The Application Potential of Hop Sediments from Beer Production for Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstocks
2.2. Composting Conditions—Experimental Set-Up
- I—Control (K; maize straw);
- II—Hot trub (HT) + maize straw (K);
- III—Spent hops (SH) + maize straw (K).
2.3. Chemical Analysis
2.4. Microbiological Analyses
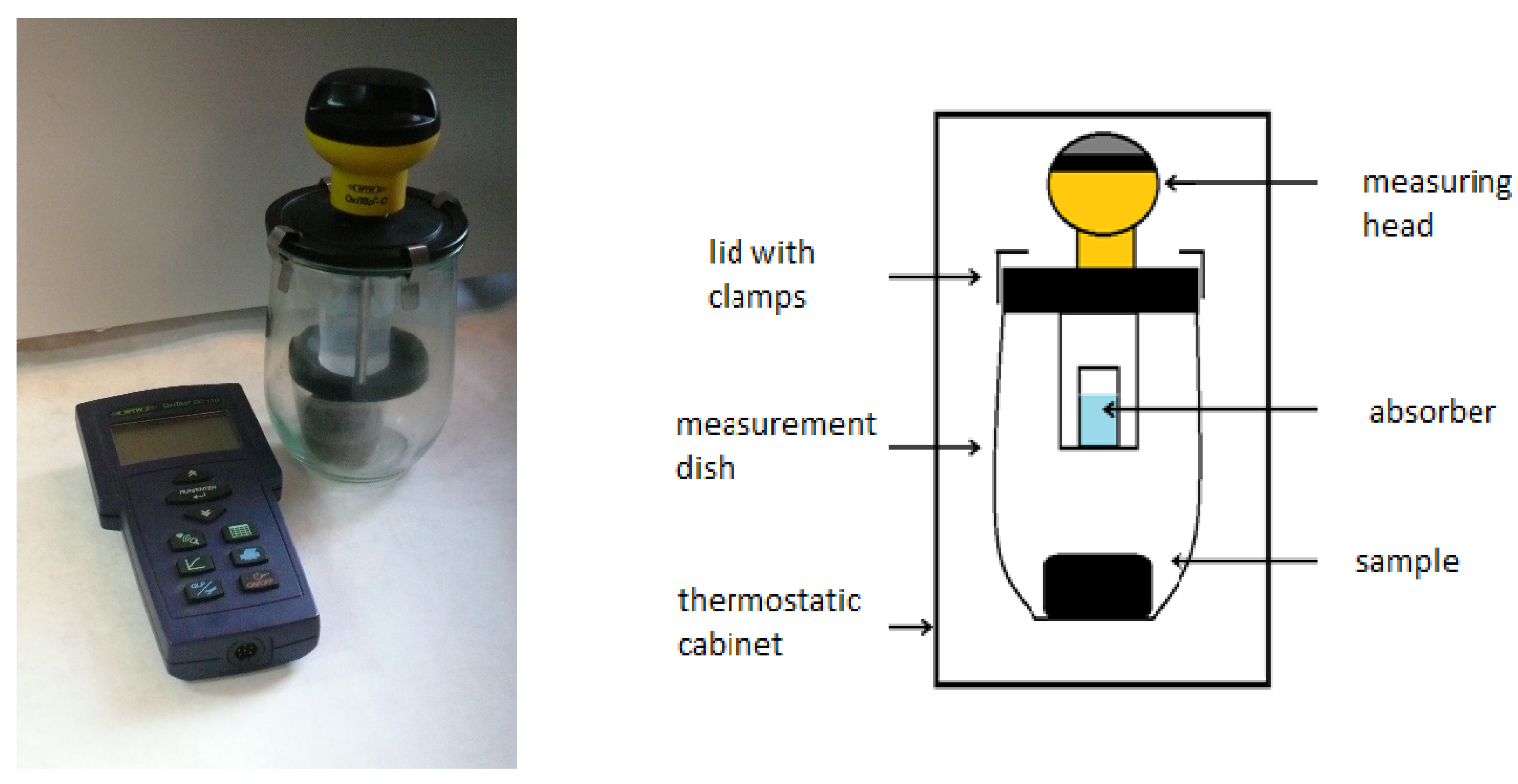
2.5. Respiratory Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Hop Sediments before Composting
