Potentialities of the Asian Watergrass (Hygroryza aristata) as Feed in Aquaculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Plantation of Asian Watergrass
2.3. Analysis of Water Quality Parameters
2.4. Analysis of Soil Quality Parameters
2.5. Determination of the Growth Performance of Asian Watergrass
2.6. Analysis of Proximate Composition
2.7. Data Analysis
3. Results
3.1. Water Quality Parameters
3.2. Soil Quality Parameters
3.3. Growth and Production of the Grass
3.4. Proximate Composition of the Watergrass
4. Discussion
4.1. Ecology and Growth Performance of the Asian Watergrass
4.2. Nutrient Compositions of the Asian Watergrass
4.3. The Asian Watergrass Used as Direct Fish Feed
4.4. Use of Asian Watergrass in the Formulation of Aqua Feeds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hossain, M.M.; Ali, M.L.; Khan, S.; Haque, M.M.; Shahjahan, M. Use of Asian water grass as feed of grass carp. Aquacult. Rep. 2020, 18, 100434. [Google Scholar] [CrossRef]
- Sofia, F. State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2018. [Google Scholar]
- Uddin, M.N.; Rahman, M.S.; Shahjahan, M. Effects of duckweed (Lemna minor) as supplementary feed on monoculture of GIFT strain of tilapia (Oreochromis niloticus). Progress. Agric. 2007, 18, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, M.Z.H.; Shahjahan, M.; Rahman, M.S. Suitability of duckweed (Lemna minor) as feed for fish in polyculture system. Int. J. Agric. Res. Innov. Technol. 2012, 2, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.R.; Rina, C. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture: A Review; FAO Fisheries and Aquaculture Technical Paper. No. 531; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2009. [Google Scholar]
- Wersal, R.M.; Madsen, J.D. Aquatic Plants Their Uses and Risks. A Review of the Global Status of Aquatic Plants; FAO: Rome, Italy, 2012. [Google Scholar]
- Mondal, K.; Payra, P. A review on use of plant protein sources in diets for fish feed formulation. J. Int. Acad. Res. Multidiscipl. 2015, 3, 257–264. [Google Scholar]
- Gangadhar, B.; Sridhar, N.; Saurabh, S.; Raghavendra, C.H.; Hemaprasanth, K.P.; Raghunath, M.R.; Jayasankar, P. Growth response of Cirrhinus mrigala fry to Azolla (Azolla pinnata) incorporated diets. Fish Technol. 2014, 51, 156–161. [Google Scholar] [CrossRef]
- Dorothy, M.S.; Raman, S.; Nautiyal, V.; Singh, K.; Yogananda, T.; Kamei, M. Use of potential plant leaves as ingredient in fish feed—A review. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 112–125. [Google Scholar] [CrossRef]
- Hossain, M.M.; Rahman, M.H.; Ali, M.L.; Khan, S.; Haque, M.M.; Shahjahan, M. Development of a low-cost polyculture system utilizing Hygroryza aristata floating grass in the coastal wetlands of Bangladesh. Aquaculture 2020, 527, 1–9. [Google Scholar] [CrossRef]
- Sipauba-Tavares, L.H.; Favero, E.G.P.; Braga, F.D.S. Utilization of macrophyte biofilter in effluent from aquaculture: I. Floating plant. Braz. J. Microbiol. 2002, 62, 713–723. [Google Scholar] [CrossRef] [Green Version]
