Abstract
Biostimulants are a novel and eco-friendly agronomic tool with practical applications in alleviating negative effects of environmental stressors. The present work studied the effects of three biostimulant products (Nomoren (N), Twin-Antistress (TW), and X-Stress (XS)) under normal irrigation (W+) and water deficit irrigation conditions (W−) on the nutritional, chemical composition and bioactive properties of common bean fresh pods. A variable effect of biostimulants and water deficit irrigation was observed on nutritional value parameters, while fructose and sucrose were the main detected sugars, especially in NW+ and CW− treatments. Oxalic, malic, and citric acid were the main detected organic acids, while γ- and total tocopherol content was the highest in TWW+. (+)-Catechin and (−)-epicatechin were the most abundant phenolic compounds, especially in the NW− treatment. A variable antioxidant capacity was observed for the Thiobarbituric Acid Reactive Substances (TBARS) and Oxidative Haemolysis assays (OxHLIA), while TWW+ extracts showed the best overall results against the tested fungi. In conclusion, the tested biostimulants had a positive effect on chemical composition and bioactivities of purple bean depending on the irrigation regime.
1. Introduction
Common bean (Phaseolus vulgaris L.) is one of the most important vegetable crops, which is widely used for its edible fruit (pods) and seeds (pulses). It is widespread and one of the main crops cultivated in many tropical, subtropical, and temperate areas of the Americas, Europe, Africa, and Asia [1]. The limited agricultural land, crop security, pests and diseases, and the ongoing climatic change are the major causes of reduction in crop productivity that put in risk food security [2]. Particularly, water scarcity associated with climatic change is a severe risk to global agriculture because the crop productivity is highly dependent on irrigation management, regimes, and water quality [3]. This problem is aggravated in horticultural crops, which are more sensitive to water deficit than other crops, especially under greenhouse conditions where water requirements are considerably higher than open field conditions [4,5,6].
Above all, agricultural and horticultural crops are the main source of vitamins, minerals, carbohydrates, and natural proteins on a daily basis and in this sense, they play a major role in human nutrition [2]. To address the pressure associated with increasing agricultural productivity to subsequently meet the rising global demands for food, producers have turned to excessive applications of chemical fertilizers and pesticides [7]. These chemicals substances pose threats to the health of the entire biosphere. Due to the development of new cultivation technologies, some alternatives have been suggested to attenuate the negative effects of drought and reduce the dependency on synthetic fertilizers and pesticides in the growth and yield of crops [8,9]. Among the most widespread cultural practices, plant biostimulants are natural compounds that may promote crop growth without the harmful side effects of chemical substances or decrease the severe effects of water stress and other abiotic stressors, improve soil water-holding capacity and physicochemical properties, and increase root growth with beneficial effects on nutrient and water use efficiency and yield [10,11,12,13].
Concerning plant biostimulants, arbuscular mycorrhizal fungi may promote many aspects of plant growth and development via improved nutrition, better growth, stress tolerance, and disease resistance, while they may decrease nutrient leaching from the soil, contributing to sustainable nutrients management of crops [14]. Natural microorganisms like bacteria and fungi are also used in the biocontrol of plant pathogens, improving plants growth and decreasing symptoms of abiotic stress caused by weather or soil [15,16]. According to Sarma and Saikia [17], the application of plant growth promoting rhizobacteria (PGPR) may alleviate the water stress effects on mung bean (Vigna radiata L.) through the accumulation of antioxidant enzymes and osmolytes, as well as via the up-regulation of stress-related genes. Seed inoculation with beneficial bacteria (Rhizobium tropici and Paenibacillus polymyxa) also had positive effects on water stress alleviation [18]. On the other hand, the use of chelated fertilizers has increased in recent years, and they were developed to increase micronutrient utilization efficiency and, actually, only the metallic micronutrients, such as such as Cu, Fe, Mn, and Zn are used in chelated forms as fertilizers in agriculture [19]. The activity of biostimulants appears to be associated with the production of phytohormones, improving the contribution of nutrients, inducing root growth and antioxidant response systems [8].
A simple method to identify water stress effects on common bean is the use of indices related with plant growth traits and pod characteristics aiming to breeding induced resistance [20], whereas the evaluation of the effects on pod quality and biochemical parameters is less common. Therefore, the present study aimed to evaluate the use of natural biostimulants as a simple cultivation practice that could alleviate the possible negative effects that water deficit irrigation may have on bean pods quality. For this purpose, a drought sensitive species, e.g., common bean (Phaseolus vulgaris L., cv. “Purple Queen”), was selected and grown in a greenhouse under water deficit irrigation conditions and the nutritional value and chemical composition of pods was studied.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
The experiment was carried out during the growing period of spring–summer 2019. Sowing took place on 22/03/2019 and seeds of dwarf French bean (Phaseolus vulgaris L. cv. Purple Queen; Sutton Seeds, Devon, UK) were sown within an unheated plastic greenhouse at the experimental farm of the University of Thessaly, Greece. Seeds were sown directly in the soil in single rows with a spacing of 50 cm within each row and 80 cm between the rows. Plant density was 2.5 plants/m2 (25,000 plants/ha), while each treatment consisted of 12 plants and was replicated three times (144 plants in total). Two factors were applied in a split-plot factorial design, namely water deficit irrigation and biostimulants. Biostimulants treatments included: (1) Control (C: no biostimulants added), (2) Nomoren, (3) Twin-Antistress (TW), and (4) X-stress (XS) [21].
Regarding the detailed composition of each product (Supplementary Material: Table S1), Nomoren contains 20% of arbuscular mycorrhizal fungi (AMF) (Glomus spp.); Twin-Antistress contains AMF (Glomus spp.), various strains of rhizobacteria (e.g., Bacillus sp.), and yeast and Ascophyllum nodosum extracts, as well as nitrogen (organic): 1%, organic carbon: 10%, and organic matter (<50 kDa): 30%. X-stress contains 0.5% Cu, 2.0% Fe, 1.0% Mn and 2.0% Zn, all chelated with glycine [15,21]. Nomoren was provided by Anthis S.A., Greece; Twin-Antistress was provided by Microspore Hellas—Sacom Hellas, Greece, and X-stress was provided by Agrofarm S.A., Greece.
