3.1. I Stage—Nitrification of the Effluent after Anaerobic Treatment
In the nitrifying reactors, the n was increased for over 35 days from 0.1 1/d to the adjusted final value of 0.3 1/d, and then it was maintained at this level to the end of the experiment. The reactors were operated at ambient temperature (20 ± 2 °C), which ensures efficient nitrification. The average pollutant concentrations are shown in
Figure 1. In the effluent from anaerobic digestion that was supplied to both nitrifying reactors, the organics concentration averaged 1530 mg COD/L. In the adaptation phase at the n of 0.1 1/d, the average COD concentration in the effluent from the nitrifying reactors was 167 mg COD/L, and this increased to 241 mg COD/L when the n of 0.3 1/d was attained (
Figure 1).
As the organics concentration expressed as BOD7 was very low in the effluent from the nitrifying reactors, it can be assumed that the remained organics were in a difficult-to-degrade form, called the inert fraction. All the pollutant concentrations in the liquid were measured after it had been subjected to filtration; thus, the inert fraction of COD constituted soluble compounds that were unavailable to the microorganisms.
The total Kjeldahl nitrogen in the effluent after anaerobic digestion was around 500 mg TKN/L, of which ammonium nitrogen constituted 46%. During the adaptation phase, the TKN concentration in the effluent from the nitrifying reactors remained at 3.5 mg/L; then, at the n of 0.3 1/d, it remained stable at 5.6 mg TKN/L. This means that organic nitrogen, constituting more than half of the TKN, was converted into ammonium, which was simultaneously oxidized. As a result of biological ammonium transformation at increasing n, nitrite appeared during the adaptation period, indicating partial, incomplete nitrification.
Then, ammonium was completely oxidized, and nitrite was only a short-term intermediate product, which was converted to nitrate. During the period with the lowest n, the nitrite concentration reached up to 58 mg N-NO
2/L, whereas nitrate made up the rest of the oxidized nitrogen forms generated from ammonium. However, in the effluent, the ammonium concentration was very low and did not exceed 3 mg N-NH
4/L. At the adjusted n of 0.3 1/d, the concentration of nitrate increased to ca. 460 mg N-NO
3/L, and no nitrite remained in the effluent from the nitrifying reactors (
Figure 1).
3.2. Kinetics in Nitrifying Reactors
Figure 2 presents the changes in the concentrations of organic and nitrogen compounds that took place during the working cycle in the nitrifying reactors while the n of 0.3 1/d was maintained. As the pollutant concentrations from both reactors were similar, the average concentrations are shown (
Figure 2). At the beginning of the reactor cycle, the COD concentration was 630 mg/L, including COD concentration from the organics remaining after the previous cycle and that from the influent. The COD concentration decreased most intensively during the first 4 h of the cycle, then it stabilized and remained at about 250 mg COD/L. Changes in the COD concentration followed 1st order kinetics, and the initial organics removal rate (r
COD) was 287.9 mg/(L·h).
It should be emphasized that the content of easily biodegradable compounds (BOD7) was very low, less than 5 mg/L, so the organics in the effluent after nitrification were resistant to biodegradation and consisted mainly of the inert fraction.
Ammonium oxidation proceeded according to zero-order kinetics. This means that the rate of ammonium removal rN-NH4 is equal to the kinetic constant of ammonium removal kN-NH4 and the process rate is constant over time and does not depend on the substrate concentration. The ammonium was completely oxidized in the first 4 h of the working cycle with a rate of 15.5 mg N-NH4/(L·h) (5.4 mg N-NH4/(g VSS·h). As a result of the n, the ammonium concentration at the beginning of the cycle was ca. 70 mg/L, which decreased to 3.8 mg/L, and then remained at this level until the end of the cycle. The decrease in ammonium concentration was accompanied by an increase in both nitrite and nitrate concentrations. The changes in nitrite concentrations also proceeded with zero-order kinetics. During the first 4 h of aeration, the rate of nitrite formation was around 14 mg N-NO2/(L·h). From 4 to 11 h of the working cycle, the nitrite concentration started to decrease at a rate of 7.8 mg N-NO2/(L·h). From the beginning of the cycle, the nitrate concentration continuously increased (the increase proceeded with first-order kinetics), and the initial rate of nitrate formation was around 30 mg N-NO3/(L·h).
