using low-molecular-weight organic acids for remediating vanadium- and chromium-contaminated soil. Environ. Sci. Pollut. Res. 2019, 26, 15406–15413. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Mitton, F.M.; Mihlioranza, K.S.B.; Peña, A. Role of a non-ionic surfactant and carboxylic acids on the leaching of aged DDT residues in undisturbed soil columns. J. Soils Sediments 2019, 19, 1745–1755. [Google Scholar] [CrossRef]
- Kobayashi, T.; Wu, Y.; Lu, Z.; Xu, K. Characterization of anaerobic degradability and kinetics of harvested submerged aquatic weeds used for nutrient phytoremediation. Energies 2015, 8, 304–318. [Google Scholar] [CrossRef]
- Qin, Y.; Shi, X.; Wang, Z.; Pei, C.; Cao, M.; Luo, J. Influence of planting density on the phytoremediation efficiencyof Festuca arundinacea in cd-polluted soil. Bull. Environ. Contam. Toxicol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yang, G.; Qin, Y.; Wang, H.; Cao, M.; Luo, J. Impact of O-3 on the phytoremediation effect of Celosia argentea in decontaminating Cd. Chemosphere 2021, 266, 128940. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Skuza, L.; Zhang, Q. Phytoremediation of two ecotypes cadmium hyperaccumulator Bidens pilosa L. sourced from clean soils. Chemosphere 2021, 273, 129652. [Google Scholar] [CrossRef]
- Dou, X.; Dai, H.; Skuza, L.; Wei, S. Strong accumulation capacity of hyperaccumulator Solanum nigrum L. for low or insoluble Cd compounds in soil and its implication for phytoremediation. Chemosphere 2020, 260, 127564. [Google Scholar] [CrossRef]
- Lin, H.; Lin, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, W.; Meng, G.; Guo, R.; Wang, Y. Phytoremediation of alkaline soils co-contaminated with cadmium and tetracycline antibiotics using the ornamental hyperaccumulators Mirabilis jalapa L. and Tagetes patula L. Environ. Sci. Pollut. Res. 2020, 27, 14175–14183. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Z.; Gu, D.; Li, D.; Tao, Y.; Zhang, D.; Su, L.; Ao, Y. Characterization of cadmium-resistant rhizobacteria and their promotion effects on Brassica napus growth and cadmium uptake. J. Basic Microbiol. 2019, 59, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Xu, Y.; Chi, S.; Li, T.; Li, Y.; He, Z.; Yang, M.; Feng, D. Resistance of alfalfa and Indian mustard to Cd and the correlation of plant Cd uptake and soil Cd form. Environ. Sci. Pollut. Res. 2019, 26, 13804–13811. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Zhou, D.; Zhang, Z.; Ai, Y.; Zhang, W.; Shi, G.; Tong, F.; Liu, L.; Chen, W.; Li, J.; et al. Effect of two-dimensional electric field on the growth and cadmium uptake of Sedum plumbizincicola. Sep. Purif. Technol. 2021, 259, 118121. [Google Scholar] [CrossRef]
- Shu, H.; Zhang, J.; Liu, F.; Bian, C.; Liang, J.; Liang, J.; Liang, W.; Lin, Z.; Shu, W.; Li, J.; et al. Comparative transcriptomic studies on a cadmium hyperaccumulator Viola baoshanensis and its non-tolerant counterpart V. inconspicua. Int. J. Mol. Sci. 2019, 20, 1906. [Google Scholar] [CrossRef]
- Ye, W.; Guo, G.; Wu, F.; Fan, T.; Lu, H.; Chen, H.; Li, X.; Ma, Y. Absorption, translocation, and detoxification of Cd in two different castor bean (Ricinus communis L.) cultivars. Environ. Sci. Pollut. Res. 2018, 25, 28899–28906. [Google Scholar] [CrossRef]
- Wu, K.; Luo, J.; Li, J.; An, Q.; Yang, X.; Liang, Y.; Li, T. Endophytic bacterium Buttiauxella sp SaSR13 improves plant growth and cadmium accumulation of hyperaccumulator Sedum alfredii. Environ. Sci. Pollut. Res. 2018, 25, 21844–21854. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Yin, Y.; Ji, R.; Wu, J.; Wang, X.; Guo, H. Ethyl lactate-EDTA composite system enhances the remediation of the cadmium-contaminated soil by Autochthonous Willow (Salix x aureo-pendula CL ‘J1011’) in the lower reaches of the Yangtze River. J. Hazard. Mater. 2010, 181, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, K.; Xu, X.; Cai, C. Interaction of Fe-Mn plaque and Arthrobacter echigonensis MN1405 and uptake and translocation of Cd by Phytolacca acinosa Roxb. Chemosphere 2017, 174, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 2020, 242, 125112. [Google Scholar] [CrossRef]
- Gerhardt, K.; Gerwing, P.; Greenberg, B. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, G.; Qin, S. Remediation effect of chemical leaching on Cr (VI)-contaminated fine soil and its economic cost. Chin. J. Ecol. 2020, 39, 2309–2315. (In Chinese) [Google Scholar] [CrossRef]
- Xie, Y.; Ji, X.; Wu, J.; Liu, S.; Pan, S.; Yi, H. The “three highs” hyperaccumulators screening and repair cost analysis of cadmium and arsenic contaminate soil. Environ. Sci. Technol. 2020, 43, 116–121. (In Chinese) [Google Scholar] [CrossRef]
- Thanappan, S.; Hosamani, S.R.; Chandrappa, M.N. Rill treatments to enhance nutrient rich soil, a case study. Asian J. Agric. Biol. 2020, 8, 186–193. [Google Scholar] [CrossRef]
- Dhiman, S.; Baliyan, N.; Maheshwari, D.K. Bufalo dung-inhabiting bacteria enhance the nutrient enrichment of soil and proximate contents of Foeniculum vulgare Mill. Arch. Microbiol. 2020, 202, 2461–2470. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Ma, J.; Ma, Z.; Li, G.; Zheng, J. Combining infiltration holes and level ditches to enhance the soil water and nutrient pools for semi-arid slope shrubland revegetation. Sci. Total Environ. 2020, 729, 138796. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).