The soil at 0–30 cm depth was clay (26% sand, 32% silt, and 42% clay). Τhree samples of soil were air-dried, passed through a 2-mm sieve and soil physicochemical properties were as follows: pH: 8.0 (1:1 soil/H2O); organic matter content: 3.1%; CaCO3: 10.8%; available P: 70.9 mg/kg; total N: 1.8 g/kg; exchangeable K2O: 195 mg/kg; electrical conductivity (ECe): 0.95 dS/m. The measurements of pH and EC were performed according to the protocols described by Rowell [22]. Calcium carbonate (CaCO3) was measured with a calcimeter [23]. Organic matter content (OC) was determined with the Walkley and Black wet oxidation method according to the protocol of Nelson and Sommers [24]. Available P was measured with the Olsen method [22]. Total N was determined with the Kjeldahl method [25]. Exchangeable K2O was measured with the ammonium acetate method according to the protocol described by Rowell [22].
Water deficit treatments included normally irrigated plants (W+) where irrigation was applied twice a week, and water deficit treated plants (W−) where water holding was applied with irrigation being implemented once a week and based on tensiometer readings (Irrometer-Moisture Indicator, Irrometer, Riverside, CA) that were in the range of 10–15% for the control treatment (W+) and 50–60% for the water deficit treatments (W−), considering that 0% refers to field capacity and 100% to dry soil [15]. Biostimulants were applied with irrigation water and according to the directions for use of each product at 20, 30, and 40 days after sowing (DAS) as following: N treatment was applied at 5 L/ha for each dose; TW was applied at 5 L/ha for each dose; XS was applied at 1 L/ha for each dose. The irrigation system is described in detail by the authors in a previously published report [10].
Water deficit started after the second application of biostimulants (30 days after sowing; DAS). Fertilization was applied through the irrigation water from two different tanks that contained the adequate amount of nutrients in order to achieve similar nutrients application, regardless of water treatment (Figure 1). Pods were harvested when achieved marketable maturity starting 13 June 2019 (84 DAS) until 29 June 2019 (100 DAS) (Figure 2). After harvest, batch samples of pods were put in deep-freezing conditions, then lyophilized, ground with a mortar and pestle, and stored at freezing conditions (−80 °C) until further analyses.

Figure 1.
Common bean plants during the cultivation period.

Figure 2.
Common bean pods ready to harvest.
2.2. Nutritional and Energetic Value
Protein, fat, and ash contents (g/100 g of dry weight (dw)) were evaluated following the AOAC official methods of food analysis [26]. The macro-Kjeldahl method was used to estimate the crude protein content (N × 6.25); the crude fat was extracted in a Soxhlet apparatus using petroleum ether as a solvent; the ash content was assessed by incineration of the plant samples at 550 ± 10 °C. The total carbohydrate content (g/100 g of dw) was determined by difference as follows: 100—(g protein + g fat + g ash). The energy (kcal/100 g of dw) was calculated according to the Regulation (EU) No 1169/2011 [27] as follows: 4 × (g protein + g carbohydrates) + 9 × (g fat).
2.3. Analysis of Hydrophilic Compounds
The free sugars profile was characterized by a high-performance liquid chromatography (HPLC) system coupled with a refraction index (RI) detector, as previously described [28]. The identification was made by relating the retention times of the authentic standards with those of the samples, while quantification was made by the internal standard method (IS, melezitose; Sigma-Aldrich, St. Louis, MO, USA), with calibration curves constructed with standards. The free sugars content was expressed in g per 100 g of dw.
The organic acids profile was characterized by ultra-fast liquid chromatography (UFLC; Shimadzu 20A series, Kyoto, Japan) following a procedure earlier described and optimized by the authors [29]. Detection was performed by a photo-diode array detector (PDA), using 280 nm as preferable wavelengths. Quantification was performed by comparing the peak area of the samples with calibration curves constructed with commercial standards. The organic acids content was expressed in g per 100 g dw.
2.4. Analysis of Lipophilic Compounds
The profile in fatty acid methyl esters (FAME) was characterized after transesterification of the lipid fraction obtained after Soxhlet extraction [30]. A YOUNG IN Chromass 6500 Gas Chromatography System (YL Instruments, Anyang, Korea) equipped with a split/splitless injector, a flame ionization detector (FID), and a Zebron-Fame column (30 m × 0.25 mm × 0.20 μm, Phenomenex, Lisbon, Portugal) was used in the analysis [28]. The elution and operation conditions were previously described by Spréa et al. [28]. Identification was performed by comparing the relative retention times of the FAME peaks of the samples with those of the standard 47885-U (Sigma-Aldrich, St. Louis, MO, USA). The Clarity DataApex 4.0 Software (DataApex, Prague, Czech Republic) was used for data processing. The results were expressed in relative percentage of each fatty acid.
The tocopherols profile was characterized following an analytical procedure previously reported by the authors [28]. An HPLC system coupled to a fluorescence detector (FP-2020; Jasco) programmed for excitation at 290 nm and emission at 330 nm was used. The isoforms identification was achieved by chromatographic comparison with authentic standards and the quantification was based on the fluorescence signal response of each standard, using the internal standard (IS; tocol (50 mg/mL); Matreya, Pleasant Gap, PA, USA) method and calibration curves constructed with commercial standards. The results were expressed in mg per 100 g of dw.
2.5. Phenolic Profile Characterization
2.5.1. Preparation of Extracts
The lyophilized samples (~2.5 g) were submitted to maceration at room temperature with the addition of 30 mL of an ethanol/water solution (80:20 v/v; for the anthocyanin extraction, 0.5% trifluoroacetic acid (TFA) was added to the extraction solvent). Then, the extracts were filtered through filter paper (Whatman No. 4) and the retained residue was re-extracted following the same procedure [31]. The combined extracts were concentrated at 40 °C under reduced pressure (rotary evaporator Büchi R-210, Flawil, Switzerland) and the aqueous phase was frozen and freeze-dried (FreeZone 4.5, Labconco, Kansas City, MO, USA).
2.5.2. Analysis of Phenolic Compounds
Phenolic compounds were analysed in the hydroethanolic extracts, which were redissolved in ethanol/water (20:80, v/v), to a final concentration of 10 mg/mL and filtered thought 0.22-μm disposable filter disks. The chromatographic analysis for phenolic compounds (non-anthocyanin and anthocyanin compounds) was achieved using an HPLC system (Dionex Ultimate 3000 UPLC, Thermo Scientific, San Jose, CA, USA) coupled with a diode-array detector (DAD) and a Linear Ion Trap (LTQ XL) mass spectrometer (MS, Thermo Finnigan, San Jose, CA, USA) equipped with an electrospray ionization (ESI) source, working in negative mode for non-anthocyanin compounds and positive mode for anthocyanin compounds. For non-anthocyanin phenolic compounds analysis, a Waters Spherisorb S3 ODS-2 reverse phase C18 column (4.6 × 150 mm, 3 µm; Waters, Milford, MA, USA) and an elution gradient using as mobile phase formic acid/water (0.1%) and acetonitrile recorded at 280 and 330 nm as preferred wavelengths, as previously described by the authors [32].
