Inactivation of Algae and Plankton by Ultrasonic Cavitation
Abstract
:1. Introduction
2. Ultrasonic Destruction of Algae and Microcapsule
2.1. Experimental Setup for Algae and Microcapsules
2.1.1. Algae
2.1.2. Microcapsules
2.1.3. Sonication
2.1.4. Evaluation Methods
2.2. Algae and Microcapsule Results
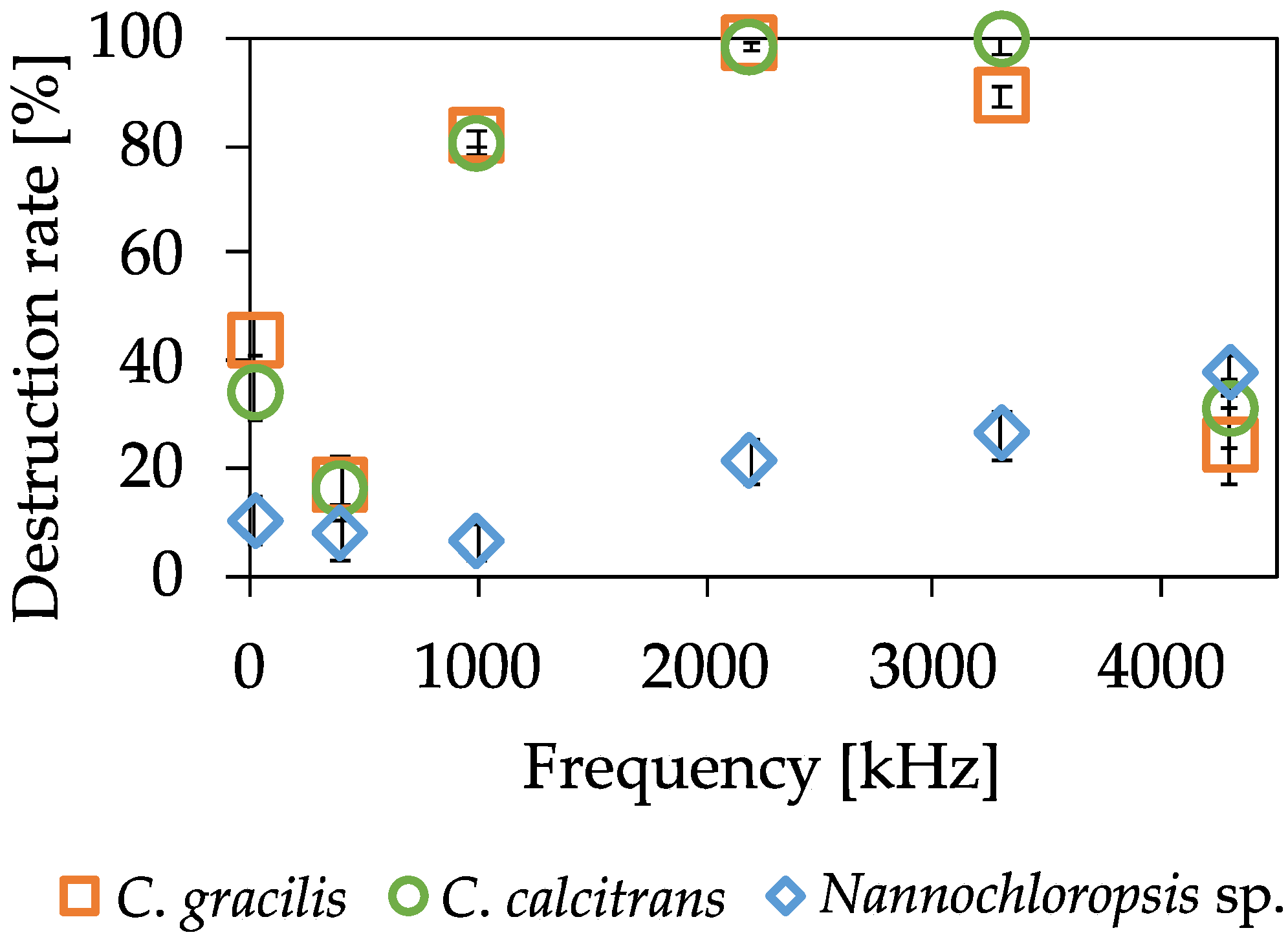
2.2.1. Algae Results
2.2.2. Microcapsule Results
2.3. Discussion of Algae and Microcapsules
3. Ultrasonic Destruction of Plankton
3.1. Experimental Setup for Plankton
3.1.1. Plankton
3.1.2. Sonication
3.1.3. Evaluation Methods
3.2. Results for Plankton
3.3. Discussion of Plankton
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.-J.; Yeh, H.-H. The mechanisms of potassium permanganate on algae removal. Water Res. 2005, 39, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Ma, J.; Fang, J.; Guan, Y.; Yue, S.; Li, X.; Chen, L. Comparison of Permanganate Preoxidation and Preozonation on Algae Containing Water: Cell Integrity, Characteristics, and Chlorinated Disinfection Byproduct Formation. Environ. Sci. Technol. 2013, 47, 14051–14061. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, C.; Xie, C.; Shao, C.; Yu, S.; Zhao, L.; Gao, N. Kinetics and mechanism of Pseudoanabaena cell inactivation, 2-MIB release and degradation under exposure of ozone, chlorine and permanganate. Water Res. 2018, 147, 422–428. [Google Scholar] [CrossRef]
- Bishop, W.M.; Richardson, R.J.; Willis, B.E. Comparison of Partitioning and Efficacy between Copper Algaecide Formulations: Refining the Critical Burden Concept. Water Air Soil Pollut. 2018, 229, 300. [Google Scholar] [CrossRef]
- Ellgehausen, H.; Guth, J.A.; Esser, H.O. Factors determining the bioaccumulation potential of pesticides in the individual compartments of aquatic food chains. Ecotoxicol. Environ. Saf. 1980, 4, 134–157. [Google Scholar] [CrossRef]
- Mahvi, A.H. Application of Ultrasonic Technology for Water and Wastewater Treatment. Iran. J. Public Health 2009, 38, 1–17. [Google Scholar]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Suslick, K.S.; Flannigan, D.J. Inside a Collapsing Bubble: Sonoluminescence and the Conditions during Cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [Green Version]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Suslick, K.S.; Hammerton, D.A.; Cline, R.E. Sonochemical hot spot. J. Am. Chem. Soc. 1986, 108, 5641–5642. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Grieser, F.A. Comparison between Multibubble Sonoluminescence Intensity and the Temperature within Cavitation Bubbles. J. Am. Chem. Soc. 2005, 127, 5326–5327. [Google Scholar] [CrossRef]