Studies on the kinetics of nitrification of the effluent from the AD of the leachate from aerobic stabilization of OFMSW are scarce. Most of these studies have concerned nitrification in different types of wastewater or landfill leachate, including artificial ones. For example, in the study of Kulikowska et al. [
9], nitrogen removal from municipal landfill leachate (with ammonium concentration of ca. 900 mg N-NH
4/L) was carried out in a two-stage system, in which denitrification was preceded by nitrification. Similar to this study, during the adaptation period, nitrite was formed as an intermediate product of nitrification. However, the ammonium concentration in the effluent did not exceed 1 mg N-NH
4/L, meaning that the effectiveness of nitrification was almost 100%. In the SBR cycle, the ammonium removal also proceeded according to zero-order kinetics, but the removal rate was 2-times higher, 31 mg N-NH
4/(L∙h). Nitrite accumulated only temporarily. However, in the study of Kulikowska et al. [
9], the main component of TKN was ammonium. Whereas, in this study, organic nitrogen prevailed (N-NH
4/TKN ratio 0.46). The high share of organic nitrogen in TKN influenced the rate of ammonium removal. The lower rate of ammonium removal may have been caused by the simultaneous conversion of the organic nitrogen into ammonium. The low ratio of N-NH
4/TKN in the effluent after AD may have resulted from the fact that TKN in the leachate from aerobic stabilization of OFMSW consisted of ca. 30% ammonium, which was stated also in a previous study [
10]. Koc-Jurczyk and Jurczyk [
11] treated old landfill leachate (4125 mg COD/L, 636 mg BOD
5/L (BOD
5/COD ratio of 0.15), 775 mg N-NH
4/L and 850 mg TKN/L) in three SBRs, one contained suspended activated sludge, whereas two SBRs additionally contained foam carriers. The effectiveness of ammonium removal in the SBR with suspended activated sludge was 46.3%; however, it should be mentioned that in the influent, the concertation was ca. 800 mg N-NH
4/L. In contrast to the present study, ammonium removal proceeded with first-order kinetics (the initial rate of the process was 50–68 mg N-NH
4/(L∙h)).
3.3. II Stage—Denitrification of the Effluent from Nitrifying Reactors
During denitrification, n increased from 0.2 to 0.5 1/d. The reactors were operated at ambient temperature (20 ± 2 °C) for 80 days. As the COD concentration in the effluent after nitrification was low, and it was composed of mainly difficult-to-degrade organics, external carbon sources had to be added. The appropriate dosage of ACS as beet molasses, crude glycerine, or distillery stillage was supplied into the denitrifying reactors to ensure that the adjusted COD/N-NO3 ratio was maintained. At the n of 0.35 1/d, which was maintained until day 40 of the experiment, the COD/N-NO3 ratio was kept at 8. Then, at the n of 0.5 1/d, the ratio was decreased to 7.
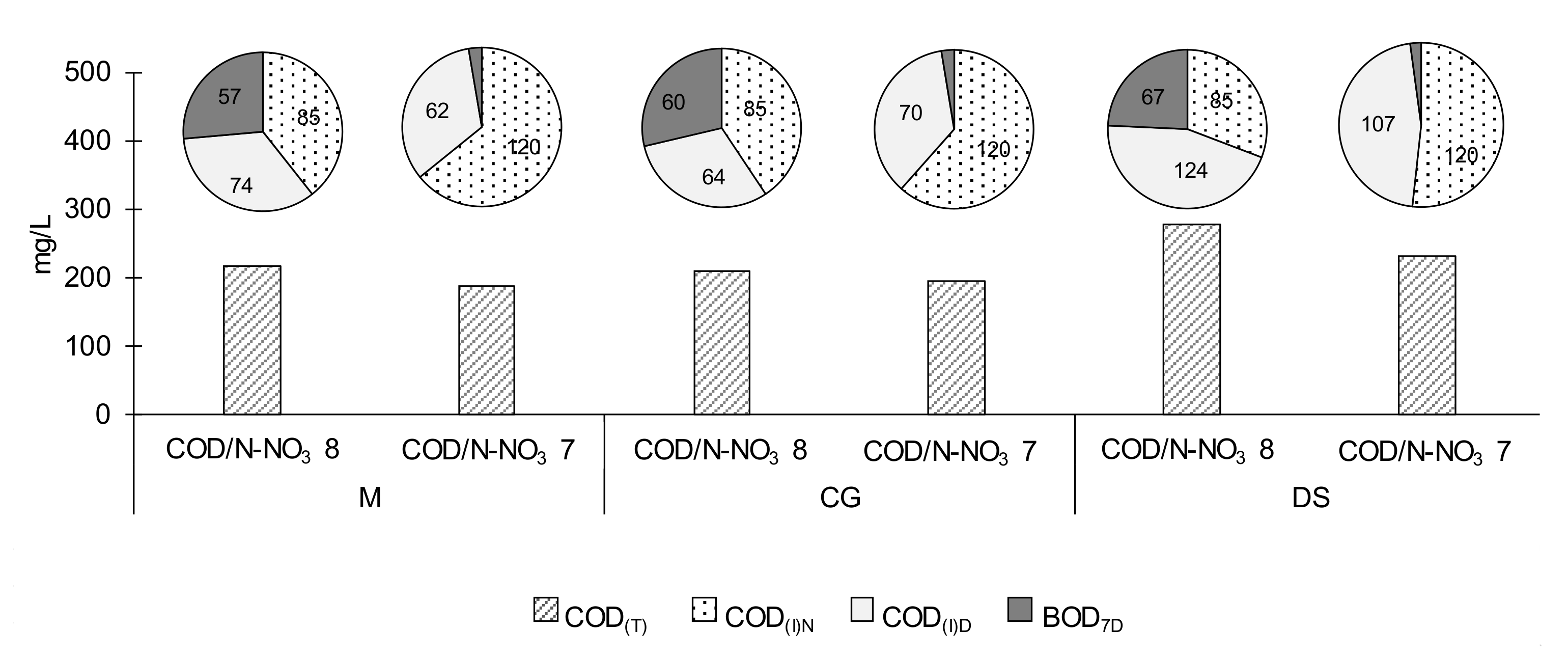
Figure 3 shows the COD concentrations in wastewater after denitrification. It was assumed that the total COD (COD
T) comprises the organics introduced into the denitrifying reactor with wastewater after nitrification COD
(I)N, and the organics remaining from ACS after denitrification, which were calculated as the sum of COD
(I)D, and BOD
7D.