For anthocyanin phenolic compounds, a reverse phase AQUA® C18 column (5 µm, 150 mm × 4.6 mm i.d., Phenomenex, Torrance, CA, USA) and an elution gradient using as mobile phase 0,1% TFA in water, and 100% acetonitrile were used, and recorded at 520 nm as the preferred wavelength, as previously described by the authors [33]. For the identification of the compounds, the data obtained (retention times, UV-Vis spectra, and mass spectra) were compared with data available in the literature and, when available, with the standards (Extrasynthèse, Genay, France). In cases where no standard compound was available, the quantification was performed using the calibration curve of a compound within the same phenolic group. For quantitative analysis of anthocyanins, 7-level calibration curves were obtained by injection of standard (Polyphenols, Sandnes, Norway) solutions with known concentrations: pelargonidin-3-O-glucoside. The results were expressed as mg per g of extract.
2.6. Antioxidant Activity Evaluation
2.6.1. Assessment of the Capacity to Inhibit the Formation of Thiobarbituric Acid Reactive Substances (TBARS)
The hydroethanolic extracts were re-dissolved in water and subjected to dilutions from 2.5 mg/mL to 0.3125 mg/mL. The lipid peroxidation inhibition in porcine brain cell homogenates was evaluated by the decrease in TBARS; the colour intensity of malondialdehyde–thiobarbituric acid (MDA–TBA) was measured at 532 nm; the inhibition ratio (%) was calculated using the following formula: [(A − B)/A] × 100%, where A and B correspond to the absorbance of the control and extract sample, respectively [28]. The results were expressed in IC50 values (mg/mL, sample concentration providing 50% of antioxidant activity). Trolox (Sigma-Aldrich, St. Louis, MO, USA) was used as positive control.
2.6.2. Assessment of the Capacity to Inhibit the Oxidative Haemolysis
The antihaemolytic activity of the hydroethanolic extracts re-dissolved in phosphate-buffered saline (pH 7.4) was evaluated by the oxidative haemolysis inhibition assay (OxHLIA) using red blood cells isolated from the blood of healthy sheep, as described by the authors [31]. Extract concentration to obtained IC50 values (mg/mL) was calculated for a Δt of 60 min, i.e., extract concentration required to protect 50% of the erythrocyte population from the haemolytic action of 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH; Sigma-Aldrich, St. Louis, MO, USA). Trolox was used as a positive control.
2.7. Antimicrobial Activity Evaluation
Staphylococcus aureus American Type Culture Collection; Bacillus cereus food isolate, Manassas, VA, USA, ATCC 6538; Listeria monocytogenes National Collection of Type Cultures, London, UK, NCTC 7973; Escherichia coli ATCC 25922; Enterobacter cloacae ATCC 35030; and Salmonella typhimurium ATCC 13311 were selected to test the antibacterial activity of the hydroethanolic extracts. For antifungal activity, six micromycetes were used, namely Aspergillus fumigatus ATCC 9197; Aspergillus niger ATCC 6275; Aspergillus versicolor ATCC 11730; Penicillium funiculosum ATCC 36839; Penicillium verrucosum var. cyclopium food isolate; and Trichoderma viride IAM 5061. The microdilution method was performed as previously described [34,35]. The results were presented as the extract concentrations that resulted in complete inhibition of the microbial growth (i.e., minimum inhibitory concentration, MIC), determined through the colorimetric microbial viability assay [34,35], as well as minimum bactericidal and minimal fungicidal concentrations (MBC and MFC values, respectively). The food preservatives sodium benzoate (E211) and potassium metabisulfite (E224) were used as positive controls, whereas the negative control was 5% dimethyl sulfoxide (DMSO).
2.8. Statistical Analysis
Throughout the manuscript, all data obtained from assays carried out in triplicate, are expressed as mean ± standard deviation. Mean and standard deviations were determined from the obtained data using Microsoft Excel (Microsoft Corp., Redmond, WA, USA). All statistical tests were performed using SPSS Statistics software (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY, USA: IBM Corp.). The results were obtained through the analysis of variance (two-way ANOVA), and were compared using the Tukey’s HSD test when significant differences were detected (α = 0.05).
Moreover, principal component analysis (PCA) was performed based on the studied chemical composition components (proximate analysis, free sugars, tocopherols, organic acids, the main fatty acids and the main phenolic compounds) and antioxidant activity (TBARS and OxHLIA) in order to depict relationships among the tested treatments and the studied variables as well as to identify those variables that were most effective in discriminating between irrigation regime and biostimulant application. The analysis was performed with the use of Statgraphics 5.1.plus (Statpoint Technologies, Inc., Warrenton, VA, USA).
3. Results and Discussion
The statistical analysis of the data showed a significant interaction between the tested factors, therefore, the means from all the treatments combinations were compared simultaneously according to the Tukey’s HSD test.
3.1. Nutritional Composition and Hydrophilic Compounds Content
The nutritional composition and hydrophilic compounds (free sugars and organic acids) content are presented in Table 1. Fat content was the highest in normally irrigated plants that either received no biostimulants (CW+; 1.05 g/100 g dw) or Nomoren (NW+; 1.04 g/100 g dw) and X-Stress (XSW+; 1.09 g/100 dw) biostimulants were applied. On the other hand, water deficit resulted in reduced content of fat in plants that received no biostimulants (CW−) and X-Stress treatment, whereas the application of Nomoren (NW+) alleviated the negative effect of water stress since no differences were observed from normally irrigated plants of the same treatment (1.04 g/100 g dw and 1.08 g/100 g dw, respectively). On the other hand, proteins content increased in water deficit treated plants, regardless of biostimulant treatment, especially in plants that received Twin-Antistress (TWW−) and X-Stress (XSW−) treatments (20.02 g/100 g dw and 19.6 g/100 g dw, respectively). The application of water deficit and biostimulants showed no clear effect on the ash content and variable trends were observed. However, the highest content was observed for the NW− treatment (3.44 g/100 g dw), indicating a positive effect of this biostimulant on this parameter under stress conditions. Carbohydrates and energetic content decreased under water deficit irrigation conditions when biostimulants were applied, whereas no significant differences were observed between CW− and CW+ treatments in both cases. The highest carbohydrates content was recorded for the TWW+ treatment (79.5 g/100 g dw), while energetic value was the highest for the XSW+ treatment (393.4 kcal/100 g dw). According to the literature, a varied effect of biostimulants on the nutritional value of common bean pods has been reported under water stress conditions [10]. In particular, Petropoulos et al. [10] indicated a higher and lower content of carbohydrates and proteins in water-stressed plants that received specific biostimulant treatments, respectively. The opposite trend was observed in our study and the differences between the two studies could be associated to the application of different biostimulant products and the different bean cultivar, since according to the literature, the stress alleviating effects of biostimulants are highly dependent on the crop and the biostimulant product and the combination of different biostimulants on the same crop is also suggested for better results [36,37,38]. Moreover, the application of Ascophyllum nodosum extracts on common bean plants subjected to water stress showed positive results not only in terms of yield components through the reduction of oxidative stress [7], but also on nutritional parameters [10,21]. However, there are contrasting reports in the literature regarding the effects of seaweed extracts, which could be due to compositional differences since many of the products contain compounds with hormone-like activities that could affect plants response to water stress [37,38]. The findings of this study are of high interest considering the importance of protein content for bean pods quality, since the use of cost-effective cultivation practices such as biostimulant application that may increase this parameter under water deficit irrigation conditions could improve the added value of the final product.