- Schmitt, F.O.; Johnson, C.H.; Olson, A.R. Oxidations Promoted by Ul-Trasonic Radiation. J. Am. Chem. Soc. 1929, 51, 370–375. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P.; Bates, D.M.; Zhao, Y. Dosimetry in sonochemistry: The use of aqueous terephthalate ion as a fluorescence monitor. Ultrason. Sonochem. 1994, 1, S91–S95. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Grieser, F. Ultrasound assisted chemical processes. Rev. Chem. Eng. 1999, 15, 41–83. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry, Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; John Wiley & Sons Inc.: New York, NY, USA, 2007; pp. 516–541. [Google Scholar]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Kozuka, T.; Towata, A. Protein release from yeast cells as an evaluation method of physical effects in ultrasonic field. Ultrason. Sonochem. 2008, 15, 995–1000. [Google Scholar] [CrossRef]
- Bai, L.; Xu, W.; Tian, Z.; Li, N. A High-Speed Photographic Study of Ultrasonic Cavitation near Rigid Boundary. J. Hydrodynam. B 2008, 20, 637–644. [Google Scholar] [CrossRef]
- Holzfuss, J.; Rüggeberg, M.; Billo, A. Shock Wave Emissions of a Sonoluminescing Bubble. Phys. Rev. Lett. 1998, 81, 5434–5437. [Google Scholar] [CrossRef] [Green Version]
- Lamminen, M.O.; Walker, H.W.; Weavers, L.K. Mechanisms and factors influencing the ultrasonic cleaning of particle-fouled ceramic membranes. J. Membr. Sci. 2004, 237, 213–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wu, J.; Liu, Y.; Zhang, M.; Yang, C.; He, M.; Gong, X.; Zhang, Z.; Wang, Z.; et al. Study on fracture of tungsten wire induced by acoustic cavitation at different hydrostatic pressures and driving electric powers. Ultrason. Sonochem. 2020, 68, 105232. [Google Scholar] [CrossRef]
- Keswani, M.; Raghavan, S.; Deymier, P.; Verhaverbeke, S. Megasonic cleaning of wafers in electrolyte solutions: Possible role of electro-acoustic and cavitation effects. Microelectron. Eng. 2009, 86, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Mason, T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016, 29, 519–523. [Google Scholar] [CrossRef]
- Shiba, K.; Takemura, Y.; Mizukoshi, Y.; Yamamoto, K. Effects of primary C1–C6 linear alcohol addition and sonochemically decomposed products on multi-bubble sonoluminescence. Jpn. J. Appl. Phys. 2019, 58, SGGD14. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, C.; Wang, B.; Yagoub, A.E.-G.A.; Ma, H.; Zhang, X.; Wu, M. Study of ultrasonic cavitation during extraction of the peanut oil at varying frequencies. Ultrason. Sonochem. 2017, 37, 106–113. [Google Scholar] [CrossRef]