3.2. Microbiological Analysis
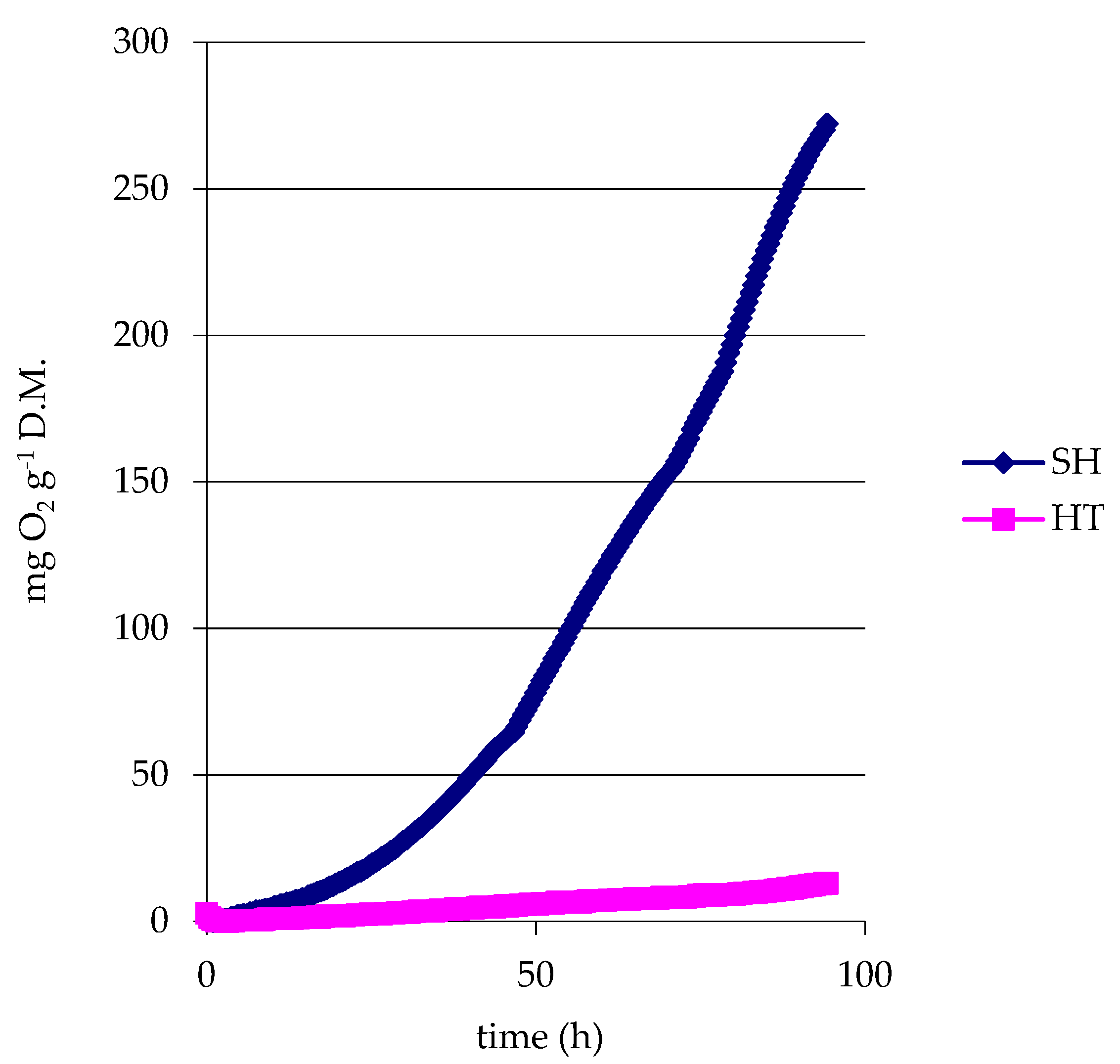
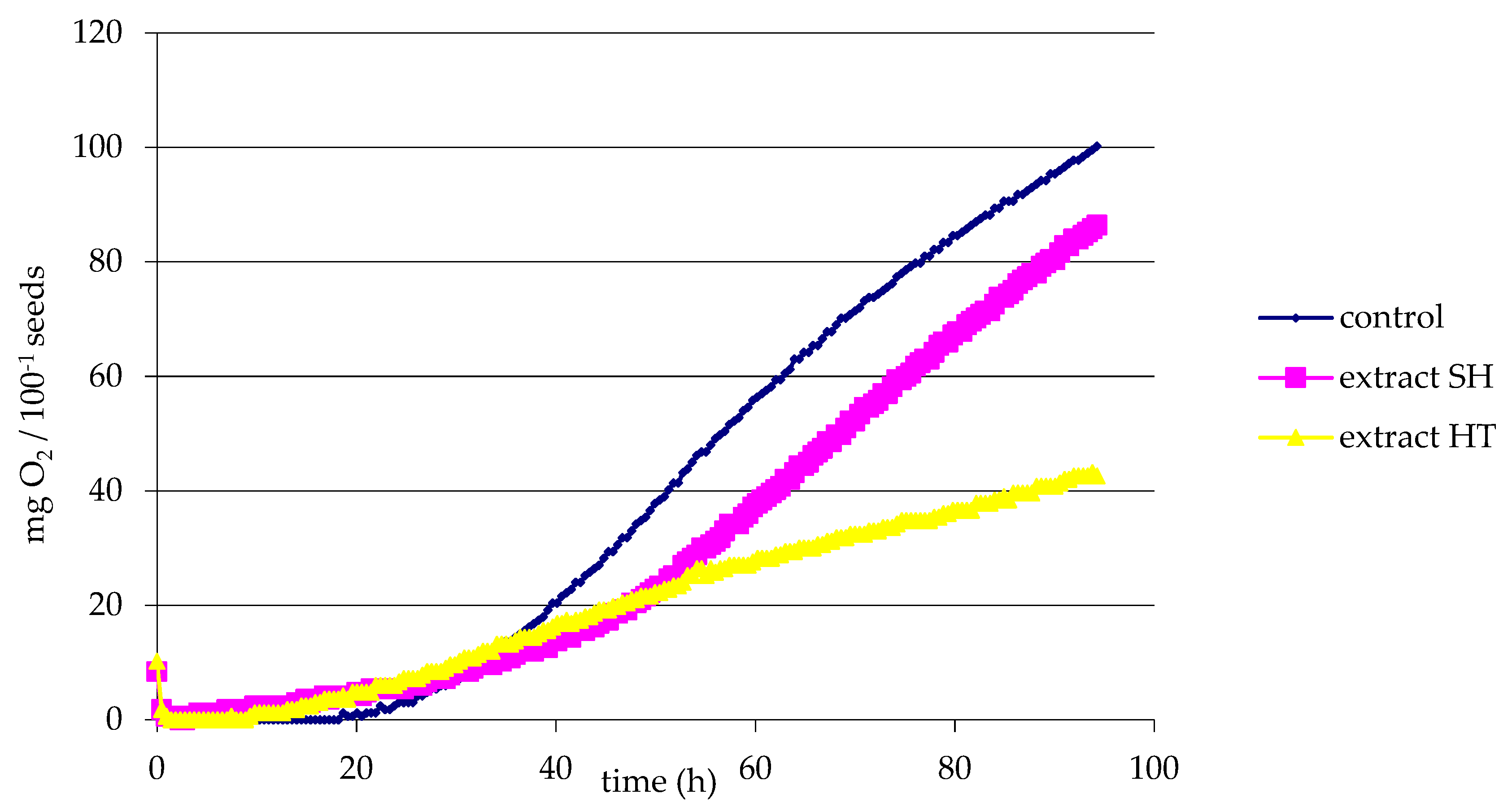
3.3. Respiratory Activity of Sediments before Composting
- y(HT) = 0.136x − 0.9672 R2 = 0.9711;
- y(SH) = 4.082x − 123.51 R2 = 0.9887.
- y(control) = 1.414x − 29.725 R2 = 0.9899;
- y(HT) = 0.454x + 0.286 R2 = 0.9942;
- y(SH) = 1.458x − 49.967 R2 = 0.9993.
3.4. Respiratory Activity of the Composted Hop Sediments
- y(K) = 0.0002x + 0.0023 for x = 96 y = 0.0215 mg O2·g−1 D.M.;
- y(K + HT) = (4 × 105)x + 0.0015 for x = 96 y = 0.00534 mg O2·g−1 D.M.;
- y(K + SH) = (8 × 10−5)x + 0.0024 for x = 96 y = 0.01008 mg O2·g−1 D.M., where K is maize straw.
4. Conclusions
- Hop sediments produced at various stages of the brewing process differ significantly in chemical and biological reactivity, which affects their possible biological processing.
- Hop sediments were free from microbiological contamination that may pose an epidemiological threat.
- The SH sediment was very rich in total nitrogen (50 g/kg D.M.), which affected the composting process. Composting this sediment required the selection of substrates with a wide C:N ratio. However, the sediment is suitable for biological processing.
- In the case of HT, plant growth inhibitory properties were found both before and after composting, which were most likely not due to the mineral composition. Organic compounds and the sterilization of the substrate as a result of wort boiling may be inhibitors of biological changes.
- Stable compost with hop sediments could be obtained after 60 days of an intensive aerobic transformation, with the appropriate quantitative and qualitative selection of substrates. In the last 4 days of the experiment, the oxygen demand was very low and amounted to below 0.0215 mg O2 g−1 D.M.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schisler, D.O.; Ruocco, J.J.; Mabee, M.S. Wort trub content and its effects on fermentation and beer flavor. J. Am. Soc. Brew. Chem. 1982, 40, 57–61. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; de Mello, P.P.M.; Sérvulo, E.F.C. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar]
- Oladokun, O.; James, S.; Cowley, T.; Smart, K.; Hort, J.; Cook, D. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Sterczyńska, M.; Stachnik, M. Technical and technological aspects of clarifying beer wort. Food Process Eng. 2017, 4, 24–27. [Google Scholar]
- Sterczyńska, M.; Stachnik, M.; Poreda, A.; Jakubowski, M. Hot sludge—Waste material in the production of clear beer wort. Food Process Eng. 2018, 2, 36–41. [Google Scholar]
- Guidelines on the Use of by-Products and Recommended Waste Management in Agriculture and the Agri-Food Industry; Ministry of Agriculture and Rural Development, Falenty—Warsaw Institute of Technology and Life Sciences: Warsaw, Poland, 2010; p. 103.
- Kerby, C.; Vriesekoop, F. An overview of the utilisation of brewery by-products as generated by british craft breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Mathias, T.R.D.S.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Girardi, J.; Cosci, F.; Conti, B. Not just for beer: Evaluation of spent hops (Humulus lupulus L.) as a source of eco-friendly repellents for insect pests of stored foods. J. Pest Sci. 2015, 88, 583–592. [Google Scholar] [CrossRef]
- Pal, J.; Piotrowska, A.; Adamiak, J.; Czerwińska-Ledwig, O. P Beer and brewing raw materials in cosmetology as well as beer baths as a treatment form. Postępy Fitoter. 2019, 20, 145–153. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. (Eds.) Methods of Analysis and Evaluation of Soil Properties and Plant—Catalogue; Institute of Environmental Protection: Warsaw, Poland, 1991; p. 331. (In Polish) [Google Scholar]
- Notes and Memoranda: Dr. Koch’s New Method of Pure Cultivation of Bacteria. J. Cell Sci. 1881, 2–21, 650–654.
- Koch, R. Sechster Bericht der deutschen wissenschaftlichen Commission zur Erforschung der Cholera. Dtsch. Med. Wochenscrift 1884, 10, 191–192. [Google Scholar]
- Wolny-Koładka, K.; Żukowski, W. Mixed municipal solid waste hygienisation for refuse-derived fuel production by ozonation in the novel configuration using fluidized bed and horizontal reactor. Waste Biomass Valorization 2019, 10, 575–583. [Google Scholar] [CrossRef]
- Malinowski, M.; Wolny-Koładka, K.; Vaverková, M.D. Effect of biochar addition on the OFMSW composting process under real conditions. Waste Manag. 2019, 84, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, M.; Wolny-Koładka, K. Microbiological and energetic assessment of the effects of the biodrying of fuel produced from waste. Ecol. Chem. Eng. 2017, 24, 551–564. [Google Scholar] [CrossRef][Green Version]
- ISO 14855-1:2005. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method; International Organization for Standardization: Genève, Switzerland, 2005. [Google Scholar]
- Jindo, K.; Sonoki, T.; Matsumoto, K.; Canellas, L.; Roig, A.; Sanchez-Monedero, M.A. Influence of biochar addition on the humic substances of composting manures. Waste Manag. 2016, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- OxiTop®. Measuring System; Wageningen University and NMI: Wageningen, The Netherlands, 2003; p. 13. [Google Scholar]
- Kopeć, M.; Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Chmiel, M.J. Effect of the addition of biochar and coffee grounds on the biological properties and ecotoxicity of composts. Waste Biomass Valorization 2018, 9, 1389–1398. [Google Scholar] [CrossRef]
- Poreda, A.; Stefaniuk, K.; Hoc, J.; Zdaniewicz, M. Improving the efficiency of malt wort supplementation with zinc ions. Ferment. Fruit Veg. Ind. 2014, 2, 4–8. [Google Scholar]
- Oszust, K.; Frąc, M.; Gryta, A.; Bilińska, N. The influence of ecological and conventional plant production systems on soil microbial quality under hops (Humulus lupulus). Int. J. Mol. Sci. 2014, 15, 9907–9923. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Jarosz, R. Biological activity of composts obtained from hop waste generated during the brewing. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
| Parameter | Unit | HT | SH |
|---|---|---|---|
| pH (1:10) | 4.96 (0.09) | 5.19 (0.08) | |
| EC (1:10) * | mS·cm−1 | 1.855 (0.06) | 1.282 (0.08) |
| N | g·kg−1 | 27.84 (0.12) | 49.96 (0.35) |
| C | g·kg−1 | 522.9 (10.5) | 490.3 (8.20) |
| C:N ratio | 18.78 | 9.81 | |
| Total Na | g·kg−1 | 0.12 (0.00) | 0.10 (0.00) |
| Total K | g·kg−1 | 9.92 (0.26) | 4.67 (0.05) |
| Total Mg | g·kg−1 | 2.41 (0.03) | 4.39 (0.01) |
| Total Ca | g·kg−1 | 8.04 (0.05) | 9.08 (0.01) |
| Total P | g·kg−1 | 3.36 (0.12) | 9.36 (0.05) |
| Total Cd | mg·kg−1 | 0.03 (0.00) | 0.10 (0.00) |
| Total Cr | mg·kg−1 | 0.68 (0.07) | 0.64 (0.09) |
| Total Cu | mg·kg−1 | 5.74 (0.05) | 124.88 (2.01) |
| Total Fe | mg·kg−1 | 314.75 (9.55) | 307.25 (17.00) |
| Total Mn | mg·kg−1 | 37.68 (0.57) | 48.34 (0.05) |
| Total Ni | mg·kg−1 | 1.10 (0.00) | 0.44 (0.02) |
| Total Pb | mg·kg−1 | 0.45 (0.00) | 0.53 (0.07) |
| Total Zn | mg·kg−1 | 30.13 (0.21) | 216.48 (0.78) |
| Parameter | Unit | Control | HT Extract | SH Extract |
|---|---|---|---|---|
| pH | - | 6.27 (0.09) ** | 4.70 (0.21) | 5.64 (0.11) |
| EC | mS·cm−1 | 0.062 (0.002) | 0.478 (0.010) | 0.275 (0.020) |
| Na | µg·cm−3 | 0.575 (0.021) | 3.591 (0.054) | 2.928 (0.092) |
| K | µg·cm−3 | 8.321 (0.321) | 134.4 (0.028) | 51.89 (0.120) |
| Mg | µg·cm−3 | 0.208 (0.011) | 16.44 (0.087) | 36.93 (0.890) |
| Ca | µg·cm−3 | 2.634 (0.121) | 18.93 (0.08) | 37.25 (0.43) |
| P | µg·cm−3 | 1.215 (0.012) | 37.16 (0.07) | 89.62 (1.23) |
| Cd | µg·cm−3 | 0.001 (0.001) | 0.001 (0.001) | 0.001 (0.001) |
| Cr | µg·cm−3 | 0.001 (0.001) | 0.005 (0.001) | 0.004 (0.001) |
| Cu | µg·cm−3 | nd * | 0.008 (0.001) | 0.057 (0.009) |
| Fe | µg·cm−3 | 0.010 (0.001) | 0.109 (0.001) | 0.083 (0.003) |
| Mn | µg·cm−3 | 0.072 (0.002) | 0.183 (0.002) | 0.252 (0.019) |
| Ni | µg·cm−3 | 0.002 (0.001) | 0.001 (0.001) | 0.001 (0.001) |
| Pb | µg·cm−3 | 0.004 (0.001) | 0.005 (0.001) | 0.008 (0.001) |
| Zn | µg·cm−3 | 0.106 (0.003) | 0.124 (0.009) | 0.177 (0.019) |
| Treatment | Day of Composting | |||
|---|---|---|---|---|
| 0 | 30 | 60 | 90 | |
| K | 100 a,* | 32.14 f | 23.36 g | 22.77 g |
| K + HT | 100 a | 56.89 b | 55.61 b,c | 53.62 c |
| K + SH | 100 a | 48.26 d | 41.67 e | 40.65 e |
| Parameter | Process Day | K | K + HT | K + SH |
|---|---|---|---|---|
| Total N | 0 | 10.5 a | 18.4 a | 30.4 b |
| 30 | 31.2 b | 33.0 b | 45.1 c,d | |
| 60 | 36.3 b,c | 32.2 b | 49.5 d | |
| 90 | 37.6 b,c | 33.8 b | 52.0 d | |
| Total C | 0 | 429.8 a,b | 513.8 e | 475.3 b,c,d,e |
| 30 | 426.4 a,b,c | 494.3 e | 462.5 c,d,e | |
| 60 | 406.7 a,b | 483.2 d,e | 458.0 c,d,e | |
| 90 | 386.3 a | 482.6 d,e | 434.9 a,b,c,d | |
| C:N ratio | 0 | 40.9 e | 27.9 d | 15.6 c |
| 30 | 13.6 b,c | 15.0 c | 10.2 a,b,c | |
| 60 | 11.2 a,b,c | 14.9 c | 9.3 a,b | |
| 90 | 10.3 a,b,c | 14.3 b,c | 8.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopeć, M.; Mierzwa-Hersztek, M.; Gondek, K.; Wolny-Koładka, K.; Zdaniewicz, M.; Suder, A. The Application Potential of Hop Sediments from Beer Production for Composting. Sustainability 2021, 13, 6409. https://doi.org/10.3390/su13116409
Kopeć M, Mierzwa-Hersztek M, Gondek K, Wolny-Koładka K, Zdaniewicz M, Suder A. The Application Potential of Hop Sediments from Beer Production for Composting. Sustainability. 2021; 13(11):6409. https://doi.org/10.3390/su13116409
Chicago/Turabian StyleKopeć, Michał, Monika Mierzwa-Hersztek, Krzysztof Gondek, Katarzyna Wolny-Koładka, Marek Zdaniewicz, and Aleksandra Suder. 2021. "The Application Potential of Hop Sediments from Beer Production for Composting" Sustainability 13, no. 11: 6409. https://doi.org/10.3390/su13116409
APA StyleKopeć, M., Mierzwa-Hersztek, M., Gondek, K., Wolny-Koładka, K., Zdaniewicz, M., & Suder, A. (2021). The Application Potential of Hop Sediments from Beer Production for Composting. Sustainability, 13(11), 6409. https://doi.org/10.3390/su13116409