Table 1.
Nutritional, energetic value, and hydrophilic compounds content of the studied purple bean samples in relation to biostimulant application and irrigation management (mean ± SD, n = 3).
Regarding free sugars composition, three sugars were identified in all the tested samples, namely fructose, sucrose, and glucose (Table 1). A varied response to biostimulant and irrigation treatments was observed in the studied fresh pods. For example, decreasing trends for fructose content under water deficit irrigation conditions and biostimulant application were observed (except for the case of TW where no differences were observed between normally irrigated and water deficit treated plants), whereas for glucose and sucrose a treatment dependent effect was observed. The highest amount of fructose and sucrose was observed in the treatments of NW+ (3.73 g/100 g dw) and CW− (5.63 g/100 g dw), respectively, resulting in the highest amount of total sugars content for the same treatments (9.4 g/100 g dw and 9.74 g/100 g dw, respectively). This result indicates a positive effect on free sugars accumulation under water deficit irrigation, which was also observed in spinach leaves [15] and common bean pods [10], although they suggest a variable effect of biostimulants depending on the applied product. According to the literature, this is a common response in water stressed plants, which accumulate free sugars either as osmotic and growth regulators or as carbon pools [39,40,41]. On the other hand, glucose content was the highest in the treatments of NW+ and TW (regardless of irrigation treatment), with no significant differences among them, which highlights the protective effects of these products and TW in particular under stress conditions. Moreover, sucrose and glucose content showed similar increasing or decreasing trends under stress when plants where applied with the same biostimulant or did not receive any biostimulant treatments. According to the literature, the positive effects of seaweed extracts in stress alleviation are associated with the induction of stress related genes that regulate the biosynthesis of sugars that act as osmolytes to retain cell turgor or as substrates for cellular respiration [42]. Similarly, the inoculation of stressed plants with AMF is also associated with increased soluble sugars content which may act as osmoregulators or as carbon sources to compensate for energy expenditure under stress conditions [43,44]. Gupta and Kaur [45] noted that both sugars may act as hormones and transfer the signals that regulate the expression of stress related genes or being used as cellular respiration substrates [39].
The main detected organic acids were oxalic, malic, and citric acid, while traces of fumaric acid were also identified (Table 1). Biostimulant application resulted in a decrease of oxalic and malic acid and total organic acids when plants were subjected to water deficit regime, while the same trend was also observed in the case of citric acid except for the XS biostimulant where no differences were observed among the irrigation treatments. The same treatment was responsible for the highest content of malic, citric acid (no differences between normally irrigated and water deficit treated stressed plants in this case), and total organic acids in normally irrigated plants, whereas the highest oxalic acid content was recorded in water deficit treated plants that received no biostimulants (CW−). Moreover, all the biostimulant treatments resulted in a decrease of oxalic acid content under water deficit irrigation conditions when compared to the corresponding control irrigation treatment (CW−). This finding is crucial for the nutritional value of the edible product, especially under water deficit irrigation conditions where oxalic acid tends to accumulate in fresh pods and cost-effective means that may reduce its content are needed. The same trend was observed in the study reported by the authors [10], where most of the tested biostimulant products resulted in a decrease of oxalic acid content in common bean pods grown under water stress conditions when compared with the corresponding biostimulant treatments of normally irrigated plants. Moreover, several other studies reported the significant effect of biostimulants on organic acids composition [15], whereas Zushi et al. [46] associated compositional changes with concentration effects that result in increased dry matter content. According to other studies, biostimulant products that contain microorganisms may affect the organic acids composition since organic acids are involved in microorganism metabolism [47].
3.2. Lipophilic Compounds Content
Fatty acid composition and contents are presented in Table 2. The main detected compounds were linoleic and palmitic acid, followed by α-linolenic and oleic acid, while saturated and polyunsaturated fatty acids (SFA and PUFA, respectively) were the most abundant classes of fatty acids. Similar results were reported in the study of Petropoulos et al. [10], where different biostimulant products were tested on common bean plants grown under tow irrigation regimes. Regarding the biostimulants effect, variable response to the tested treatments was observed in individual fatty acids content with linoleic and α-linolenic acids being higher under normal irrigation conditions (CW+ and XSW+ in the first case and NW+ in the second one), whereas palmitic and oleic acids were the highest under water deficit irrigation conditions (CW− and XSW− for palmitic acid and TWW− for oleic acid). Similarly, SFA, monounsaturated fatty acids (MUFA) and PUFA content were the highest for XSW−, TWW−, and XSW+ treatments, respectively. The regulation of fatty acid composition through mobilization and unsaturation in plant tissues is associated with the adjustment of membrane fluidity as a mechanism of adaptation against environmental stressors [48]. Moreover, plants subjected to drought stress usually present lower α-linolenic acid levels, while PUFA content is associated with salt tolerance [48]. Therefore, as already reported in the literature [10], the presented results indicate that biostimulatory products (e.g., XS) may affect the composition of fatty acids and increase the quality of the final product, especially when considering the improvement in n6/n3 and PUFA/SFA ratios under water deficit irrigation and normal irrigation conditions (1.32 and 1.08, respectively) [10,49]. The observed changes in fatty acid composition related to the biostimulant application could be associated with the induction of the antioxidant mechanisms of plants that inhibit lipid peroxidation, as well as to the fact that fatty acids could be used as carbon sources [43,50,51].

Table 2.
Chemical composition of lipophilic compounds of the studied purple bean samples in relation to biostimulant application and irrigation management (mean ± SD, n = 3).
Tocopherols content and composition are presented in Table 2. The only detected vitamin E isoforms were α- and γ-tocopherols. The latter was the most abundant in all the tested samples. In contrast to our study, Petropoulos et al. [10] detected not only the two abovementioned compounds but also δ-tocopherol. However, similarly to the present study, the previous authors [10] as well as Chen et al. [52] also suggested γ-tocopherol as the main vitamin E vitamer [10]. In regards to the biostimulant effect, a variable response was observed depending on the biostimulant product composition. For example, the highest content of α-tocopherol was recorded in the TWW− treatment, whereas the highest γ- and total tocopherols was observed for the same biostimulant under the normal irrigation regime (TWW+). α-tocopherol is considered an important antioxidant compound and the fact that its content was the highest in the TWW− treatment indicates that the specific biostimulant may have a protective role through the induction of the antioxidant defense mechanism and the production of tocopherols [53,54]. Considering the contrasting reports in the literature regarding the effect of biostimulant products on tocopherols content and composition it could be suggested that there is a species-specific response to biostimulants, which also depends on stress severity [55].
3.3. Phenolic Compounds Composition and Quantification
The parameters of the detected phenolic compounds are presented in Table 3. Twelve individual compounds in total were identified in all the studied samples, including two phenolic acids, nine flavonoids, and one anthocyanin. With regard to non-anthocyanin compounds, peaks 1 ([M−H]− at m/z 387) and 2 ([M−H]− at m/z 341) were tentatively identified as p-coumaric acid derivative and caffeoyl-O-hexoside, respectively; from a more comprehensive perspective, these compounds have been described in agreement with previous reports in the literature [56]. (+)-Catechin (peak 3), (−)-epicatechin (peak 4), quercetin-3-O-rutinoside (peak 9), quercetin-3-O-glucoside (peak 10) and isorhamnetin-3-O-rutinoside (peak 11), were positively identified according to their retention time (Rt), mass spectra, and maximum absorption wavelength in the UV-Vis region (λ max) by comparison with commercial standards. For the rest of the detected compounds, peaks 5–8 were identified by comparison with literature reports, taking into account the fragmentation patter, retention time, and UV-Vis spectra [21]. Regarding the anthocyanin compounds, only one molecule was identified in the common bean’s samples, being assigned as malvidin 3,5-di-O-glucoside (Peak 12). Kan et al. [57] investigated 26 kidney bean cultivars and sixteen anthocyanins were identified in total, with malvidin derivatives being found in three cultivars. In the recent study of Petropoulos et al. [21], it was reported that fresh pods of common beans contain only flavonoids while phenolic acids were detected in seeds. This contrast could be associated partly with the harvesting stage since, in our study, the harvest took place late in the growing season (84 DAS to 100 DAS) compared to 60 and 70 DAS in the study of Petropoulos et al. [21], as well as with the genotype tested.

Table 3.
Retention time (Rt), wavelengths of maximum absorption in the visible region (λmax), and mass spectral data of the identified phenolic compounds in the studied purple bean hydroethanolic extracts in relation to biostimulant application and irrigation management.
Phenolic compounds profile concerning the applied treatments is presented in Table 4. Regarding non-anthocyanin phenolic compounds, (−)-epicatechin (peak 4) and (+)-catechin (peak 3) were the most abundant compound, with the highest contents being observed in TWW− treatment (1.3 and 0.9 mg/g of extract, respectively). The second most abundant compounds were caffeoyl-O-hexoside (peak 2) and p-coumaric acid derivative (peak 1), which were the richest in XSW+ and NW− treatments, respectively. Consequently, the highest amounts of total flavonoids and total phenolic compounds were detected in TWW− treatment, while XSW+ treatment resulted in the highest amount of total phenolic acids (1.28 ± 0.02 mg/g extract). Regarding the anthocyanin compounds content, the NW+ treatment recorded the highest amount of malvidin 3,5-di-O-glucoside (peak 12) (0.60 mg/g of extract) while the rest of the treatments contained similar amounts, which ranged between 0.348 and 0.398 mg/g of extract.

Table 4.
Content (mg/g of extract) of the non-anthocyanin and anthocyanin phenolic compounds identified in the studied purple bean hydroethanolic extracts in relation to biostimulant application and irrigation management (mean ± SD, n = 3).
These results come in agreement with the previous finding regarding α-tocopherol content, which was also the highest in TWW− (see Table 2) and further confirmed the protective role of the specific biostimulant against oxidative stress since α-tocopherol is not consumed due to the high content of phenolic compounds, which serve as antioxidants instead [53]. Moreover, the abundance of (+)-catechin and (−)-epicatechin in our study could be justified by late harvesting, as already reported by Petropoulos et al. [21]. The varied response of phenolic compounds composition to biostimulant products has been previously reported by Kałużewicz et al. [62], who suggested a combinatory effect of biostimulants and growing conditions as well as to biostimulant product composition, e.g., products that contained Ascophyllum nodosum filtrates (see TW product in our study) increased phenolic compounds content. Moreover, according to Szparaga et al. [63] and Kocira et al. [38], a cultivar and dose-dependent response were observed regarding the biostimulant application on common bean plants. Therefore, the proper selection of biostimulant product and the dose application can be cost-effective cultivation practices to increase the quality of the final product.
3.4. Antioxidant Activity
The results of the antioxidant activity determined with the TBARS and OxHLIA assays are presented in Table 5. The TBARS assay revealed no significant differences among most of the studied treatments. In contrast, TW treatment consistently showed significantly lower antioxidant activity than XSW+, CW−, and NW− treatments, regardless of the irrigation regime. In any case, the IC50 values of all samples were considerably higher than those of Trolox, which was used as positive control. Regarding the OxHLIA assay, a different response was observed, and XSW+ treatment had the lowest IC50 values indicating better antioxidant potential than the rest of the treatments. The positive effect of this treatment could be associated with the beneficial effects that minerals such as Zn may have on plants subjected to stress through the alleviation of oxidative damage as well as to hormonal balance since Zn is involved in auxin biosynthesis [64,65,66,67]. However, the recorded IC50 values were also higher than those of Trolox, showing moderate antioxidant activity. According to Gan et al. [68], pigmented beans are a good source of antioxidant compounds such as polyphenols, especially flavonoids, while Cho et al. [69] came to the same conclusion regarding the importance of flavonoids in the antioxidant mechanisms of spinach. This report agrees with our study where TW treatment showed consistent antioxidant activity in both irrigation regimes and the lowest overall IC50 values in TWW− treatment that contained the highest amounts of flavonoids. Moreover, the variable results of the tested assays are prevalent in natural matrices since other compounds are involved in each antioxidant mechanism tested [21].

Table 5.
Antioxidant activity of the studied purple bean hydroethanolic extracts in relation to biostimulant application and irrigation management (mean ± SD, n = 3).
3.5. Antimicrobial Properties
The antimicrobial properties of the tested samples against relevant foodborne pathogens (bacteria and fungi) are presented in Table 6. Bean pod extracts were more effective than the food additive E224 against Bacillus cereus ATCC 6538 but less effective than the other positive control used, namely E211. The treatments that showed the highest effectiveness were N and TW (regardless of the irrigation regime) and XSW−, with no significant differences between them. Moreover, CW− treatment was the most effective against Salmonella typhimurium ATCC 13311, having similar MIC and MBC values to E211 and the same MIC values as E244 positive control. The extracts’ effectiveness against Listeria monocytogenes NCTC 7973 was lower than the positive controls, while specific extracts were more effective than E211 against Staphylococcus aureus and Enterobacter cloacae ATCC 35030 and similarly effective against Escherichia coli ATCC 25922). According to the literature, pigmented coat extracts from various bean species and cultivars showed effectiveness against two Gram-positive bacteria (B. cereus and S. aureus), although only two species were effective against Gram-negative bacteria (E. coli and S. typhimurium) [68]. The same authors suggested that the effectiveness against Gram-positive bacteria is partly associated with the high polyphenol content of the extracts, which was also the case for NW+ and TWW− treatments in our study, whereas Gram-negative bacteria are less prone to the extracts due to differences in their cell membranes [70]. Furthermore, they highlighted that differences in antibacterial effects might be due to polyphenol composition since differences in compounds’ structure may result in different activities.

Table 6.
Antimicrobial activity (minimal inhibition concentration (MIC), minimal bactericidal concentration (MBC), and minimal fungicidal concentration (MFC) mg/mL) of the studied purple bean hydroethanolic extracts in relation to biostimulant application and irrigation management.
In contrast to the antibacterial effects, the tested extracts showed great potency against various fungi, which in most cases was similar or higher than that of the positive controls (E211 and E244; Table 6). In particular, specific extracts were more effective than the positive controls in the case of Aspergillus fumigatus ATCC 9197 (TWW+), A. niger ATCC 6275 (TWW+ and CW−), A. versicolor ATCC 11730 (C and N regardless of the irrigation regime and TWW+), and Penicillium verrucosum var. cyclopium (TWW+ and NW−), while the had similar MIC values to positive controls in the case of Penicillium funicolosum ATCC 36839 (C and N treatments regardless of the irrigation regime and TWW+) and Trichoderma viride (CW+). Similar to our study, Petropoulos et al. [21] also recorded significant fungicidal effects of common bean pods and they also suggested a varied response depending on the biostimulant treatment and the harvesting stage. The exerted antimicrobial effects could be partly attributed to the polyphenols content (as in the case of NW+ of our study), as well as to the presence of other compounds with fungicidal activity such as defensins or other compounds which were not determined in our study [71,72,73].