- Inui, A.; Honda, A.; Yamanaka, S.; Ikeno, T.; Yamamoto, K. Effect of ultrasonic frequency and surfactant addition on microcapsule destruction. Ultrason. Sonochem. 2021, 70, 105308. [Google Scholar] [CrossRef]
- Joyce, E.M.; Phull, S.S.; Lorimer, J.P.; Mason, T.J. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Tsukamoto, I.; Yim, B.; Stavarache, C.E.; Furuta, M.; Hashiba, K.; Maeda, Y. Inactivation of Saccharomyces cerevisiae by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 61–65. [Google Scholar] [CrossRef]
- Gao, S.; Hemar, Y.; Ashokkumar, M.; Paturel, S.; Lewis, G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014, 60, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Lewis, G.D.; Ashokkumar, M.; Hemar, Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014, 21, 446–453. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Otani, Y.; Yabunaka, A.; Ikeuchi, R.; Yamamoto, K. Inactivation of Escherichia coli, Saccharomyces cerevisiae and Bacillus subtilis by ultrasonic cavitation. Acoust. Sci. Technol. 2020, 41, 877–884. [Google Scholar] [CrossRef]
- Kurokawa, M.; King, P.M.; Wu, X.; Joyce, E.M.; Mason, T.J.; Yamamoto, K. Effect of sonication frequency on the disruption of algae. Ultrason. Sonochem. 2016, 31, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, K.; King, P.M.; Wu, X.; Mason, T.J.; Joyce, E.M. Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason. Sonochem. 2015, 24, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.M.; Wu, X.; Mason, T.J. Effect of ultrasonic frequency and power on algae suspensions. J. Environ. Sci. Health A 2010, 45, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Peng, Y.; Zhang, Z.; Zhang, M.; Zhou, Y.; Duan, Z. Removal of Microcystis aeruginosa by ultrasound: Inactivation mechanism and release of algal organic matter. Ultrason. Sonochem. 2019, 56, 447–457. [Google Scholar] [CrossRef]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, Z.; Wang, M.; Shi, X.; Zhou, Y.; Zhou, Y.; Kong, Y. Inactivation of harmful Anabaena flos-aquae by ultrasound irradiation: Cell disruption mechanism and enhanced coagulation. Ultrason. Sonochem. 2020, 69, 105254. [Google Scholar] [CrossRef]
- Wei, Z.; Kosterman, J.A.; Xioa, R.; Pee, G.Y.; Cai, M.; Weavers, L.K. Designing and characterizing a multi-stepped ultrasonic horn for enhanced sonochemical performance. Ultrason. Sonochem. 2015, 27, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Calinescu, I.; Vartolomei, A.; Gavrila, A.I.; Vinatoru, M.; Mason, T.J. A reactor designed for the ultrasonic stimulation of enzymatic esterification. Ultrason. Sonochem. 2019, 54, 32–38. [Google Scholar] [CrossRef]