- Henry-Silva, G.G.; Camargo, A.F.M. Efficiency of aquatic macrophytes to treat Nile tilapia pond effluents. Sci. Agric. 2006, 63, 433–438. [Google Scholar] [CrossRef] [Green Version]
- Ferdoushi, Z.; Haque, F.; Khan, S.; Haque, M.M. The effects of two aquatic floating macrophytes (Lemna and Azolla) as biofilters of nitrogen and phosphate in fish ponds. Turkish J. Fish Aquat. Sci. 2008, 8, 253–258. [Google Scholar]
- Carlozzi, P.; Padovani, G. The aquatic fern Azolla as a natural plant-factory for ammonia removal from fish-breeding fresh waste water. Environ. Sci. Pollut. Res. 2016, 23, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; Jeppesen, E. Regime shifts in shallow lakes. Ecosystems 2007, 10, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.E.A. Encyclopedia of Biological Invasions; Simberloff, D., Rejmanek, M., Eds.; University of California Press: Los Angeles, CA, USA, 2011; pp. 11–15. [Google Scholar]
- Meerhoff, M.; Mazzeo, N.; Moss, B.; Rodríguez-Gallego, L. The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat. Ecol. 2003, 37, 377–391. [Google Scholar] [CrossRef]
- Madsen, J.D.; Chambers, P.A.; James, W.F. The interaction between water movement, sediment dynamics and submersed macrophytes. Hydrobiologia 2011, 444, 71–84. [Google Scholar] [CrossRef]
- Gupta, G.C. Use of water hyacinth in wastewater treatment. J. Environ. Health. 1982, 43, 80–82. [Google Scholar]
- Muñoz, E.M.; Voyevoda, M.; Fühner, C.; Zehnsdorf, A. Potential uses of Elodea nuttallii-harvested biomass. Energy. Sustain. Soc. 2011, 1, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Pörschmann, J.; Weiner, B.; Wedwitschka, H.; Zehnsdorf, A.; Köhler, R.; Kopinke, F.D. Characterization of biochars and dissolved organic matter phases obtained upon hydrothermal carbonization of Elodea nuttallii. Bioresour. Technol. 2015, 189, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zehnsdorf, A.; Korn, U.; Pröter, J.; Naumann, D.; Seirig, M.; Rönicke, H.; Pieper, B. Western waterweed (Elodea nuttallii) as a co-substrate for biogas plants. Agric. Eng. 2011, 66, 136–139. [Google Scholar]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W. Expanding the utilization of sustainable plant products in aqua feeds: A review. Aquacult. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Tandon, H.L.S. (Ed.) Methods of Analysis of Soils, Plants, Water and Fertilizer; Fertilizer Development of Consultation Organization: New Delhi, India, 1995. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice- Hall of India Private Limited: New Delhi, India, 1973; pp. 10–144. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular 939; US Department of Agriculture: Washington, DC, USA, 1954; p. 19. [Google Scholar]
- AOAC. Official Methods of Analysis, 7th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Edwards, P. Food Potential of Aquatic Macrophytes; ICLARM Studies and Reviews No. 5; International Center for Living Aquatic Resources: Manila, Phillipines, 1980; p. 51. [Google Scholar]
- Coche, A.G. Freshwater Aquaculture Development in China; Report of the FAO/UNDP study tour organized for French-speaking African countries, 22 April–20 May 1980; FAO Fisheries Technical Paper No. 215; FAO: Rome, Italy, 1983. [Google Scholar]
- Reddy, K.R.; DeBusk, W.F. Growth characteristics of aquatic macrophytes cultured in nutrient-enriched water: I. Water hyacinth, water lettuce, and pennywort. Econ. Bot. 1984, 38, 229–239. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Carone, M.; Colonna, T.; Fragale, C. Production of biodiesel from macro-algae by supercritical CO2 extraction and thermo-chemical liquefaction. Environmen. Chem. Lett. 2005, 3, 136–139. [Google Scholar] [CrossRef]
- Whiteman, J.B.; Room, R.M. Temperature lethal to Salvinia molesta Mitcheel. Aquat. Bot. 1991, 40, 27–35. [Google Scholar] [CrossRef]
- Cary, P.R.; Weerts, P.G.J. Growth of Salvinia molesta as affected by water temperature and nutrition I. Effects of nitrogen level and nitrogen compounds. Aquat. Bot. 1983, 16, 163–172. [Google Scholar] [CrossRef]
- Mcintosh, D.; King, C.; Fitzsimmons, K. Tilapia for biological control of Giant Salvinia. J. Aquat. Plant. Manag. 2003, 41, 28–31. [Google Scholar]
- Imaoka, T.; Teranishi, S. Rates of nutrient uptake and growth of the water hyacinth (Eichhornia crassipes (Mart. Solms). Water Res. 1988, 22, 943–951. [Google Scholar] [CrossRef]
- Kola, K. Aspects of the ecology of water hyacinth Eichhornia crassipes (Martius) solms in the Lagos lagoon system. In Proceedings of the International Workshop on Water Hyacinth-Menace and Resource; Farri, T.A., Ed.; Nigerian Federal Ministry of Science and Technology: Lagos, Nigerian, 1988; pp. 80–84. [Google Scholar]
- Olivares, E.; Colonnello, G. Salinity gradient in the Manamo River, a dammed distributary of the Orinoco Delta, and its influence on the presence of Eichhornia crassipes and Paspalumrepens. Interciencia 2000, 25, 242–248. [Google Scholar]
- Khondker, M.; Islam, A.K.M.N.; Makhnun, A.D. Lemna perpusilla: Screening on habitat limnology. Bangladesh J. Bot. 1994, 23, 99–106. [Google Scholar]
- Stephenson, M.; Turner, G.; Pope, P.; Colt, J.; Knight, A.; Tchobanoglous, G. The Use and Potential of Aquatic Species for Wastewater Treatment; Appendix A: The environmental requirements of aquatic plants; California State Water Resources Control Board: Sacramento, CA, USA, 1980; p. 655. [Google Scholar]
- Islam, A.K.M.N.; Khondker, M. Preliminary limnological investigations of some polluted waters covered by duckweeds. Bangladesh J. Bot. 1991, 20, 73–75. [Google Scholar]
- Carr, G.M.; Chambers, P.A. Macrophyte growth and sediment phosphorus and nitrogen in a Canadian Prairie River. Freshwater Biol. 1998, 39, 525–536. [Google Scholar] [CrossRef]
- Clarke, S.J.; Wharton, G. Sediment nutrient characteristics and aquatic macrophytes in lowland English rivers. Sci. Total Environ. 2001, 266, 103–112. [Google Scholar] [CrossRef]
- Barko, J.W.; Smart, R.M. Sediment-based nutrition of submersed macrophytes. Aquat. Bot. 1981, 10, 339–352. [Google Scholar] [CrossRef]
- Holmes, N.T.H.; Newbold, C. River plant communities—Reflectors of water and substrate chemistry. Focus Nat. Conserv. 1984, 9, 535–539. [Google Scholar]
- Chambers, P.A.; Prepas, E.E.; Bothwell, M.L.; Hamilton, H.R. Roots versus shoots in nutrient uptake by aquatic macrophytes in flowing waters. Can. J. Fish. Aquat. Sci. 1989, 46, 435–439. [Google Scholar] [CrossRef]
- Pelton, D.K.; Levine, S.N.; Braner, M. Measurements of phosphorus uptake by macrophytes and epiphytes from the La Platte River (VT) using 32P in streams microcosms. Freshwater Biol. 1998, 39, 285–299. [Google Scholar] [CrossRef]
- Agami, M.; Waisel, Y. The ecophysiology of roots of submerged vascular plants. Physiol. Veg. 1986, 24, 607–624. [Google Scholar]
- Wilson, J.R.; Rees, M.; Holst, N.; Thomas, M.B.; Hill, G. Water Hyacinth Population Dynamics. In Biological and Integrated Control of Water Hyacinth, Eichhornia crassipes; Hill, M.H., Centre, T.D., Jianqing, D., Eds.; ACIAR Proceedings 102; ACIAR: Canberra, Australia, 2001; Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.513.2771&rep=rep1&type=pdf (accessed on 26 April 2021).
- Wilson, J.R.; Holst, N.; Rees, M. Determinants and patterns of population growth in water hyacinth. Aquat. Bot. 2005, 81, 51–67. [Google Scholar] [CrossRef]
- Ray, A.K.; Das, I. Apparent digestibility of some aquatic macrophytes in rohu, Labeo rohita (Ham.) fingerlings. J. Aquacult. Trop. 1994, 9, 335–342. [Google Scholar]
- Ward, D.; Newman, R.M. Fish predation on eurasian water milfoil (Myriophyllum spicatum) herbivores and indirect effects on macrophytes. Can. J. Fish. Aquat. Sci. 2006, 63, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, E.A.T.; Amiza, M.A.; Bakhsh, H.K.; Abol-Munafi, A.B. Apparent digestibility coefficients of pelleted feed incorporated with water hyacinth Eichhornia crassipes fed to red tilapia, [Oreochromis mossambicus (Peters, 1852) X Oreochromis niloticus (Linnaeus, 1758)]. Agric. J. 2011, 6, 322–326. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Mamauag, R.E.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Comparative effects of dietary supplementation levels of Eucheuma denticulatum and Sargassum fulvellum in diet of juvenile Japanese flounder Paralichthys olivaceus. Aquac. Sci. 2013, 61, 27–37. [Google Scholar]
- Dhamayanti, R.R.; Nursyam, H.; Hariati, A.M. Utilization of Hydrillaverticillata fermented meal as alternative sources of protein in feed formulation for Tilapia (Oreochromis sp.) growth. Int. J. Sci. Technol. Res. 2016, 5, 34–35. [Google Scholar]
- Beaune, D.; Sellier, Y.; Lambert, É.; Grandjean, F. The use of Chara spp. (Charales: Characeae) as a bioindicator of physico-chemical habitat suitability for an endangered crayfish Austropotamobius pallipes in lentic waters. Aquatic Conserv. Mar. Freshw. Ecosyst 2017, 1–6. [Google Scholar] [CrossRef]
- Rath, R.K. Freshwater Aquaculture; Scientific Publishers: New Delhi, India, 2000; pp. 98–102. [Google Scholar]
- Nielsen, M.M.; Bruhn, A.; Rasmussen, M.B.; Olesen, B.; Larsen, M.M.; Møller, H.B. Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production. J. Appl. Phycol. 2012, 24, 449–458. [Google Scholar] [CrossRef]
- Cole, A.J.; de Nys, R.; Paul, N.A. Biorecovery of nutrient waste as protein in freshwater macroalgae. Algal. Res. 2015, 7, 58–65. [Google Scholar] [CrossRef]
- Angell, A.R.; Angell, S.F.; de Nys, R.; Paul, N.A. Seaweed as a protein source for mono-gastric livestock. Trends Food Sci. Technol. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.K.; Jena, J.K. Use of Nonconventional Dietary Ingredients in Fish Feed Formulations; Ichthyology, Recent Research Advances, D.N. Saksenaedn; Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 1995; p. 225. [Google Scholar]
- Edwards, P.; Hassan, M.S.; Chao, C.H.; Pacharaprakiti, C. Cultivation of duckweeds in septage-loaded earthen ponds. Bioresour. Technol. 1992, 40, 109–117. [Google Scholar] [CrossRef]
- Pipalova, I. A review of grass carp use for aquatic weed control and its impact on water bodies. J. Aquat. Plant. Manag. 2006, 44, 1–12. [Google Scholar]
- Srisuwantach, V. Induced Breeding of Thai Silver Barb (Puntius gonionotus, Bleeker); SAFIS manual No. 10; Eng. Transl.; South-East Fish. Dev. Center: Bangkok, Thailand, 1981. [Google Scholar]
- Bentsen, H.B.; Gjedrem, T.; Hao, N.V. Breeding plan for silver barb (Puntius gonionotus) in Vietnam: Individual (mass) selection to improve growth rate. In International Network on Genetics in Aquaculture (INGA) 3; The WorldFish Center: Penang, Malaysia, 1996; pp. 1–12. [Google Scholar]
- Moriaty, D.J.W. The physiology of digestion of blue-green in cichlid fish, Tilapia nilotica. J. Zool. 1973, 171, 25–39. [Google Scholar] [CrossRef]
- Getachew, T. A study of an herbivorous fish, Oreochromis niloticus L., diet and its quality in two Ethiopi an Rift valley Lakes, Awasa and Zwai. J. Fish Biol. 1987, 30, 439–449. [Google Scholar]
- Diana, J.S.; Lin, C.K.; Schneeberger, P.J. Relationships among nutrient inputs, water nutrient concentrations, primary production, and yield of Oreochromis niloticus in ponds. Aquaculture 1991, 92, 323–341. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agriculture Organization). The State of World Fisheries and Aquaculture; Sustainability in action; FAO: Rome, Italy, 2020. [Google Scholar]
- Abdelghany, A.E. Partial and complete removal of fish meal with gambusia meal in diets for red tilapia, Oreochromis niloticus × O. mossambicus. Aquacult. Nutr. 2003, 9, 145–154. [Google Scholar] [CrossRef]
- Gallagher, M.L. The use of soybean meal as a replacement for fish meal in diets for hybrid striped bass (Murunescrrarilis × M. chrysops). Aquaculture 1994, 126, 114–127. [Google Scholar] [CrossRef]
- Bahnasy, S.A.; Kamel, G.A.; Saaffan, S.E. The nutritive value of aquatic plants and their utilization in fish and animal feed. Arab J. Sci. Rese. Publ. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Rumsey, G.L.; Hughes, S.G.; Winfree, R.A. Chemical and nutritional evaluation of soya protein preparations as primary nitrogen sources for rainbow trout (Oncorhynchus mykiss). Anim. Feed Sci. Technol. 1993, 40, 135–151. [Google Scholar] [CrossRef]
- Hardy, R.W. Alternative protein sources for salmon and trout diets. Anim. Feed Sci. Technol. 1996, 59, 71–80. [Google Scholar] [CrossRef]
- Liti, D.M.; Waidbacher, H.; Straif, M.; Mbaluka, R.K.; Munguti, J.M.; Kyenze, M.M. Effects of partial and complete replacement of freshwater shrimp meal (Caridinea niloticus) with a mixture of plant protein sources on growth performance of Nile tilapia (Oreochromis niloticus L.) in fertilized ponds. Aquac. Res. 2006, 37, 477–483. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Sanz, A.; Gallego, M.G.; Suarez, M.D.; De la Higuera, M. Feeding of the European eel Anguilla anguilla. I. Influence of dietary carbohydrate level. Comp. Biochem. Physiol. 1993, 105, 165–169. [Google Scholar] [CrossRef]
- Iskandar, I.; Andriani, Y.; Rostika, R.; Zidni, I.; Riyanti, N.A. Effect of using fermented Lemna spp. in fish feed on growth rate of Nilem carp (Osteochilus hasselti). World News Nat. Sci. 2019, 26, 157–166. [Google Scholar]
- Mandal, S.; Ghosh, K. Utilization of fermented Pistia leaves in the diet of rohu, Labeo rohita (Hamilton): Effects on growth, digestibility and whole body composition. Waste Biomass Valor 2019, 10, 3331–3342. [Google Scholar] [CrossRef]
- Yousif, R.A.; Abdullah, O.J.; Ahmed, M.; Adam, M.I.; Ahmed, F.A.M.; Omer, A.; Idam, O.A. Effect of replacing fishmeal with water spinach (Ipomoea aquatica) on growth, feed conversion and carcass composition for Nile tilapia fry (Oreochromis niloticus). J. Aquat. Sci. Marine Biol. 2019, 2, 13–20. [Google Scholar]
- Rath, S.S.; Dutta, H. Use of water hyacinth, Eichhornia crassipes as an ingredient in the feed of Clarias batrachus. Proc. Nat. Symp. Freshwat. Aquacult. 1991, 5, 98–99. [Google Scholar]
- Patnaik, S.; Swami, D.N.; Rout, M.; Das, K.M. Water hyacinth leaf meal as a protein source in pelleted feed for Indian major carps. Proc. Nat. Sem. Fresh Wat. Aquacult. 1989, 2, 136–138. [Google Scholar]


| Parameters | CWL | ACC | URP |
|---|---|---|---|
| Water temperature (°C) | 27.9 ± 4.3 (18.0–32.5) | 28.1 ± 4.3 (18.5–32.8) | 27.4 ± 6.1 (15.0–34.1) |
| Salinity (%) | 1.2 ± 0.4 (1.0–2.0) | 1.2 ± 0.4 (1.0–2.0) | 1.0 ± 0.0 (1.0–1.0) |
| Dissolved oxygen (mg L−1) | 5.4 ± 0.2 (5.1–5.8) | 5.4 ± 0.2 (5.0–5.8) | 4.6 ± 0.2 (4.2–4.9) |
| pH | 7.1 ± 0.1 (6.8–7.3) | 7.0 ± 0.2 (6.8–7.4) | 7.9 ± 0.2 (7.6–8.2) |
| Nitrate-nitrogen (mg L−1) | 0.06 ± 0.02 (0.02–0.08) | 0.06 ± 0.03 (0.01–0.10) | 0.05 ± 0.01 (0.02–0.08) |
| Nitrite-nitrogen (mg L−1) | 0.010 ± 0.002 (0.003–0.013) | 0.010 ± 0.002 (0.005–0.014) | 0.010 ± 0.006 (0.003–0.027) |
| Phosphate-phosphorous (mg L−1) | 0.28 ± 0.07 (0.14–0.45) | 0.34 ± 0.11 (0.14–0.63) | 0.19 ± 0.09 (0.09–0.54) |
| Total alkalinity (mg L−1) | 108.0 ± 16.9 (72.0–134.0) | 105.5 ± 16.7 (64.0–130.0) | 172.3 ± 19.9 (142.0–208.0) |
| Area | pH | Electric Conductivity (dS/m) | Exchangeable Na (mg kg−1) | Exchangeable K (mg kg−1) | Exchangeable Ca (mg kg−1) | Exchangeable Mg (mg kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available S (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| CWL | 5.9 ± 0.6 b (5.2–5.9) | 0.4 ± 0.2 (0.3–0.5) | 83.6 ± 11.7 (71.0–94.1) | 81.4 ± 10.7 b (71.8–92.9) | 683.9 ± 24.9 b (71.0–94.1) | 25.2 ± 0.2 (25.0–25.4) | 0.085 ± 0.006 (0.081–0.092) | 300.2 ± 25.1 a (274.0–324.1) | 141.0 ± 14.5 (124.3–149.7) |
| ACC | 6.0 ± 0.3 b (5.6–6.2) | 0.2 ± 0.1 (0.1–0.2) | 73.1 ± 3.7 (70.8–77.3) | 78.2 ± 4.7 b (74.6–83.5) | 684.0 ± 32.2 b (652.0–716.4) | 25.3 ± 0.2 (25.1–25.5) | 0.087 ± 0.009 (0.076–0.095) | 45.1 ± 6.4 b (39.1–51.8) | 117.8 ± 29.1 (85.4–141.8) |
| URP | 7.3 ± 0.2 a (7.0–7.5) | 0.2 ± 0.1 (0.2–0.3) | 87.2 ± 4.6 (81.9–89.9) | 117.7 ± 9.6 a (110.9–128.7) | 823.2 ± 16.0 a (810.1–840.9) | 25.1 ± 0.3 (24.9–25.4) | 0.097 ± 0.002 (0.095–0.098) | 27.6 ± 1.8 b (25.5–28.8) | 136.9 ± 22.9 (121.4–163.2) |
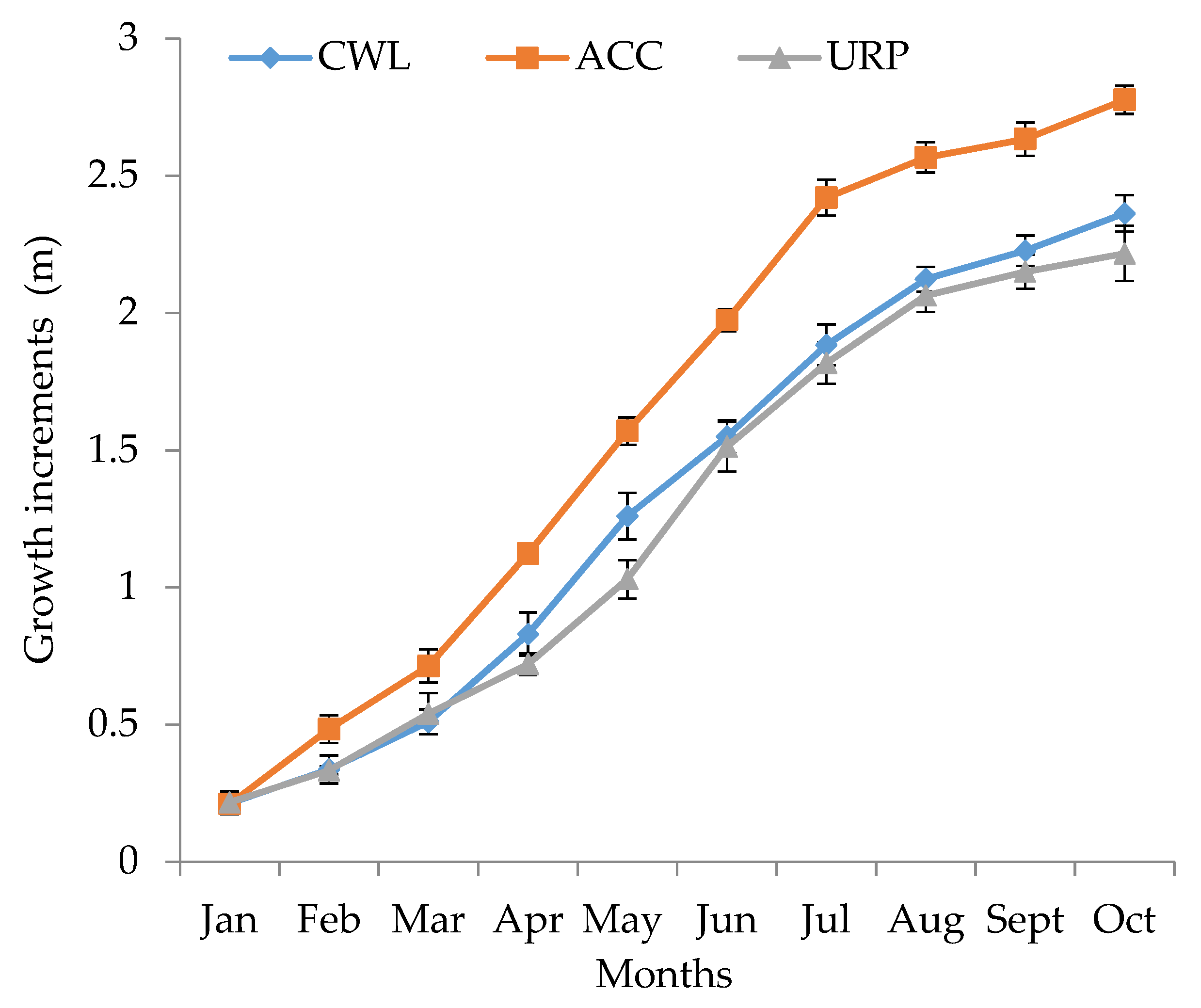
| Months | CWL | ACC | URP |
|---|---|---|---|
| January | 0.21 ± 0.04 | 0.21 ± 0.03 | 0.22 ± 0.04 |
| February | 0.34 ± 0.05 b | 0.48 ± 0.05 a | 0.33 ± 0.02 b |
| March | 0.51 ± 0.05 b | 0.71 ± 0.06 a | 0.54 ± 0.08 b |
| April | 0.83 ± 0.08 b | 1.12 ± 0.04 a | 0.72 ± 0.04 b |
| May | 1.26 ± 0.09 b | 1.57 ± 0.05 a | 1.03 ± 0.07 c |
| June | 1.55 ± 0.06 b | 1.97 ± 0.04 a | 1.51 ± 0.09 b |
| July | 1.88 ± 0.08 b | 2.42 ± 0.07 a | 1.82 ± 0.08 b |
| August | 2.12 ± 0.05 b | 2.57 ± 0.06 a | 2.06 ± 0.06 b |
| September | 2.23 ± 0.06 b | 2.63 ± 0.06 a | 2.15 ± 0.06 b |
| October | 2.36 ± 0.07 b | 2.78 ± 0.05 a | 2.22 ± 0.10 b |
| Proximate Composition (%) | Leaves | Roots | Stems |
|---|---|---|---|
| Moisture | 11.49 ± 0.18 | 9.28 ± 0.24 | 10.44 ± 0.23 |
| Crude protein | 17.49 ± 0.21 | 12.17 ± 0.65 | 9.39 ± 0.23 |
| Crude lipid | 2.15 ± 0.02 | 1.07 ± 0.02 | 2.07 ± 0.09 |
| Crude fiber | 8.47 ± 0.07 | 7.85 ± 0.12 | 9.53 ± 0.11 |
| Carbohydrate | 50.96 ± 0.65 | 56.97 ± 0.97 | 53.33 ± 0.61 |
| Ash | 20.93 ± 0.47 | 21.95 ± 0.33 | 25.68 ± 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.M.; Shahjahan, M.; Khan, S.; Juraimi, A.S.; Uddin, M.K.; Hasan, M. Potentialities of the Asian Watergrass (Hygroryza aristata) as Feed in Aquaculture. Sustainability 2021, 13, 6559. https://doi.org/10.3390/su13126559
Hossain MM, Shahjahan M, Khan S, Juraimi AS, Uddin MK, Hasan M. Potentialities of the Asian Watergrass (Hygroryza aristata) as Feed in Aquaculture. Sustainability. 2021; 13(12):6559. https://doi.org/10.3390/su13126559
Chicago/Turabian StyleHossain, Md. Moazzem, Md. Shahjahan, Saleha Khan, Abdul Shukor Juraimi, Md. Kamal Uddin, and Mahmudul Hasan. 2021. "Potentialities of the Asian Watergrass (Hygroryza aristata) as Feed in Aquaculture" Sustainability 13, no. 12: 6559. https://doi.org/10.3390/su13126559