3.6. Principal Components Analysis (PCA)
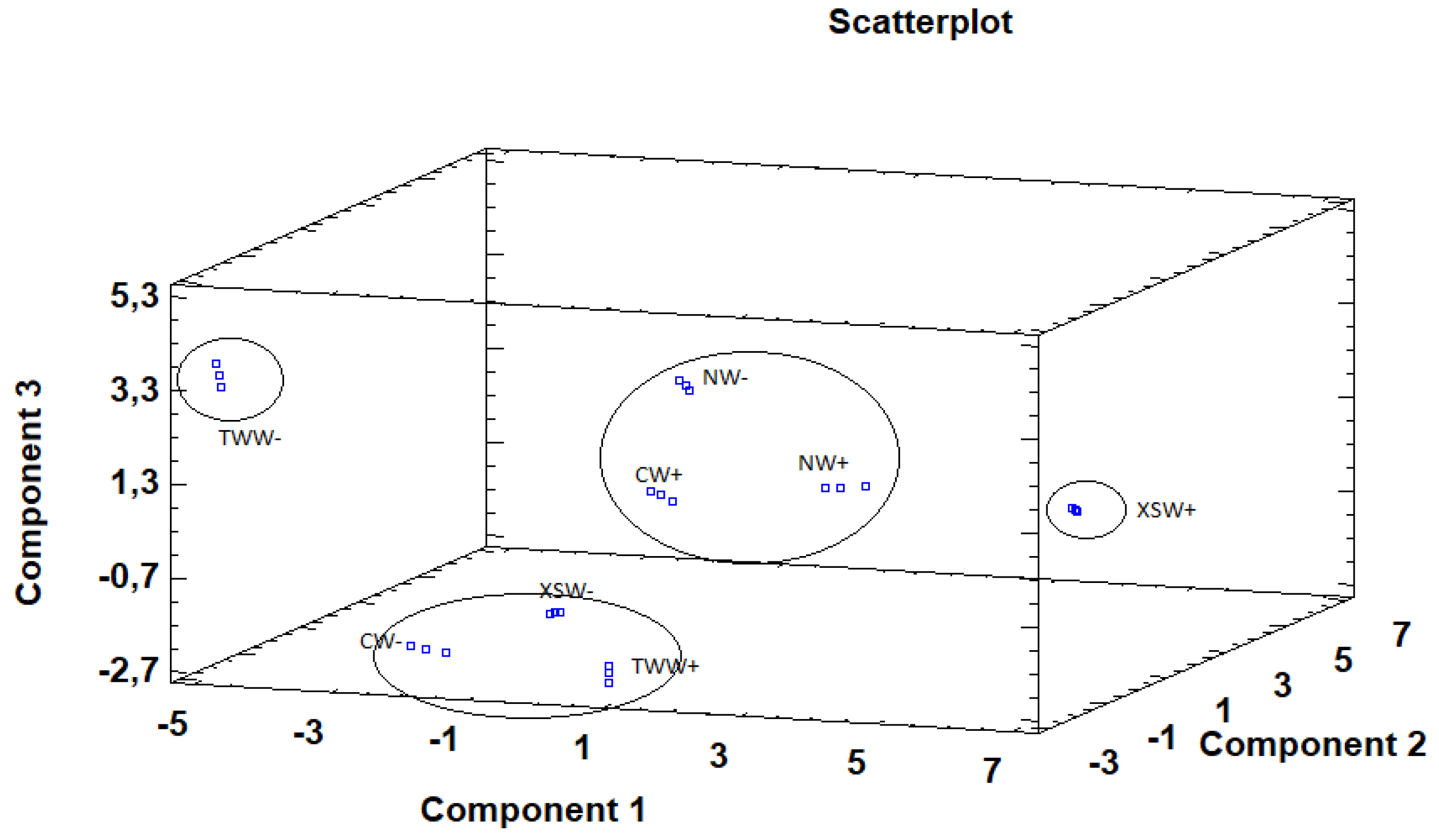
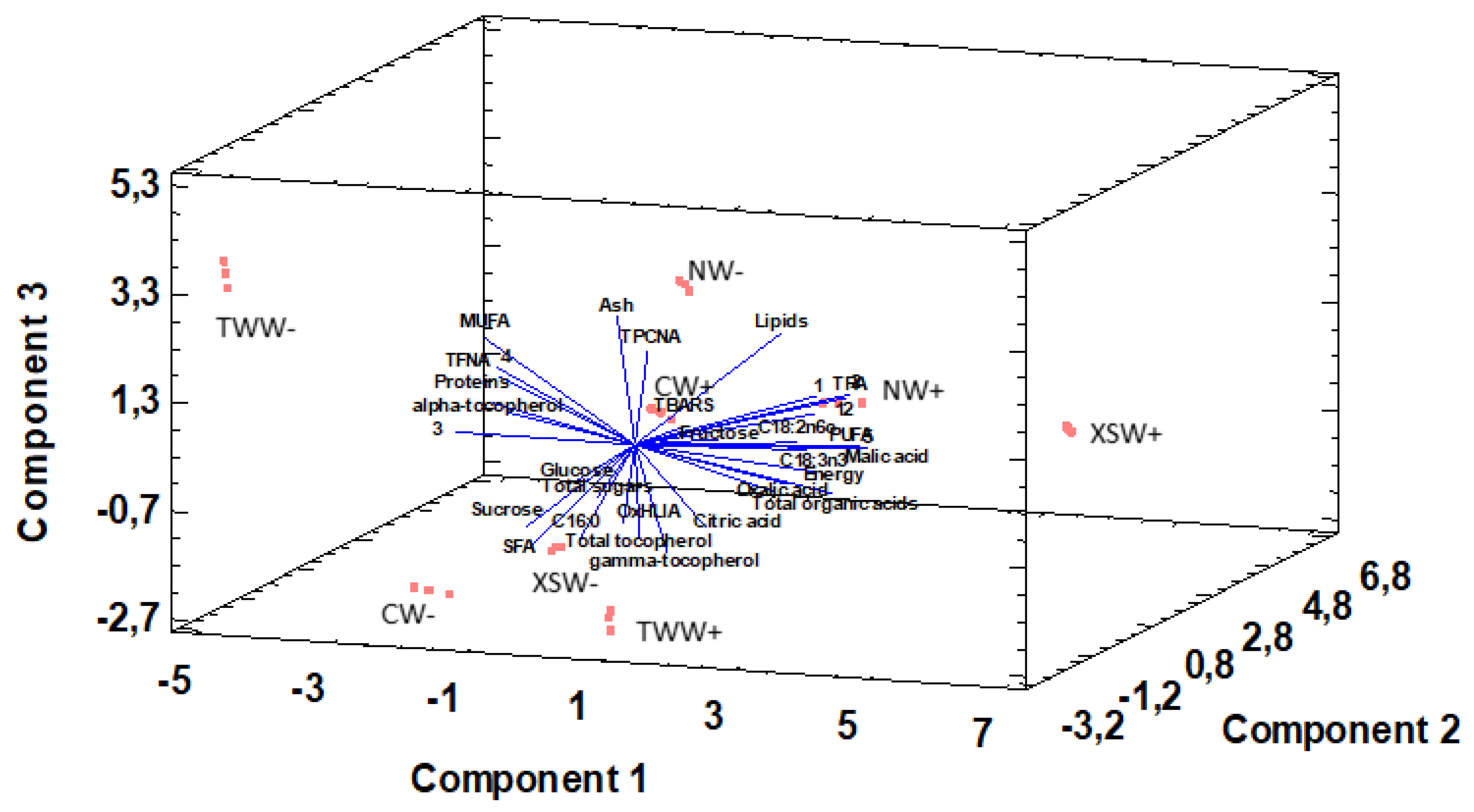
Principal component analysis (PCA) is used to reduce multivariate data complexity as a method of identifying patterns and expressing data in ways that highlight similarities and differences, and further identify groups of samples according to the biostimulant application and irrigation treatments [74,75]. Therefore, PCA results showed that the first six principal components (PCs) were associated with Eigen values higher than 1 and they explained 97.05% of the cumulative variance, while PC1 accounted for 36.4%, PC2 for 21.9%, and PC3 for 14.8% of total variance. PC1 was positively correlated to fructose, oxalic acid, malic acid, total organic acids, γ-tocopherol, linoleic acid, α-linolenic acid, PUFA, caffeoyl-O-hexoside, B-type (epi)catechin dimer, malvidin 3,5-di-O-glucoside and TPA, whereas it was negatively correlated to proteins, MUFA, (+)-Catechin and (−)-Epicatechin. Moreover, PC2 was positively correlated only to TBARS and negatively correlated to energy, fructose, glucose, sucrose, total sugars, α-tocopherol, γ-tocopherol, total tocopherols, α-linolenic acid, and TFNA. Finally, PC3 was positively correlated to lipids, ash, and MUFA, whereas it was negatively correlated to malice acid, citric acid, total organic acids, palmitic acid, and SFA. These results indicate a correct application of the PCA allowing differentiation between the tested treatments, as shown in the corresponding scatterplot (Figure 3). Moreover, the loading plot suggests that the differences in the chemical composition and antioxidant activity of the tested samples are correlated to the biostimulant application and the irrigation regime and provide four distinct groups (Figure 4). The combinations of TWW− and XSW+ are clearly distinct from the rest of the combinations, while CW−, XSW−, and TWW+ form the third distinct group and CW+, NW+, and NW− compile the final group. The presented plots suggest that MUFA, TFNA, proteins, α-tocopherol, (+)-Catechin, and (−)-Epicatechin were responsible for the discrimination of TWW− from the rest of the combinations, whereas TPA, PUFA, malic acid, total organic acids, energy, linoleic, α-linolenic acid, Caffeoyl-O-hexoside, -type (epi)catechin dimer and Malvidin 3,5-di-O-glucoside were responsible for the discrimination of XSW+. On the other hand, CW−, XSW−, and TWW+ formed a distinct group due to SFA, sucrose, palmitic acid, γ-tocopherol, total tocopherols, whereas ash, lipids, and TPCNA were responsible for the formation of a distinct group by CW+, NW+, and NW−.