The concentration of COD(I)N depended on the n in the denitrifying reactor. At the lower n of 0.35 1/d, the concentration of organics as COD(I)N was ca. 85 mg COD/L, whereas at the higher n of 0.5 1/d, it was proportionally higher, ca. 120 mg COD/L. These organics can be assumed to be the difficult-to degrade inert fraction because of their negligible content of BOD after nitrification. The remaining COD concentration depended on the COD/N-NO3 ratio used during the denitrification step. At a COD/N-NO3 ratio of 8, easily biodegradable compounds were present in the effluent after denitrification, indicating that, although a part of the organics was easily available to the microorganisms, they were not used for denitrification. For this reason, the COD/N-NO3 ratio was decreased to 7 at the higher n of 0.5 1/d. Under those conditions, the effluent had a negligible content of BOD, and all the COD consisted of the inert fraction of the organic compounds. Taking into consideration all of the COD fractions, including BOD, and the type of ACS used as the carbon source, it can be concluded that the effluent concentrations of COD were higher at the larger COD/N-NO3 ratio. Regardless of the COD/N-NO3 ratio, the COD concentration in the effluent after denitrification was highest when distillery stillage was used. When beet molasses or crude glycerine were used, the effluent COD content was lower, and it did not differ between these two carbon sources.
Regardless of the COD/N-NO
3 ratio and the external carbon source that was used, the sum of the concentrations of nitrates and nitrites in the effluent did not exceed 1 mg/L. This means that waste carbon sources, with COD/N-NO
3 ratios of 8 and 7, ensured effective denitrification. Similar results were obtained by Shao et al. [
12]. The authors stated that for denitrification with waste sludge alkaline fermentation liquid as the external carbon source, the optimal was a C/N ratio of 7. They reported that the nitrate removal efficiency was 96.4%, and no nitrite accumulation was found.
Figure 4 presents the changes in the concentrations of nitrogen compounds that took place during the working cycle in the denitrifying reactors while maintaining the n of 0.35 1/d and the COD/N-NO
3 ratio 8, or the n of 0.5 1/d and the COD/N-NO
3 ratio of 7. At the COD/N-NO
3 ratio of 7, nitrite accumulation during denitrification was substantial, but this was not as noticeable at the higher COD/N-NO
3 ratio.
The high nitrite accumulation influenced the rate of denitrification (the decrease in the concentration of all oxidized forms of nitrogen, calculated as the sum of nitrite and nitrate, rN-NOx) and the time for complete removal of nitrite and nitrate during the cycle. This time extended when the nitrite accumulation was more substantial. Irrespective of the COD/N-NO3 ratio, the decrease in the concentration of all oxidized forms of nitrogen (as the sum of nitrite and nitrate) followed zero-order kinetics.
With an excess of organic compounds, i.e., at the COD/N-NO3 ratio of 8, the oxidized forms of nitrogen were removed very quickly (for no longer than 2 h of the cycle). The highest rate of nitrite and nitrate removal, 160 mg N-NOx/(L·h) (57 mg N-NOx/(g VSS·h)), was observed when beet molasses was used. The removal rate was 1.67 times lower with crude glycerine, and it was lowest with distillery stillage (72 mg N-NOx/(L·h); 26 mg N-NOx/(g VSS·h)). The rate of nitrite and nitrate removal decreased when the COD/N-NO3 ratio was decreased to 7, and the n was concurrently increased, causing the nitrate content at the beginning of the cycle to be higher (225 mg N-NO3/L vs. 160 mg N-NO3/L). Similar to what was observed at the higher COD/N-NO3 ratio, the rate of nitrite and nitrate removal at the lower COD/N-NO3 ratio was highest (45 mg N-NOx/(L·h); 16 mg N-NOx/(g VSS·h)) when beet molasses was used the carbon source, and lowest when distillery stillage was used (30 mg N-NOx/(L·h); 11 mg N-NOx/(g VSS·h)). With these respective alternative carbon sources, the time for complete removal of nitrite and nitrate during the cycle ranged from 5 h to more than 8 h. This would affect the total length of the cycle in denitrifying reactors.
The effect of the external carbon source on the kinetics of denitrification has been also reported by other authors, but during nitrogen removal from synthetic or municipal wastewater, not from AD effluent. For example, Horova et al. [
13] analyzed the effect of ethanol, glycerol, molasses, and whey on nitrate removal from highly contaminated synthetic wastewater (1500 mg N-NO
3/L; COD/N ratio 4). They showed that the time necessary for complete removal of total nitrogen was 8.3 h for ethanol and whey, 7.1 h for molasses, and 10.4 h for glycerol. However, in all cases, the formation of nitrite was observed after ca. 5 h, and the maximum concentration reached 544 mg N-NO
2/L with ethanol and ca. 400 mg N-NO
2/L with whey and molasses. The lowest nitrite accumulation, 200 mg N-NO
2/L, was with glycerol. The N-NOx removal rates ranged from 58.2 mg/(g VSS·h) to 62.3 mg/(g VSS
·h). These values were similar to the values obtained in the present study when beet molasses was used as a carbon source, at a COD/N-NO
3 ratio of 8.
In another study, Smyk and Ignatowicz [
14] tested molasses as an external carbon source during municipal wastewater treatment in SBR reactors. They found that after the addition of molasses, COD concentration increased and the COD/TKN ratio also increased from 4.5 to 5.3, resulted in a higher efficiency of nitrogen (by 6%) and ammonium (by 0.8%) removal than in the reactor with no supplementation of the additional carbon source. The use of molasses caused a decrease in the nitrate concentration in the effluent [
14]. Guo et al. [
15] found that at the COD/N-NO
3 ratio of 7–8, the effectiveness of denitrification in synthetic wastewater supplemented by fermentation liquid and waste sludge thermal hydrolysate was 92.3 and 98.9%, respectively. The COD/N-NO
3 ratio was similar to that used in the present study, but in contrast, no nitrite accumulation was observed.
Based on the changes in concentrations of organic compounds, measured as COD, the removal rates and the rate constants were determined (
Figure 5). The decrease in the concentrations of COD followed first-order kinetics, and thus, in this case, the initial rate of COD removal could be determined. Similar to what was observed with the oxidized forms of nitrogen, the organics were removed quickly at the COD/N-NO
3 ratio of 8, and the time needed for complete COD removal did not exceed 4 h. After this time, the COD concentration remained on the same level. When the COD/N-NO
3 ratio decreased to 7, the initial COD concentration at the beginning of the cycle increased because the n was concurrently increased. Irrespective of the COD/N-NO
3 ratio, the rate of organics removal was highest when beet molasses was used as a carbon source, and lowest when distillery stillage was used.
As can be seen in
Figure 5, the removal rates of both the oxidized forms of nitrogen and the organics depended on the type of carbon source used in the denitrifying stage. It was found that, at the COD/N-NO
3 ratio of 8, the rates were higher than at the COD/N-NO
3 ratio of 7. Regardless of the COD/N-NO
3 ratio, the highest rates were observed with beet molasses and the lowest with distillery stillage. At the COD/N-NO
3 ratio of 8, the rate of denitrification with distillery stillage was 55% and 75% lower in comparison with molasses and crude glycerine. At the COD/N-NO
3 ratio of 7, it was 65% and 74% lower, respectively. Distillery stillage is a waste product from the production of ethyl alcohol from substrates that are rich in simple and complex sugars. Although distillery stillage has high concentrations of biodegradable organic compounds, these compounds are mostly present in the form of suspended solids [
16]. Therefore, they require a longer period of degradation before their assimilation by microorganisms. Similar results with distillery stillage as a source of organic carbon were reported in a study on post-denitrification of effluent (NO
2-/NO
3- ratio of 0.45) from the treatment of nitrogen-rich digester supernatant, in which an initial lag-phase during NOx removal was observed [
17]. In a study by Miao et al. [
18], the denitritation rates with the use of distillery stillage were like those observed during denitritation with the use of other complex carbon sources, such as starch. In the study presented here, it can be concluded that denitrification with distillery stillage was limited by the biodegradability level of this carbon source because, after the denitrification stage, the highest contribution of COD came from the external carbon source that was not utilized in nitrite/nitrate reduction (almost half of total COD).