- Vinatoru, M.; Toma, M.; Radu, O.; Filip, P.I.; Lazurca, D.; Mason, T.J. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason. Sonochem. 1997, 4, 135–139. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uchida, T. Calorimetric method for measuring high ultrasonic power using water as a heating material. J. Phys. Conf. Ser. 2011, 279, 012012. [Google Scholar] [CrossRef]
- Zinin, P.V.; Allen, J.S., III; Levin, V.M. Mechanical resonances of bacteria cells. Phys. Rev. E 2005, 72, 061907. [Google Scholar] [CrossRef]
- Zinin, P.V.; Allen, J.S., III. Deformation of biological cells in the acoustic field of oscillating bubble. Phys. Rev. E 2009, 79, 021910. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.Y.J.; Hébraud, P.; Hemar, Y.; Ashokkumar, M. Quantification of high-power ultrasound induced damage on potato starch granules using light microscopy. Ultrason. Sonochem. 2012, 19, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.B.; Miksis, M. Bubble oscillations of large amplitude. J. Acoust. Soc. 1980, 68, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Tsochatzidis, N.A.; Guiraud, P.; Wilhelm, A.M.; Delmas, H. Determination of Velocity, Size and Concentration of Ultrasonic Cavitation Bubbles by the Phase-Doppler Technique. Chem. Eng. Sci. 2001, 56, 1831–1840. [Google Scholar] [CrossRef]
- Brotchie, A.; Grieser, F.; Ashokkumar, M. Effect of Power and Frequency on Bubble-Size Distributions in Acoustic Cavitation. Phys. Rev. Lett. 2009, 102, 084302. [Google Scholar] [CrossRef]













| Algae | Resonance Frequency [kHz] | Best Frequency [kHz] |
|---|---|---|
| C. gracilis | 4000 | 2200 |
| C. calcitrans | 4400 | 3300 |
| Nannochloropsis sp. | 4900 | 4300 |
| Microcapsule | Resonance frequency [kHz] | Best frequency [kHz] |
| Microcapsules A | 1900 | 2200 |
| Microcapsules B | 5000 | 4300 |
| Parameters | Value |
|---|---|
| c [m/s] | 1520 |
| ρ [kg/] | 1027 |
| [Pa] | 1.013 × |
| σ [N/m] | 0.0735 |
| [m] | 0.1~150 × in 0.1 µm increments |
| γ | 1.4 |
| μ [Pa·s] | 0.001036 |
| [Pa] | 2.1 × (at 10 W) |
| ω [rad/s] | Frequency dependent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, A.; Sugino, F.; Yamamoto, K. Inactivation of Algae and Plankton by Ultrasonic Cavitation. Sustainability 2021, 13, 6769. https://doi.org/10.3390/su13126769
Honda A, Sugino F, Yamamoto K. Inactivation of Algae and Plankton by Ultrasonic Cavitation. Sustainability. 2021; 13(12):6769. https://doi.org/10.3390/su13126769
Chicago/Turabian StyleHonda, Atsushi, Fumiya Sugino, and Ken Yamamoto. 2021. "Inactivation of Algae and Plankton by Ultrasonic Cavitation" Sustainability 13, no. 12: 6769. https://doi.org/10.3390/su13126769