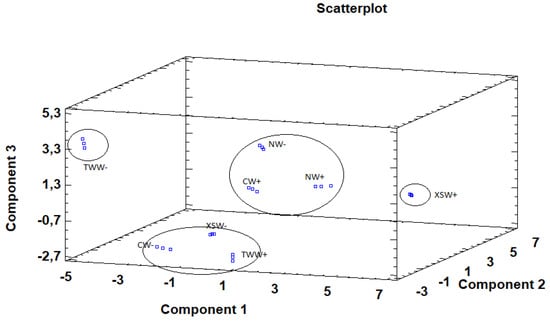
Figure 3.
3D scatter plot constructed based on the first (36.4%), second (21.9%), and third (14.8%) components using the principal components analysis (PCA) as a function of biostimulant (C: Control; N: Nomoren; TW: Twin-Antistress; XS: X-stress) and irrigation treatments (W+: normal irrigation; W−: deficit irrigation).
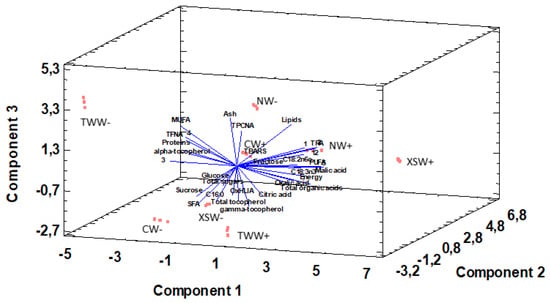
Figure 4.
Principal component loading plot of chemical composition parameters and antioxidant activity as function of biostimulant and irrigation treatments. 1: p-Coumaric acid derivative; 2: Caffeoyl-O-hexoside; 3: (+)-Catechin; 4: (−)-Epicatechin; 5: B-type (epi)catechin dimer; 12: Malvidin 3,5-di-O-glucoside; SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids; TPA: total phenolic acids; TFNA: total flavonoids non-anthocyanins; TPCNA: total phenolic compounds non-anthocyanins.
4. Conclusions
The implementation of agronomic activities, based on the use of biostimulants, is an important element of sustainable agroecological practices that goes hand in hand with non-stop research, especially on the wild plant species useful for man [76], which are compatibles with the pedological and climatic of local territory [77]. In this study, nutritional value, chemical composition and bioactivities of purple bean fresh pods were positively affected by biostimulants application, although a product specific effect was recorded depending on the irrigation regime. The application of seaweed extracts or microοrganisms (e.g., TW and N treatments of our study) are implicated in the induction of the plants’ antioxidant mechanisms through the production of antioxidant compounds as well as the increased biosynthesis of compounds with osmoregulation activity, the use of produced compounds for energy purposes or finally the production of compounds with hormone-like activity. Moreover, the positive effects of minerals application (e.g., XS treatment of our study) could be associated with the beneficial effects that minerals such as Zn may have on plants subjected to stress through the alleviation of oxidative damage as well as to the regulation of plant hormonal balance. Promising results were also recorded regarding the alleviation of negative effects of drought stress since the application of biostimulants improved specific quality parameters of purple bean fresh pods, such as the protein and phenolic compounds content and the antifungal activities of the tested pod extracts. However, considering that the observed effects were product specific, further studies are needed with more common bean genotypes in order to evaluate the application doses and the timing of application of biostimulant products and further suggest application protocols under specific conditions for common bean cultivation. Moreover, the possibility to obtain increased contents of specific compounds through the application of biostimulants should be further studied aiming to isolate these compounds in pure form for applications in the food and nutraceutical industry.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su13126869/s1, Table S1: Detailed composition and application guides for the tested biostimulants.
Author Contributions
Â.F.: formal analyses, data curation, methodology, prepared the original draft, reviewed and edited the final manuscript; S.F.: formal analysis; T.C.F.: formal analyses, data curation, reviewed and edited the final manuscript; J.P., M.I., M.S.: formal analysis; N.T.: reviewed and edited the final manuscript; I.C.F.R.F.: obtained funding, administered and supervised the project; S.A.P.: conceived and designed the research, administered and supervised the project, carried out the cultivation, wrote the original draft, reviewed and edited the final manuscript; L.B.: reviewed and edited the final manuscript, supervision, project administration, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support by national funds FCT/MCTES to CIMO (UIDB/00690/2020) and national funding by FCT, P.I., through the institutional scientific employment program-contract for L. Barros, A. Fernandes, and through the individual scientific employment program-contract for J. Pinela (CEECIND/01011/2018). The authors are also grateful to FEDER-Interreg España-Portugal programme for financial support through the project TRANSCoLAB 0612_TRANS_CO_LAB_2_P and to European Regional Development Fund (ERDF) through the Regional Operational Program North 2020, within the scope of Project Norte-01-0145-FEDER-000042: GreenHealth.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Devi, B.; Singh, G.; Dash, A.K.; Gupta, S.K. Chemically induced systemic acquired resistance in the inhibition of French bean rust. Curr. Plant Biol. 2020, 23, 100151. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Khan, A. Optimization of PGPR and silicon fertilization using response surface methodology for enhanced growth, yield and biochemical parameters of French bean (Phaseolus vulgaris L.) under saline stress. Biocatal. Agric. Biotechnol. 2020, 23, 101463. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Postel, S.L. Entering an era of water scarcity: The challenges ahead. Ecol. Appl. 2000, 10, 941–948. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 1–22. [Google Scholar] [CrossRef]
- Galvão, Í.M.; dos Santos, O.F.; de Souza, M.L.C.; de Jesus Guimarães, J.; Kühn, I.E.; Broetto, F. Biostimulants action in common bean crop submitted to water deficit. Agric. Water Manag. 2019, 225, 105762. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.; Barros, L.; Ferreira, I.C.F.R. Biostimulants application alleviates water stress effects on yield and chemical composition of greenhouse green bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Cirillo, C. Biochemical, physiological, and molecular aspects of ornamental plants adaptation to deficit irrigation. Horticulturae 2021, 7, 107. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi—From ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The effects of biostimulants, biofertilizers and water-stress on nutritional value and chemical composition of two spinach genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Biotechnol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Burity, H.A.; Martínez, C.R.; Chanway, C.P. Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl. Soil Ecol. 2008, 40, 182–188. [Google Scholar] [CrossRef]
- