Abandoned Mine Lands Reclamation by Plant Remediation Technologies
Abstract
1. Introduction
2. Occurrence of AMLs and Their Ecological Impact Worldwide
3. Plant Remediation Technologies for AML Restoration
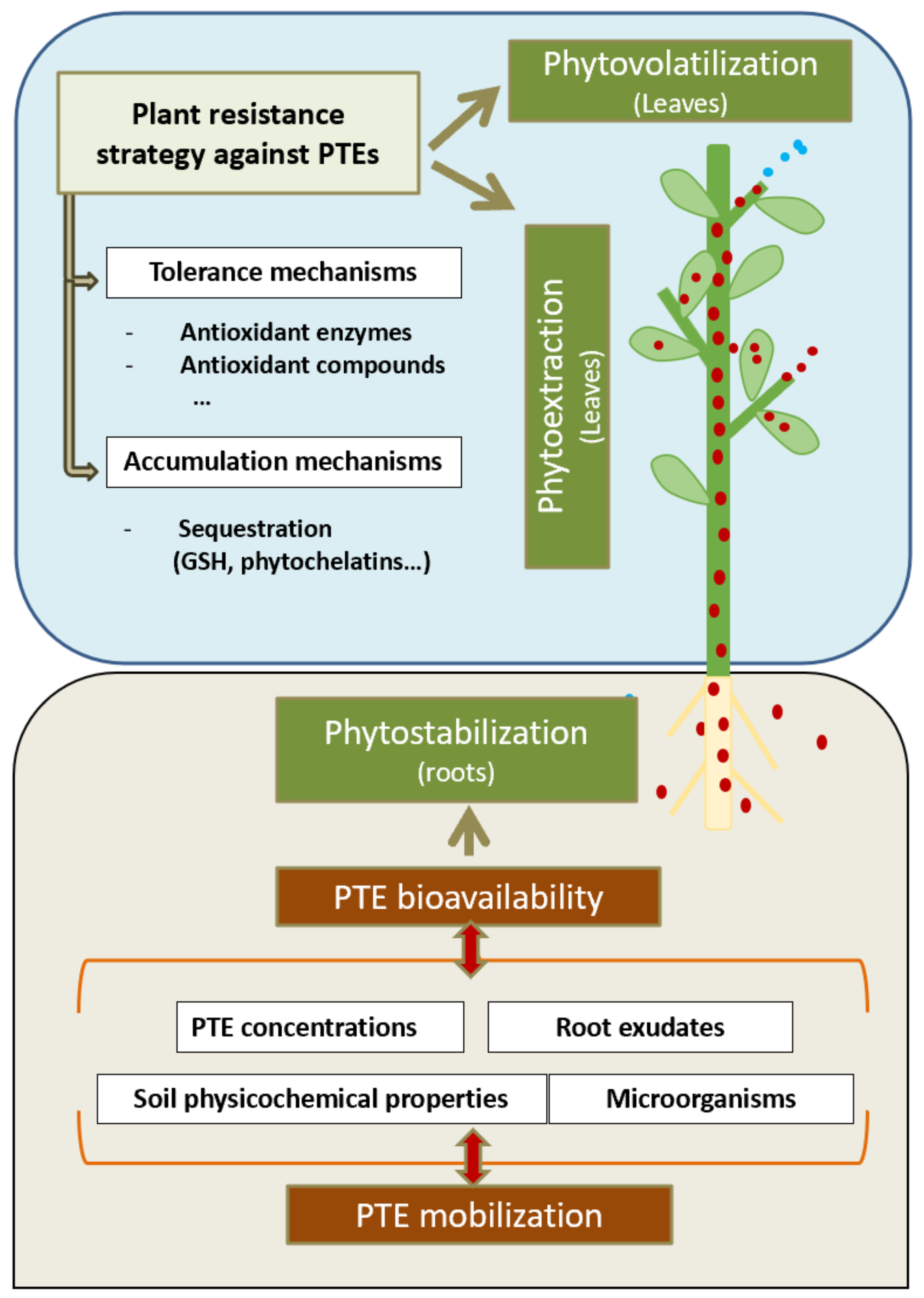
3.1. Clean-Up Techniques
3.2. On-Site Stabilization Techniques
4. Selection of Suitable Plants for AML Restoration
4.1. Wild Plant Species
4.1.1. Metal Uptake and Accumulation Mechanisms
4.1.2. Tolerance Mechanisms
4.2. Genetically Modified Plants
5. Real Examples of Using Plants for AML Restoration
| Species | Maximum Data of PTEs Concentration in Leaves (mg kg−1 DW) | References | |
|---|---|---|---|
| Alyssum sp. | Ni | 20,100 | [190] |
| Arabidopsis halleri | Cd | 5722 | [191] |
| Zn | 9491 | ||
| Arabis gemmifera | Cd | 1810 | [192] |
| Zn | 20,300 | ||
| Atriplex halimus | Cd | 850 * | [193] |
| Berkheya coddii | Ni | 31,300 | [194] |
| Bidens pilosa | Cd | 144.1 | [195] |
| Biscutella laevigata | Tl | 66.7 | [196] |
| Brassica pekinensis | Pb | 2670 | [197] |
| Carpobrotus rossi | Cd | 172 | [198] |
| Lantana cámara | Cd | 423.06 | [46] |
| Leersia hexandra | Cr | 5005 | [199] |
| Lonicera japonica | Cd | 57.22 | [200] |
| Phytolacca acinosa | Mn | 19,300 | [201] |
| Polygonum lapathifolium | Mn | 18,841.7 | [202] |
| Pteris vittata | As | 1530 | [203] |
| Schima superba | Mn | 9975.61 | [204,205] |
| Sedum alfredii | Cd | 9000 | [206] |
| Zn | 4807 | [207] | |
| Sesbania drummondi | Pb | 5000 * | [208] |
| Solanum sp. | Cd | 142.7 | [209] |
| Thlaspi sp. | Cd | 164 | [210] |
| Ni | 16,600 | [190] | |
| Zn | 19,070 | [210] | |
| Species | PTEs | References |
|---|---|---|
| Pteris vittata | As | [211] |
| Arundo donax | [56] | |
| Transgenic Arabidopsis thaliana (fungal gene WaarsM) | [212] | |
| Dittrichia viscosa | [213] | |
| Oryza sativa | [214] | |
| Zea mays | Hg | [57] |
| Transgenic Nicotiana tabacum (bacterial gene merA and merB) | [215] | |
| Haliminone portulacoides | [216] | |
| Arabidopsis thaliana | [217] | |
| Brassica juncea | Se | [218] |
| Oryza sativa Brassica oleracea Daucus carota Hordeum vulgare Medicago sativa Lycopersicon esculentum Cucumis sativus Gossypium hirsutum Solanum melongena Zea mays | [219] | |
| Populus tremula x alba | [220] | |
| Stanleya pinnata | [221] | |
| Agrostis tenuis Hordeum vulgare | [222] |
| Species | PTEs | References |
|---|---|---|
| Acanthus ilicifolius | Cd | [223] |
| Agrostis capillaris | Pb | [224] |
| Arundo donax | Ni, Pb and Hg | [225] |
| Atriplex halimus | Pb and Cd | [49,94] |
| Biscutella auriculata | Pb, Cu and Cd | [19,69,70] |
| Brassica juncea | Cu and Zn | [63] |
| Brassica juncea Dactylis glomerata | Cd, Pb and Zn | [226] |
| Casdaminopsis arenosa | Zn, Cd and Pb | [227] |
| Conocarpus erectus Populus deltoides | As | [228] |
| Dahlia pinnata | As | [229] |
| Imperata condensata | Cu | [230] |
| Iris lactea | Cd | [126] |
| Jatropha curcas | Al, Zn and Cu | [231] |
| Lolium perenne | Cu | [232] |
| Lupinus albus | Cd, As and Pb | [156,233] |
| Miscanthus sacchariflorus | Cd | [234] |
| Miscanthus sinensis x giganteus | Zn, Cd and Pb | [235] |
| Pistacia lentiscus | Pb | [236] |
| Pteridium aquilinum | Cd, Cu, Pb and Zn | [236] |
| Quercus ilex | Cd | [162] |
| Ricinus communis | Pb | [237] |
| Salix purpurea | As | [171] |
| Typha latifolia | Co, Cd, Ni, Mn, Cr and As | [238] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elmayel, I.; Esbrí, J.M.; García-Ordiales, E.; Elouaer, Z.; Garcia-Noguero, E.M.; Bouzid, J.; Campos, J.A.; Higueras, P.L. Biogeochemical assessment of the impact of Zn mining activity in the area of the Jebal Trozza mine, Central Tunisia. Environ. Geochem. Health 2020, 42, 3529–3542. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Das, A. Impact of Mine Waste Leachates on Aquatic Environment: A Review. Curr. Pollut. Rep. 2017, 3, 31–37. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Nirola, R.; Kuppusamy, S.; Thavamani, P.; Naidu, R.; Megharaj, M. Abandoned metalliferous mines: Ecological impacts and potential approaches for reclamation. Rev. Environ. Sci. Biotechnol. 2016, 15, 327–354. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Sengupta, M. Environmental Impacts of Mining: Monitoring, Restoration, and Control; Routledge: London, UK, 2018; ISBN 9781351450539. [Google Scholar]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 27 May 2021).
- Xie, L.; van Zyl, D. Distinguishing reclamation, revegetation and phytoremediation, and the importance of geochemical processes in the reclamation of sulfidic mine tailings: A review. Chemosphere 2020, 252, 126446. [Google Scholar] [CrossRef] [PubMed]
- Dada, E.; Njoku, K.; Osuntoki, A.; Akinola, M. A review of current techniques of in situ Physico-chemical and biological remediation of heavy metals polluted soil. Ethiop. J. Environ. Stud. Manag. 2015, 8, 606. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Rodríguez, L.; Gómez, R.; Sánchez, V.; Villaseñor, J.; Alonso-Azcárate, J. Performance of waste-based amendments to reduce metal release from mine tailings: One-year leaching behaviour. J. Environ. Manag. 2018, 209, 1–8. [Google Scholar] [CrossRef]
- Rodríguez, L.; Gómez, R.; Sánchez, V.; Alonso-Azcárate, J. Chemical and plant tests to assess the viability of amendments to reduce metal availability in mine soils and tailings. Environ. Sci. Pollut. Res. 2016, 23, 6046–6054. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodríguez, L.; Alonso-Azcárate, J.; Rincón, J. Phytoextraction of metal polluted soils around a Pb-Zn mine by crop plants. Int. J. Phytoremediat. 2009, 11, 360–384. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost-benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef]
- Dickinson, N. Phytoremediation. Encycl. Appl. Plant Sci. 2016, 3, 327–331. [Google Scholar]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- Peco, J.D.; Campos, J.A.; Romero-Puertas, M.C.; Olmedilla, A.; Higueras, P.; Sandalio, L.M. Characterization of mechanisms involved in tolerance and accumulation of Cd in Biscutella auriculata L. Ecotoxicol. Environ. Saf. 2020, 201, 110784. [Google Scholar] [CrossRef]
- Sanz-Fernández, M.; Rodríguez-Serrano, M.; Sevilla-Perea, A.; Pena, L.; Mingorance, M.D.; Sandalio, L.M.; Romero-Puertas, M.C. Screening Arabidopsis mutants in genes useful for phytoremediation. J. Hazard. Mater. 2017, 335, 143–151. [Google Scholar] [CrossRef]
- Macek, T.; Kotrba, P.; Svatos, A.; Novakova, M.; Demnerova, K.; Mackova, M. Novel roles for genetically modified plants in environmental protection. Trends Biotechnol. 2008, 26, 146–152. [Google Scholar] [CrossRef]
- Ferreira, R.A.; Pereira, M.F.; Magalhães, J.P.; Maurício, A.M.; Caçador, I.; Martins-Dias, S. Assessing local acid mine drainage impacts on natural regeneration-revegetation of São Domingos mine (Portugal) using a mineralogical, biochemical and textural approach. Sci. Total Environ. 2021, 755, 142825. [Google Scholar] [CrossRef]
- Moreno González, R.; Cánovas, C.R.; Olías, M.; Macías, F. Seasonal variability of extremely metal rich acid mine drainages from the Tharsis mines (SW Spain). Environ. Pollut. 2020, 259, 113829. [Google Scholar] [CrossRef]
- Pavoni, E.; Covelli, S.; Adami, G.; Baracchini, E.; Cattelan, R.; Crosera, M.; Higueras, P.; Lenaz, D.; Petranich, E. Mobility and fate of Thallium and other potentially harmful elements in drainage waters from a decommissioned Zn-Pb mine (North-Eastern Italian Alps). J. Geochem. Explor. 2018, 188, 1–10. [Google Scholar] [CrossRef]
- Sánchez-Donoso, R.; Martín-Duque, J.F.; Crespo, E.; Higueras, P.L. Tailing’s geomorphology of the San Quintín mining site (Spain): Landform catalogue, aeolian erosion and environmental implications. Environ. Earth Sci. 2019, 78, 166. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- United States Department of Agriculture of Soil Health. NRCS Soils. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/main/soils/health/ (accessed on 2 March 2021).
- Gallego, S.; Esbrí, J.M.; Campos, J.A.; Peco, J.D.; Martin-Laurent, F.; Higueras, P. Microbial diversity and activity assessment in a 100-year-old lead mine. J. Hazard. Mater. 2020, 420, 124618. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of heavy metals: Mechanisms, methods and enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Ghori, Z.; Iftikhar, H.; Bhatti, M.F.; Nasar-um-Minullah; Sharma, I.; Kazi, A.G.; Ahmad, P. Phytoextraction; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780128031582. [Google Scholar]
- Grobelak, A. Organic soil amendments in the phytoremediation process. In Phytoremediation: Management of Environmental Contaminants; Springer International Publishing: Cham, Switzerland, 2016; Volume 4, pp. 21–39. ISBN 9783319418117. [Google Scholar]
- Jadia, C.D.; Fulekar, M.H. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8, 921–928. [Google Scholar]
- Zhao, X.; Sun, Y.; Huang, J.; Wang, H.; Tang, D. Effects of soil heavy metal pollution on microbial activities and community diversity in different land use types in mining areas. Environ. Sci. Pollut. Res. 2020, 27, 20215–20226. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Soil Reclamation of Abandoned Mine Land by Revegetation: A Review. Int. J. Soil Sediments Water 2010, 3, 13. [Google Scholar]
- Buta, M.; Blaga, G.; Paulette, L.; Păcurar, I.; Roșca, S.; Borsai, O.; Grecu, F.; Sînziana, P.E.; Negrușier, C. Soil Reclamation of Abandoned Mine Lands by Revegetation in Northwestern Part of Transylvania: A 40-Year Retrospective Study. Sustainability 2019, 11, 3393. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev. Environ. Sci. Biotechnol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Black, H. Absorbing possibilities: Phytoremediation. Environ. Health Perspect. 1995, 103, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Chalot, M.; Blaudez, D.; Rogaume, Y.; Provent, A.S.; Pascual, C. Fate of trace elements during the combustion of phytoremediation wood. Environ. Sci. Technol. 2012, 46, 13361–13369. [Google Scholar] [CrossRef]
- Corzo Remigio, A.; Chaney, R.L.; Baker, A.J.M.; Edraki, M.; Erskine, P.D.; Echevarria, G.; van der Ent, A. Phytoextraction of high value elements and contaminants from mining and mineral wastes: Opportunities and limitations. Plant Soil. 2020, 449, 11–37. [Google Scholar] [CrossRef]
- Novo, L.A.B.; Castro, P.M.L.; Alvarenga, P.; da Silva, E.F. Phytomining of rare and valuable metals. In Phytoremediation: Management of Environmental Contaminants; Springer International Publishing: Cham, Switzerland, 2017; Volume 5, pp. 469–486. ISBN 9783319523811. [Google Scholar]
- Li, Y.M.; Chaney, R.L.; Brewer, E.P.; Angle, J.S.; Nelkin, J. Phytoextraction of nickel and cobalt by hyperaccumulator Alyssum species grown on nickel-contaminated soils. Environ. Sci. Technol. 2003, 37, 1463–1468. [Google Scholar] [CrossRef]
- Nkrumah, P.N.; Echevarria, G.; Erskine, P.D.; Chaney, R.L.; Sumail, S.; van der Ent, A. Growth effects in tropical nickel-agromining ‘metal crops” in response to nutrient dosing. J. Plant Nutr. Soil Sci. 2019, 182, 715–728. [Google Scholar] [CrossRef]
- Barbaroux, R.; Plasari, E.; Mercier, G.; Simonnot, M.O.; Morel, J.L.; Blais, J.F. A new process for nickel ammonium disulfate production from ash of the hyperaccumulating plant Alyssum murale. Sci. Total Environ. 2012, 423, 111–119. [Google Scholar] [CrossRef]
- Gisbertz, K.; Hilgendorf, S.; Friedrich, B.; Heinrichs, S.; Rüßmann, D.; Pretz, T. Maximising metal recovery from incineration ashes. In Proceedings of the European Metallurgical Conference, EMC 2013, Weimar, Germany, 23–26 June 2013; pp. 1127–1132. [Google Scholar]
- Liu, S.; Ali, S.; Yang, R.; Tao, J.; Ren, B. A newly discovered Cd-hyperaccumulator Lantana camara L. J. Hazard. Mater. 2019, 371, 233–242. [Google Scholar] [CrossRef]
- Grčman, H.; Velikonja-Bolta, Š.; Vodnik, D.; Kos, B.; Leštan, D. EDTA enhanced heavy metal phytoextraction: Metal accumulation, leaching and toxicity. Plant Soil 2001, 235, 105–114. [Google Scholar] [CrossRef]
- Moslehi, A.; Feizian, M.; Higueras, P.; Eisvand, H.R. Assessment of EDDS and vermicompost for the phytoextraction of Cd and Pb by sunflower (Helianthus annuus L.). Int. J. Phytoremediat. 2019, 21, 191–199. [Google Scholar] [CrossRef]
- Acosta, J.A.; Abbaspour, A.; Martínez, G.R.; Martínez-Martínez, S.; Zornoza, R.; Gabarrón, M.; Faz, A. Phytoremediation of mine tailings with Atriplex halimus and organic/inorganic amendments: A five-year field case study. Chemosphere 2018, 204, 71–78. [Google Scholar] [CrossRef]
- Nörtemann, B. Biodegradation of Chelating Agents: EDTA, DTPA, PDTA, NTA, and EDDS. Biogeochem. Chelating Agents 2005, 150–170. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, F.J.; Lombi, E.; McGrath, S.P. Leaching of heavy metals from contaminated soils using EDTA. Environ. Pollut. 2001, 113, 111–120. [Google Scholar] [CrossRef]
- Meers, E.; Ruttens, A.; Hopgood, M.J.; Samson, D.; Tack, F.M.G. Comparison of EDTA and EDDS as potential soil amendments for enhanced phytoextraction of heavy metals. Chemosphere 2005, 58, 1011–1022. [Google Scholar] [CrossRef]
- Meers, E.; Hopgood, M.; Lesage, E.; Vervaeke, P.; Tack, F.M.G.; Verloo, M.G. Enhanced phytoextraction: In search of EDTA alternatives. Int. J. Phytoremediat. 2004, 6, 95–109. [Google Scholar] [CrossRef]
- Duquène, L.; Vandenhove, H.; Tack, F.; Meers, E.; Baeten, J.; Wannijn, J. Enhanced phytoextraction of uranium and selected heavy metals by Indian mustard and ryegrass using biodegradable soil amendments. Sci. Total Environ. 2009, 407, 1496–1505. [Google Scholar] [CrossRef]
- Sakakibara, M.; Watanabe, A.; Sano, S.; Inoue, M.; Kaise, T. Phytoextraction and phytovolatili-zation of arsenic from as-contaminated soils by Pteris vittata. In Proceedings of the Association for Environmental Health and Sciences—22nd Annual International Conference on Contaminated Soils, Sediments and Water 2006, Amherst, MA, USA, 16–19 October 2006; Volume 12, pp. 258–263. [Google Scholar]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic phytovolatilization and epigenetic modifications in Arundo donax L. assisted by a PGPR consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef]
- Mello, I.S.; Targanski, S.; Pietro-Souza, W.; Frutuoso Stachack, F.F.; Terezo, A.J.; Soares, M.A. Endophytic bacteria stimulate mercury phytoremediation by modulating its bioaccumulation and volatilization. Ecotoxicol. Environ. Saf. 2020, 202, 110818. [Google Scholar] [CrossRef]
- Raskin, I.; Ensley, B.D. Phytoremediation of Toxic Metals-Using Plants to Clean Up the Environment; Wiley Inter-Science Publication: New York, NY, USA, 2001. [Google Scholar]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of Heavy Metals: Understanding of Principles and Practices. In Plant-Metal Interactions; Springer International Publishing: Cham, Switzerland, 2019; pp. 263–282. [Google Scholar]
- Marques, A.P.G.C.; Rangel, A.O.S.S.; Castro, P.M.L. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-Up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.; Koo, N.; Hyun, S.; Hwang, A. Evaluation of the effectiveness of various amendments on trace metals stabilization by chemical and biological methods. J. Hazard. Mater. 2011, 188, 44–51. [Google Scholar] [CrossRef]
- Ruttens, A.; Mench, M.; Colpaert, J.V.; Boisson, J.; Carleer, R.; Vangronsveld, J. Phytostabilization of a metal contaminated sandy soil. I: Influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environ. Pollut. 2006, 144, 524–532. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Moliner, A.; Masaguer, A.; Ruiz-Fernández, J. Phytostabilization of metals in mine soils using Brassica juncea in combination with organic amendments. Plant Soil 2014, 377, 97–109. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J.; Lombi, E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil 2001, 232, 207–214. [Google Scholar] [CrossRef]
- Sheng, X.F.; Xia, J.J.; Jiang, C.Y.; He, L.Y.; Qian, M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef]
- Mastretta, C.; Taghavi, S.; Van Der Lelie, D.; Mengoni, A.; Galardi, F.; Gonnelli, C.; Barac, T.; Boulet, J.; Weyens, N.; Vangronsveld, J. Endophytic bacteria from seeds of Nicotiana tabacum can reduce cadmium phytotoxicity. Int. J. Phytoremediat. 2009, 11, 251–267. [Google Scholar] [CrossRef]
- Quiñones, M.A.; Ruiz-Díez, B.; Fajardo, S.; López-Berdonces, M.A.; Higueras, P.L.; Fernández-Pascual, M. Lupinus albus plants acquire mercury tolerance when inoculated with an Hg-resistant Bradyrhizobium strain. Plant Physiol. Biochem. 2013, 73, 168–175. [Google Scholar] [CrossRef]
- Rock, S.; Pivetz, B.; Madalinski, K.; Adams, N.; Wilson, T. Introduction to Phytoremediation; U.S. Environmental Protection Agency: Washington, DC, USA, 2019.
- Peco, J.D.; Higueras, P.; Campos, J.A.; Olmedilla, A.; Romero-Puertas, M.C.; Sandalio, L.M. Deciphering lead tolerance mechanisms in a population of the plant species Biscutella auriculata L. from a mining area: Accumulation strategies and antioxidant defenses. Chemosphere 2020, 261, 127721. [Google Scholar] [CrossRef]
- Peco, J.D.; Sandalio, L.M.; Higueras, P.; Olmedilla, A.; Campos, J.A. Characterization of the biochemical basis for copper homeostasis and tolerance in Biscutella auriculata L. Physiol. Plant. 2020. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef]
- Pošćić, F.; Marchiol, L.; Schat, H. Hyperaccumulation of thallium is population-specific and uncorrelated with caesium accumulation in the thallium hyperaccumulator, Biscutella laevigata. Plant Soil 2013, 365, 81–91. [Google Scholar] [CrossRef]
- McIntyre, T. Phytoremediation of heavy metals from soils. Adv. Biochem. Eng. Biotechnol. 2003, 78, 97–123. [Google Scholar] [PubMed]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The genetic basis of metal hyperaccumulation in plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Ke, W.; Xiong, Z.T.; Chen, S.; Chen, J. Effects of copper and mineral nutrition on growth, copper accumulation and mineral element uptake in two Rumex japonicus populations from a copper mine and an uncontaminated field sites. Environ. Exp. Bot. 2007, 59, 59–67. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC press: Boca Raton, FL, USA, 2001; ISBN 0849315751. [Google Scholar]
- Wang, X.P.; Shan, X.Q.; Zhang, S.Z.; Wen, B. A model for evaluation of the phytoavailability of trace elements to vegetables under the field conditions. Chemosphere 2004, 55, 811–822. [Google Scholar] [CrossRef]
- Kim, K.-R.; Owens, G.; Naidu, R. Heavy metal distribution, bioaccessibility, and phytoavailability in long-term contaminated soils from Lake Macquarie, Australia. Soil Res. 2009, 47, 166. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Romić, D.; Filipović, V.; Ondrašek, G. Organic matter and salinity modify cadmium soil (phyto) availability. Ecotoxicol. Environ. Saf. 2018, 147, 824–831. [Google Scholar] [CrossRef]
- Stafford, A.; Jeyakumar, P.; Hedley, M.; Anderson, C. Influence of Soil Moisture Status on Soil Cadmium Phytoavailability and Accumulation in Plantain (Plantago lanceolata). Soil Syst. 2018, 2, 9. [Google Scholar] [CrossRef]
- Ren, J.H.; Ma, L.Q.; Sun, H.J.; Cai, F.; Luo, J. Antimony uptake, translocation and speciation in rice plants exposed to antimonite and antimonate. Sci. Total Environ. 2014, 475, 83–89. [Google Scholar] [CrossRef]
- Kim, R.Y.; Yoon, J.K.; Kim, T.S.; Yang, J.E.; Owens, G.; Kim, K.R. Bioavailability of heavy metals in soils: Definitions and practical implementation—a critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef]
- Dong, J.; Wu, F.; Zhang, G. Influence of cadmium on antioxidant capacity and four microelement concentrations in tomato seedlings (Lycopersicon esculentum). Chemosphere 2006, 64, 1659–1666. [Google Scholar] [CrossRef]
- Yang, C.M.; Juang, K.W. Alleviation effects of calcium and potassium on cadmium rhizotoxicity and absorption by soybean and wheat roots. J. Plant Nutr. Soil Sci. 2015, 178, 748–754. [Google Scholar] [CrossRef]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M.; Sheikh Abdullah, S.R.; Idris, M.; Tangahu, B.V.; Basri, H. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Römheld, V.; Awad, F. Significance of root exudates in acquisition of heavy metals from a contaminated calcareous soil by graminaceous species. J. Plant Nutr. 2000, 23, 1857–1866. [Google Scholar] [CrossRef]
- Kim, S.; Lim, H.; Lee, I. Enhanced heavy metal phytoextraction by Echinochloa crus-galli using root exudates. J. Biosci. Bioeng. 2010, 109, 47–50. [Google Scholar] [CrossRef]
- Montiel-Rozas, M.M.; Madejón, E.; Madejón, P. Effect of heavy metals and organic matter on root exudates (low molecular weight organic acids) of herbaceous species: An assessment in sand and soil conditions under different levels of contamination. Environ. Pollut. 2016, 216, 273–281. [Google Scholar] [CrossRef]
- Martin, M.H.; Marschner, H. The Mineral Nutrition of Higher Plants. J. Ecol. 1988, 76, 1250. [Google Scholar] [CrossRef]
- Lombi, E.; Tearall, K.L.; Howarth, J.R.; Zhao, F.J.; Hawkesford, M.J.; McGrath, S.P. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2002, 128, 1359–1367. [Google Scholar] [CrossRef]
- Chen, S.; Han, X.; Fang, J.; Lu, Z.; Qiu, W.; Liu, M.; Sang, J.; Jiang, J.; Zhuo, R. Sedum alfredii SaNramp6 Metal Transporter Contributes to Cadmium Accumulation in Transgenic Arabidopsis thaliana. Sci. Rep. 2017, 7, 13318. [Google Scholar] [CrossRef]
- Feng, S.; Tan, J.; Zhang, Y.; Liang, S.; Xiang, S.; Wang, H.; Chai, T. Isolation and characterization of a novel cadmium-regulated Yellow Stripe-Like transporter (SnYSL3) in Solanum nigrum. Plant Cell Rep. 2017, 36, 281–296. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Puig, S. Function and Regulation of the Plant COPT Family of High-Affinity Copper Transport Proteins. Adv. Bot. 2014, 2014, 476917. [Google Scholar] [CrossRef]
- Andrés-Colás, N.; Sancenón, V.; Rodríguez-Navarro, S.; Mayo, S.; Thiele, D.J.; Ecker, J.R.; Puig, S.; Peñarrubia, L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006, 45, 225–236. [Google Scholar] [CrossRef]
- Dai, J.; Wang, N.; Xiong, H.; Qiu, W.; Nakanishi, H.; Kobayashi, T.; Nishizawa, N.K.; Zuo, Y. The yellow stripe-like (YSL) gene functions in internal copper transport in peanut. Genes 2018, 9, 635. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.; Zhang, X.; Yu, H.; Huang, F.; Zhang, L.; Zhang, M.; Li, T. Changes of non-protein thiols in root and organic acids in xylem sap involved in cadmium translocation of cadmium-safe rice line (Oryza sativa L.). Plant Soil 2019, 439, 475–486. [Google Scholar] [CrossRef]
- Kerkeb, L.; Krämer, U. The role of free histidine in xylem loading of nickel in Alyssum lesbiacum and Brassica juncea. Plant Physiol. 2003, 131, 716–724. [Google Scholar] [CrossRef]
- Centofanti, T.; Sayers, Z.; Cabello-Conejo, M.I.; Kidd, P.; Nishizawa, N.K.; Kakei, Y.; Davis, A.P.; Sicher, R.C.; Chaney, R.L. Xylem exudate composition and root-to-shoot nickel translocation in Alyssum species. Plant Soil 2013, 373, 59–75. [Google Scholar] [CrossRef]
- Saathoff, A.J.; Ahner, B.; Spanswick, R.M.; Walker, L.P. Detection of Phytochelatin in the Xylem Sap of Brassica napus. Environ. Eng. Sci. 2011, 28, 103–111. [Google Scholar] [CrossRef]
- Wei, Z.G.; Wong, J.W.C.; Zhao, H.Y.; Zhang, H.J.; Li, H.X.; Hu, F. Separation and determination of heavy metals associated with low molecular weight chelators in xylem saps of Indian mustard (Brassica juncea) by size exclusion chromatography and atomic absorption spectrometry. Biol. Trace Elem. Res. 2007, 118, 146–158. [Google Scholar] [CrossRef]
- Yadav, S.K.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Dago, À.; González, I.; Ariño, C.; Martínez-Coronado, A.; Higueras, P.; Díaz-Cruz, J.M.; Esteban, M. Evaluation of mercury stress in plants from the Almadén mining district by analysis of phytochelatins and their Hg complexes. Environ. Sci. Technol. 2014, 48, 6256–6263. [Google Scholar] [CrossRef] [PubMed]
- Dago, A.; Ariño, C.; Díaz-Cruz, J.M.; Esteban, M. Analysis of phytochelatins and Hg-phytochelatin complexes in Hordeum vulgare plants stressed with Hg and Cd: HPLC study with amperometric detection. Int. J. Environ. Anal. Chem. 2014, 94, 668–678. [Google Scholar] [CrossRef]
- Heiss, S.; Wachter, A.; Bogs, J.; Cobbett, C.; Rausch, T. Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J. Exp. Bot. 2003, 54, 1833–1839. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Liang, L.; Chen, C.; Wei, S.; Zhou, Q. Phytochelatin and oxidative stress under heavy metal stress tolerance in plants. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Springer International Publishing: Cham, Switzerland, 2015; pp. 191–217. ISBN 9783319204215. [Google Scholar]
- Grill, E.; Zenk, M.H.; Winnacker, E.-L. Induction of heavy metal-sequestering phytochelatin by cadmium in cell cultures of Rauvolfia serpentina. Naturwissenschaften 1985, 72, 432–433. [Google Scholar] [CrossRef]
- Klapheck, S.; Chrost, B.; Starke, J.; Zimmermann, H. γ-Glutamylcysteinylserine—A New Homologue of Glutathione in Plants of the Family Poaceae. Bot. Acta 1992, 105, 174–179. [Google Scholar] [CrossRef]
- Meuwly, P.; Thibault, P.; Rauser, W.E. γ-Glutamylcysteinylglutamic acid-a new homologue of glutathione in maize seedlings exposed to cadmium. FEBS Lett. 1993, 336, 472–476. [Google Scholar] [CrossRef]
- Kubota, H.; Sato, K.; Yamada, T.; Maitani, T. Phytochelatin homologs induced in hairy roots of horseradish. Phytochemistry 2000, 53, 239–245. [Google Scholar] [CrossRef]
- Sylwia, W.; Anna, R.; Ewa, B.; Stephan, C.; Danuta Maria, A. The role of subcellular distribution of cadmium and phytochelatins in the generation of distinct phenotypes of AtPCS1- and CePCS3-expressing tobacco. J. Plant Physiol. 2010, 167, 981–988. [Google Scholar] [CrossRef]
- Yang, X.E.; Li, T.Q.; Long, X.X.; Xiong, Y.H.; He, Z.L.; Stoffella, P.J. Dynamics of zinc uptake and accumulation in the hyperaccumulating and non-hyperaccumulating ecotypes of Sedum alfredii Hance. Plant Soil 2006, 284, 109–119. [Google Scholar] [CrossRef]
- Melo, E.E.C.; Costa, E.T.S.; Guilherme, L.R.G.; Faquin, V.; Nascimento, C.W.A. Accumulation of arsenic and nutrients by castor bean plants grown on an As-enriched nutrient solution. J. Hazard. Mater. 2009, 168, 479–483. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef]
- Fuentes, A. Phytotoxicity and heavy metals speciation of stabilised sewage sludges. J. Hazard. Mater. 2004, 108, 161–169. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Dalurzo, H.C.; Gómez, M.; Romero-Puertas, M.C.; del Río, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Kalaivanan, D.; Ganeshamurthy, A.N. Mechanisms of Heavy Metal Toxicity in Plants. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 85–102. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M.; Wani, K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J. Plant Nutr. 2018, 41, 1744–1763. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Rodríguez-Serrano, M.; Gupta, D.K.; Archilla, A.; Romero-Puertas, M.C.; Del Río, L.A. Reactive Oxygen species and Nitric Oxide in plants under cadmium stress: From toxicity to signaling. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 199–215. ISBN 9781461408154. [Google Scholar]
- Molina-Moya, E.; Terrón-Camero, L.C.; Pescador-Azofra, L.; Sandalio, L.M.; Romero-Puertas, M.C. Reactive Oxygen Species and Nitric Oxide Production, Regulation and Function During Defense Response. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; Wiley: Hoboken, NJ, USA, 2019; pp. 573–590. [Google Scholar]
- Romero-Puertas, M.C.; Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Olmedilla, A.; Sandalio, L.M. Reactive oxygen and nitrogen species as key indicators of plant responses to Cd stress. Environ. Exp. Bot. 2019, 161, 107–119. [Google Scholar] [CrossRef]
- Gupta, A.S.; Webb, R.P.; Holaday, A.S.; Allen, R.D. Overexpression of superoxide dismutase protects plants from oxidative stress. Induction of ascorbate peroxidase in superoxide dismutase-overexpressing plants. Plant Physiol. 1993, 103, 1067–1073. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, L.; Zhang, Y.-N.N.; Mao, P.-C.C.; Tian, X.-X.X.; Li, S.-S.S.; Zhang, L. Antioxidative systems, metal ion homeostasis and cadmium distribution in Iris lactea exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2017, 139, 50–55. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, L.; Dai, T.; Zhou, J.; Kang, Q.; Chen, H.; Li, K.; Li, Z. Effects of copper on the growth, antioxidant enzymes and photosynthesis of spinach seedlings. Ecotoxicol. Environ. Saf. 2019, 171, 771–780. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Singh, S.; Sounderajan, S.; Kumar, K.; Fulzele, D.P. Investigation of arsenic accumulation and biochemical response of in vitro developed Vetiveria zizanoides plants. Ecotoxicol. Environ. Saf. 2017, 145, 50–56. [Google Scholar] [CrossRef]
- Ortega, A.; Garrido, I.; Casimiro, I.; Espinosa, F. Effects of antimony on redox activities and antioxidant defence systems in sunflower (Helianthus annuus L.) plants. PLoS ONE 2017, 12, e0183991. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron-lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, A.K.; Behera, S.K.; Tiwari, P.K. Zinc Application Enhances Superoxide Dismutase and Carbonic Anhydrase Activities in Zinc-Efficient and Zinc-Inefficient Wheat Genotypes. J. Soil Sci. Plant Nutr. 2019, 19, 477–487. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Wu, Q.H.; Lv, H.Q.; Chen, W.X.; Wang, L.Z.; Shi, S.J.; Yang, J.G.; Zhao, P.P.; Li, Y.P.; Christopher, R.; et al. Toxicity of different forms of antimony to rice plants: Effects on reactive oxidative species production, antioxidative systems, and uptake of essential elements. Environ. Pollut. 2020, 263, 114544. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Irfan, Q.M.; Rehman, R.U. Characterization of mercury-induced stress biomarkers in Fagopyrum tataricum plants. Int. J. Phytoremediat. 2018, 20, 225–236. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Sandalio, L.M.; Distefano, S.; Palma, J.M.; Lupiáñez, J.A.; Del Río, L.A. A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem. J. 1998, 330 Pt 2, 777–784. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Aparicio-Chacón, M.V.; Palma, J.M.; Corpas, F.J. Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ. Exp. Bot. 2019, 161, 143–156. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Glutathione S-transferases: Role in combating abiotic stresses including arsenic detoxification in plants. Front. Plant Sci. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Namdjoyan, S.; Kermanian, H.; Abolhasani Soorki, A.; Modarres Tabatabaei, S.; Elyasi, N. Interactive effects of Salicylic acid and nitric oxide in alleviating zinc toxicity of Safflower (Carthamus tinctorius L.). Ecotoxicology 2017, 26, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Abd_Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Selenium modulates dynamics of antioxidative defence expression, photosynthetic attributes and secondary metabolites to mitigate chromium toxicity in Brassica juncea L. plants. Environ. Exp. Bot. 2019, 161, 180–192. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant. Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Terrón-Camero, L.C.; Peláez-Vico, M.Á.; Del-Val, C.; Sandalio, L.M.; Romero-Puertas, M.C.; Brouquisse, R. Role of nitric oxide in plant responses to heavy metal stress: Exogenous application versus endogenous production. J. Exp. Bot. 2019, 70, 4477–4488. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, N. Transgenic plants for phytoremediation. Int. J. Phytoremediat. 2011, 13, 264–279. [Google Scholar] [CrossRef]
- Cherian, S.; Oliveira, M.M. Transgenic plants in phytoremediation: Recent advances and new possibilities. Environ. Sci. Technol. 2005, 39, 9377–9390. [Google Scholar] [CrossRef]
- Gunarathne, V.; Mayakaduwa, S.; Ashiq, A.; Weerakoon, S.R.; Biswas, J.K.; Vithanage, M. Transgenic Plants: Benefits, Applications, and Potential Risks in Phytoremediation. Benefits, Applications, and Potential Risks in Phytoremediation. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–102. ISBN 9780128143902. [Google Scholar]
- Liu, D.; An, Z.; Mao, Z.; Ma, L.; Lu, Z. Enhanced Heavy Metal Tolerance and Accumulation by Transgenic Sugar Beets Expressing Streptococcus thermophilus StGCS-GS in the Presence of Cd, Zn and Cu Alone or in Combination. PLoS ONE 2015, 10, e0128824. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, T.; Cheng, J.S.; Yi, Y.J.; Han, J.J.; Cheng, H.L.; Li, Q.; Tang, N.; Liang, M.X. Heterologous expression of the metallothionein PpMT2 gene from Physcomitrella patens confers enhanced tolerance to heavy metal stress on transgenic Arabidopsis plants. Plant Growth Regul. 2020, 90, 63–72. [Google Scholar] [CrossRef]
- Evans, D.M.; Zipper, C.E.; Burger, J.A.; Strahm, B.D.; Villamagna, A.M. Reforestation practice for enhancement of ecosystem services on a compacted surface mine: Path toward ecosystem recovery. Ecol. Eng. 2013, 51, 16–23. [Google Scholar] [CrossRef]
- Madejón, P.; Domínguez, M.T.; Madejón, E.; Cabrera, F.; Marañón, T.; Murillo, J.M. Soil-plant relationships and contamination by trace elements: A review of twenty years of experimentation and monitoring after the Aznalcóllar (SW Spain) mine accident. Sci. Total Environ. 2018, 625, 50–63. [Google Scholar] [CrossRef]
- Del Río, M.; Font, R.; Almela, C.; Vélez, D.; Montoro, R.; De Haro Bailón, A. Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcóllar mine. In Proceedings of the Journal of Biotechnology. J. Biotechnol. 2002, 98, 125–137. [Google Scholar] [CrossRef]
- Madejón, P.; Murillo, J.M.; Marañón, T.; Cabrera, F. Bioaccumulation of trace elements in a wild grass three years after the Aznalcóllar mine spill (South Spain). Environ. Monit. Assess. 2006, 114, 169–189. [Google Scholar] [CrossRef]
- Madejón, P.; Murillo, J.M.; Marañón, T.; Lepp, N.W. Factors affecting accumulation of thallium and other trace elements in two wild Brassicaceae spontaneously growing on soils contaminated by tailings dam waste. Chemosphere 2007, 67, 20–28. [Google Scholar] [CrossRef]
- Murillo, J.M.; Marañón, T.; Cabrera, F.; López, R. Accumulation of heavy metals in sunflower and sorghum plants affected by the Guadiamar spill. Sci. Total Environ. 1999, 242, 281–292. [Google Scholar] [CrossRef]
- Madejón, P.; Murillo, J.M.; Marañón, T.; Cabrera, F.; Soriano, M.A. Trace element and nutrient accumulation in sunflower plants two years after the Aznalcóllar mine spill. Sci. Total Environ. 2003, 307, 239–257. [Google Scholar] [CrossRef]
- Clemente, R.; Walker, D.J.; Bernal, M.P. Uptake of heavy metals and As by Brassica juncea grown in a contaminated soil in Aznalcóllar (Spain): The effect of soil amendments. Environ. Pollut. 2005, 138, 46–58. [Google Scholar] [CrossRef]
- Soriano, M.A.; Fereres, E. Use of crops for in situ phytoremediation of polluted soils following a toxic flood from a mine spill. Plant Soil 2003, 256, 253–264. [Google Scholar] [CrossRef]
- Vázquez, S.; Agha, R.; Granado, A.; Sarro, M.J.; Esteban, E.; Peñalosa, J.M.; Carpena, R.O. Use of white lupin plant for phytostabilization of Cd and as polluted acid soil. Water Air Soil Pollut. 2006, 177, 349–365. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Vázquez, S.; Carpena-Ruiz, R.O.; Esteban, E.; Peñalosa, J.M. Using Mediterranean shrubs for the phytoremediation of a soil impacted by pyritic wastes in Southern Spain: A field experiment. J. Environ. Manag. 2011, 92, 1584–1590. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Marañón, T.; Murillo, J.M.; Schulin, R.; Robinson, B.H. Trace element accumulation in woody plants of the Guadiamar Valley, SW Spain: A large-scale phytomanagement case study. Environ. Pollut. 2008, 152, 50–59. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Madejón, P.; Marañón, T.; Murillo, J.M. Avorestation of a trace-element polluted area in SW Spain: Woody plant performance and trace element accumulation. Eur. J. For. Res. 2010, 129, 47–59. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Pérez-Ramos, I.M.; Murillo, J.M.; Marañón, T. Facilitating the afforestation of Mediterranean polluted soils by nurse shrubs. J. Environ. Manag. 2015, 161, 276–286. [Google Scholar] [CrossRef]
- De la Fuente, C.; Pardo, T.; Alburquerque, J.A.; Martínez-Alcalá, I.; Bernal, M.P.; Clemente, R. Assessment of native shrubs for stabilisation of a trace elements-polluted soil as the final phase of a restoration process. Agric. Ecosyst. Environ. 2014, 196, 103–111. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Madrid, F.; Marañón, T.; Murillo, J.M. Cadmium availability in soil and retention in oak roots: Potential for phytostabilization. Chemosphere 2009, 76, 480–486. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Peñalosa, J.M.; Carpena-Ruiz, R.O.; Esteban, E. Comparison of arsenic resistance in Mediterranean woody shrubs used in restoration activities. Chemosphere 2008, 71, 466–473. [Google Scholar] [CrossRef]
- Martín-Crespo, T.; Gómez-Ortiz, D.; Martín-Velázquez, S.; Esbrí, J.M.; de Ignacio-San José, C.; Sánchez-García, M.J.; Montoya-Montes, I.; Martín-González, F. Abandoned mine tailings in cultural itineraries: Don Quixote Route (Spain). Eng. Geol. 2015, 197, 82–93. [Google Scholar] [CrossRef]
- Asensio, V.; Vega, F.A.; Singh, B.R.; Covelo, E.F. Effects of tree vegetation and waste amendments on the fractionation of Cr, Cu, Ni, Pb and Zn in polluted mine soils. Sci. Total Environ. 2013, 443, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Pardo, T.; Bernal, M.P.; Clemente, R. Phytostabilisation of severely contaminated mine tailings using halophytes and field addition of organic and inorganic amendments. Chemosphere 2017, 178, 556–564. [Google Scholar] [CrossRef]
- Hernández, A.; Jébrak, M.; Higueras, P.; Oyarzun, R.; Morata, D.; Munhá, J. The Almaden mercury mining district, Spain. Miner. Depos. 1999, 34, 539–548. [Google Scholar] [CrossRef]
- Rodriguez, L.; Lopez-Bellido, F.J.; Carnicer, A.; Recreo, F.; Tallos, A.; Monteagudo, J.M. Mercury recovery from soils by phytoremediation. In Environmental Chemistry: Green Chemistry and Pollutants in Ecosystems; Springer: Berlin/Heidelberg, Germany, 2005; pp. 197–204. ISBN 3540228608. [Google Scholar]
- Naharro, R.; Esbrí, J.M.; Amorós, J.Á.; García-Navarro, F.J.; Higueras, P. Assessment of mercury uptake routes at the soil-plant-atmosphere interface. Geochem. Explor. Environ. Anal. 2018, 19, 146–154. [Google Scholar] [CrossRef]
- Pratas, J.; Prasad, M.N.V.; Freitas, H.; Conde, L. Plants growing in abandoned mines of Portugal are useful for biogeochemical exploration of arsenic, antimony, tungsten and mine reclamation. J. Geochem. Explor. 2005, 85, 99–107. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, P.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytoremediation of a former mine soil using biochar and iron sulphate: Effects on As soil immobilization and accumulation in three Salicaceae species. Sci. Total Environ. 2020, 710, 136203. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, G.; Cao, A.; Cappai, G.; Carucci, A.; Casti, M.; Fercia, M.L.; Lonis, R.; Mola, F. A field experiment on the use of Pistacia lentiscus L. and Scrophularia canina L. subsp. bicolor (Sibth. et Sm.) Greuter for the phytoremediation of abandoned mining areas. Plant Biosyst. 2012, 146, 1054–1063. [Google Scholar] [CrossRef]
- Swab, R.M.; Lorenz, N.; Byrd, S.; Dick, R. Native vegetation in reclamation: Improving habitat and ecosystem function through using prairie species in mine land reclamation. Ecol. Eng. 2017, 108, 525–536. [Google Scholar] [CrossRef]
- Gil-Loaiza, J.; White, S.A.; Root, R.A.; Solís-Dominguez, F.A.; Hammond, C.M.; Chorover, J.; Maier, R.M. Phytostabilization of mine tailings using compost-assisted direct planting: Translating greenhouse results to the field. Sci. Total Environ. 2016, 565, 451–461. [Google Scholar] [CrossRef]
- Zhao, Z.; Shahrour, I.; Bai, Z.; Fan, W.; Feng, L.; Li, H. Soils development in opencast coal mine spoils reclaimed for 1–13 years in the West-Northern Loess Plateau of China. Eur. J. Soil Biol. 2013, 55, 40–46. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, F.; Lan, M.; Liu, W.; Yang, W.; Tang, Y.; Qiu, R. Phytostabilization with tolerant plants and soil amendments of the tailings of the Dabaoshan polymetallic mine in Guangdong Province. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2019, 39, 545–552. [Google Scholar]
- Tai, Y.P.; Yang, Y.F.; Li, Z.A.; Yang, Y.; Wang, J.X.; Zhuang, P.; Zou, B. Phytoextraction of 55-year-old wastewater-irrigated soil in a Zn-Pb mine district: Effect of plant species and chelators. Environ. Technol. 2018, 39, 2138–2150. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, Y.; Yang, X.; Cui, Z. Optimization of combined phytoremediation for heavy metal contaminated mine tailings by a field-scale orthogonal experiment. Ecotoxicol. Environ. Saf. 2019, 168, 1–8. [Google Scholar] [CrossRef]
- Yu, G.; Jiang, P.; Fu, X.; Liu, J.; Sunahara, G.I.; Chen, Z.; Xiao, H.; Lin, F.; Wang, X. Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn.: A long-term field study. Environ. Pollut. 2020, 266, 115408. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, T.; Yao, S.; Liu, C.; Yin, Y.; Li, H.; Li, N. A real filed phytoremediation of multi-metals contaminated soils by selected hybrid sweet sorghum with high biomass and high accumulation ability. Chemosphere 2019, 237, 124536. [Google Scholar] [CrossRef]
- Shen, S.; Chen, J.; Chang, J.; Xia, B. Using bioenergy crop cassava (Manihot esculenta) for reclamation of heavily metal-contaminated land. Int. J. Phytoremediat. 2020, 22, 1313–1320. [Google Scholar] [CrossRef]
- Shu, W.S.; Xia, H.P.; Zhang, Z.Q.; Lan, C.Y.; Wong, M.H. Use of vetiver and three other grasses for revegetation of Pb/Zn mine tailings: Field experiment. Int. J. Phytoremediat. 2002, 4, 47–57. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, X.; Huot, H.; Liu, W.; Liu, C.; Guo, M.; Li, Y.; Fei, Y.; Chao, Y.; Wang, S.; et al. Reclamation with organic amendments and plants remodels the diversity and structure of bacterial community in ion-adsorption rare earth element mine tailings. J. Soils Sediments 2020, 20, 3669–3680. [Google Scholar] [CrossRef]
- Mi, J.; Hou, H.; Raval, S.; Yang, Y.; Zhang, S.; Hua, Y.; Wang, C.; Chen, F. Effect of crop cultivation on the soil carbon stock in mine dumps of the Loess Plateau, China. Sci. Total Environ. 2020, 741, 139809. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Mi, J.; Liu, R.; Hou, H.; Zhang, S. Effects of vegetation pattern and spontaneous succession on remediation of potential toxic metal-polluted soil in mine dumps. Sustainability 2019, 11, 397. [Google Scholar] [CrossRef]
- Singh, A.K. Toxic aqueous discharge of iron and sulphur from spoiled coal mined lands and its control by phytostabilization process. Curr. Sci. 2018, 115, 529–534. [Google Scholar] [CrossRef]
- Kamble, S.S.; Dod, R.D. Management of coal mine overburden and fly ash using bamboo cultivation. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 4223–4226. [Google Scholar]
- Jeżowski, S.; Mos, M.; Buckby, S.; Cerazy-Waliszewska, J.; Owczarzak, W.; Mocek, A.; Kaczmarek, Z.; McCalmont, J.P. Establishment, growth, and yield potential of the perennial grass Miscanthus × Giganteus on degraded coal mine soils. Front. Plant Sci. 2017, 8, 726. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Rana, V.; Maiti, S.K. Chronological Variation of Metals in Reclaimed Coal Mine Soil and Tissues of Eucalyptus Hybrid Tree After 25 Years of Reclamation, Jharia Coal Field (India). Bull. Environ. Contam. Toxicol. 2018, 101, 604–610. [Google Scholar] [CrossRef]
- Pavlova, D.; Echevarria, G.; Mullaj, A.; Reeves, R.D.; Morel, J.-L.; Sulçe, S. Nickel hyperaccumulation by the species of Alyssum and Thlaspi (Brassicaceae) from the ultramafic soils of the Balkans. Bot. Serbica 2010, 34, 3–10. [Google Scholar]
- Küpper, H.; Lombi, E.; Zhao, F.J.; McGrath, S.P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 2000, 212, 75–84. [Google Scholar] [CrossRef]
- Kubota, H.; Takenaka, C. Arabis gemmifera is a Hyperaccumulator of Cd and Zn. Int. J. Phytoremediat. 2003, 5, 197–201. [Google Scholar] [CrossRef]
- Mesnoua, M.; Mateos-Naranjo, E.; Barcia-Piedras, J.M.; Pérez-Romero, J.A.; Lotmani, B.; Redondo-Gómez, S. Physiological and biochemical mechanisms preventing Cd-toxicity in the hyperaccumulator Atriplex halimus L. Plant Physiol. Biochem. 2016, 106, 30–38. [Google Scholar] [CrossRef]
- Ugustyniak, M.; Arnawska, M.; Eimold, W. Uptake of cadmium, lead, nickel and zinc from soil and water solutions by the nickel hyperaccumulator. Acta Biol. Cracoviensia 2004, 46, 75–85. [Google Scholar]
- Sun, Y.; Zhou, Q.; Wang, L.; Liu, W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J. Hazard. Mater. 2009, 161, 808–814. [Google Scholar] [CrossRef]
- Wierzbicka, M.; Pielichowska, M.; Abratowska, A.; WiŁkomirski, B.; Wysocka, I.; Panufnik-Mędrzycka, D.; Bulska, E.; Panufnik-Mȩdrzycka, D.; Bulska, E. Thallium Hyperaccumulation in Polish Populations of Biscutella laevigata (Brassicaceae). Acta Biol. Cracoviensia Ser. Bot. 2016, 58, 7–19. [Google Scholar] [CrossRef]
- Xiong, Z.T. Lead uptake and effects on seed germination and plant growth in a Pb hyperaccumulator Brassica pekinensis Rupr. Bull. Environ. Contam. Toxicol. 1998, 60, 285–291. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, C.; Hu, P.; Luo, Y.; Wu, L.; Sale, P.; Tang, C. Influence of nitrogen form on the phytoextraction of cadmium by a newly discovered hyperaccumulator Carpobrotus rossii. Environ. Sci. Pollut. Res. 2016, 23, 1246–1253. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, J.; Huang, H.T.; Chen, J.; Zhu, Y.N.; Wang, D.Q. Chromium accumulation by the hyperaccumulator plant Leersia hexandra Swartz. Chemosphere 2007, 67, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, X.; Chen, W.; Yuan, F.; Yan, K.; Tao, D. Accumulation and tolerance characteristics of cadmium in a potential hyperaccumulator—Lonicera japonica Thunb. J. Hazard. Mater. 2009, 169, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.G.; Chen, Y.X.; Reeves, R.D.; Baker, A.J.; Lin, Q.; Fernando, D.R. Manganese uptake and accumulation by the hyperaccumulator plant Phytolacca acinosa Roxb. (Phytolaccaceae). Environ. Pollut. 2004, 131, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yu, F.; Chen, M.; Zhou, Z.; Chen, C.; Li, M.S.; Zhu, J. A newly found manganese hyperaccumulator—Polygonum lapathifolium Linn. Int. J. Phytoremediat. 2016, 18, 348–353. [Google Scholar] [CrossRef]
- Chen, T.; Wei, C.; Huang, Z.; Huang, Q.; Lu, Q.; Fan, Z. Arsenic hyperaccumulator Pteris vittata L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar] [CrossRef]
- Li, M.S.; Yang, S.X. Heavy metal contamination in soils and phytoaccumulation in a manganese mine Wasteland, South China. Air Soil Water Res. 2008, 1, 31–41. [Google Scholar] [CrossRef]
- Yang, S.X.; Deng, H.; Li, M.S. Manganese uptake and accumulation in a woody hyperaccumulator, Schima superba. Plant Soil Environ. 2008, 54, 441–446. [Google Scholar] [CrossRef]
- Yang, X.E.; Long, X.X.; Ye, H.B.; He, Z.L.; Calvert, D.V.; Stoffella, P.J. Cadmium tolerance and hyperaccumulation in a new Zn-hyperaccumulating plant species (Sedum alfredii Hance). Plant Soil 2004, 259, 181–189. [Google Scholar] [CrossRef]
- Yang, X.; Long, X.; Ni, W.; Fu, C. Sedum alfredii H: A new Zn hyperaccumulating plant first found in China. Chin. Sci. Bull. 2002, 47, 1634–1637. [Google Scholar] [CrossRef]
- Sahi, S.V.; Bryant, N.L.; Sharma, N.C.; Singh, S.R. Characterization of a lead hyperaccumulator shrub, Sesbania drummondii. Environ. Sci. Technol. 2002, 36, 4676–4680. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, P.; Mao, L.; Shi, W.J.; Zhi, Y.E. Phytoextraction of cadmium and physiological changes in Solanum nigrum as a novel cadmium hyperaccumulator. Russ. J. Plant Physiol. 2010, 57, 501–508. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Reeves, R.D.; Hajar, A.S.M. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol. 1994, 127, 61–68. [Google Scholar]
- Sakakibara, M.; Watanabe, A.; Inoue, M.; Sano, S.; Kaise, T. Phytoextraction and phytovolatilization of arsenic from As-contaminated soils by Pteris vittata. In Proceedings of the Annual International Conference on Soils, Sediments, Water and Energy, Amherst, MA, USA, 16–19 October 2010; Volume 12. [Google Scholar]
- Verma, S.; Verma, P.K.; Pande, V.; Tripathi, R.D.; Chakrabarty, D. Transgenic Arabidopsis thaliana expressing fungal arsenic methyltransferase gene (WaarsM) showed enhanced arsenic tolerance via volatilization. Environ. Exp. Bot. 2016, 132, 113–120. [Google Scholar] [CrossRef]
- Guarino, F.; Conte, B.; Improta, G.; Sciarrillo, R.; Castiglione, S.; Cicatelli, A.; Guarino, C. Genetic characterization, micropropagation, and potential use for arsenic phytoremediation of Dittrichia viscosa (L.) Greuter. Ecotoxicol. Environ. Saf. 2018, 148, 675–683. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, H.; Sun, G.X.; Zhao, F.J.; Zhu, Y.G. Pathways and relative contributions to arsenic volatilization from rice plants and paddy soil. Environ. Sci. Technol. 2012, 46, 8090–8096. [Google Scholar] [CrossRef]
- Hussein, H.S.; Ruiz, O.N.; Terry, N.; Daniell, H. Phytoremediation of mercury and organomercurials in chloroplast transgenic plants: Enhanced root uptake, translocation to shoots, and volatilization. Environ. Sci. Technol. 2007, 41, 8439–8446. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Duarte, B.; Cesário, R.; Mendes, R.; Hintelmann, H.; Eckey, K.; Dimock, B.; Caçador, I.; Canário, J. Mercury mobility and effects in the salt-marsh plant Halimione portulacoides: Uptake, transport, and toxicity and tolerance mechanisms. Sci. Total Environ. 2019, 650, 111–120. [Google Scholar] [CrossRef]
- Battke, F.; Ernst, D.; Fleischmann, F.; Halbach, S. Phytoreduction and volatilization of mercury by ascorbate in Arabidopsis thaliana, European beech and Norway spruce. Appl. Geochemistry 2008, 23, 494–502. [Google Scholar] [CrossRef]
- De Souza, M.P.; Chu, D.; Zhao, M.; Zayed, A.M.; Ruzin, S.E.; Schichnes, D.; Terry, N. Rhizosphere bacteria enhance selenium accumulation and volatilization by indian mustard. Plant Physiol. 1999, 119, 565–573. [Google Scholar] [CrossRef]
- Terry, N.; Carlson, C.; Raab, T.K.; Zayed, A.M. Rates of Selenium Volatilization among Crop Species. J. Environ. Qual. 1992, 21, 341–344. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; De Souza, M.P.; Lytle, C.M.; Shang, C.; Lugo, T.; Terry, N. Selenium volatilization and assimilation by hybrid poplar (Populus tremula x alba). J. Exp. Bot. 1998, 49, 1889–1892. [Google Scholar] [CrossRef]
- Parker, D.R.; Feist, L.J.; Varvel, T.W.; Thomason, D.N.; Zhang, Y. Selenium phytoremediation potential of Stanleya pinnata. Plant Soil 2003, 249, 157–165. [Google Scholar] [CrossRef]
- Zieve, R.; Peterson, P.J. Volatilization of selenium from plants and soils. Sci. Total Environ. 1984, 32, 197–202. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Enhanced phytostabilization of cadmium by a halophyte—Acanthus ilicifolius L. Int. J. Phytoremediat. 2017, 19, 319–326. [Google Scholar] [CrossRef]
- Nandillon, R.; Lebrun, M.; Miard, F.; Gaillard, M.; Sabatier, S.; Morabito, D.; Bourgerie, S. Contrasted tolerance of Agrostis capillaris metallicolous and non-metallicolous ecotypes in the context of a mining technosol amended by biochar, compost and iron sulfate. Environ. Geochem. Health 2019, 43, 1457–1475. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Oliveri Conti, G.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef]
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661. [Google Scholar] [CrossRef]
- Nadgórska-Socha, A.; Ptasiński, B.; Kita, A. Heavy metal bioaccumulation and antioxidative responses in Cardaminopsis arenosa and Plantago lanceolata leaves from metalliferous and non-metalliferous sites: A field study. Ecotoxicology 2013, 22, 1422–1434. [Google Scholar] [CrossRef]
- Hussain, S.; Akram, M.; Abbas, G.; Murtaza, B.; Shahid, M.; Shah, N.S.; Bibi, I.; Niazi, N.K. Arsenic tolerance and phytoremediation potential of Conocarpus erectus L. and Populus deltoides L. Int. J. Phytoremediat. 2017, 19, 985–991. [Google Scholar] [CrossRef]
- Raza, A.; Khan, A.H.A.; Nawaz, I.; Qu, Z.; Yousaf, S.; Ali, M.A.; Sayal, A.U.; Iqbal, M. Evaluation of Arsenic-Induced Stress in Dahlia pinnata Cav.: Morphological and Physiological Response. Soil Sediment Contam. 2019, 28, 716–728. [Google Scholar] [CrossRef]
- Meier, S.; Alvear, M.; Borie, F.; Aguilera, P.; Ginocchio, R.; Cornejo, P. Influence of copper on root exudate patterns in some metallophytes and agricultural plants. Ecotoxicol. Environ. Saf. 2012, 75, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization potential of Jatropha curcas L. in polymetallic acid mine tailings. Int. J. Phytoremediat. 2011, 13, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, C.; Verdugo, C.; Ginocchio, R. Phytostabilization of copper mine tailings with biosolids: Implications for metal uptake and productivity of Lolium perenne. Sci. Total Environ. 2008, 395, 1–10. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Zornoza, P.; Hernández, L.E.; Esteban, E.; Carpena, R.O. Response of Lupinus albus to Pb-EDTA indicates relatively high tolerance. Toxicol. Environ. Chem. 2017, 99, 1378–1388. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Huang, Y.; Zhou, S. The Tolerance and Accumulation of Miscanthus Sacchariflorus (maxim.) Benth., an Energy Plant Species, to Cadmium. Int. J. Phytoremediat. 2015, 17, 538–545. [Google Scholar] [CrossRef]
- Pavel, P.B.; Puschenreiter, M.; Wenzel, W.W.; Diacu, E.; Barbu, C.H. Aided phytostabilization using Miscanthus sinensis×giganteus on heavy metal-contaminated soils. Sci. Total Environ. 2014, 479–480, 125–131. [Google Scholar] [CrossRef]
- Lee, S.H.; Ji, W.H.; Lee, W.S.; Koo, N.; Koh, I.H.; Kim, M.S.; Park, J.S. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef]
- Kiran, B.R.; Prasad, M.N.V. Assisted phytostabilization of Pb-spiked soils amended with charcoal and banana compost and vegetated with Ricinus communis L. (Castor bean). Environ. Geochem. Health 2021, 43, 1507–1521. [Google Scholar] [CrossRef]
- Varun, M.; Souza, R.D.; Pratas, J.; Paul, M.S.; De Ciências, D.; Coimbra, U. De Evaluation of phytostabilization, a green technology to remove heavy metals from industrial sludge using Typha latifolia L. Biotechnol. Bioinf. Bioeng. 2011, 1, 137–145. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peco, J.D.; Higueras, P.; Campos, J.A.; Esbrí, J.M.; Moreno, M.M.; Battaglia-Brunet, F.; Sandalio, L.M. Abandoned Mine Lands Reclamation by Plant Remediation Technologies. Sustainability 2021, 13, 6555. https://doi.org/10.3390/su13126555
Peco JD, Higueras P, Campos JA, Esbrí JM, Moreno MM, Battaglia-Brunet F, Sandalio LM. Abandoned Mine Lands Reclamation by Plant Remediation Technologies. Sustainability. 2021; 13(12):6555. https://doi.org/10.3390/su13126555
Chicago/Turabian StylePeco, Jesús D., Pablo Higueras, Juan A. Campos, José M. Esbrí, Marta M. Moreno, Fabienne Battaglia-Brunet, and Luisa M. Sandalio. 2021. "Abandoned Mine Lands Reclamation by Plant Remediation Technologies" Sustainability 13, no. 12: 6555. https://doi.org/10.3390/su13126555
APA StylePeco, J. D., Higueras, P., Campos, J. A., Esbrí, J. M., Moreno, M. M., Battaglia-Brunet, F., & Sandalio, L. M. (2021). Abandoned Mine Lands Reclamation by Plant Remediation Technologies. Sustainability, 13(12), 6555. https://doi.org/10.3390/su13126555







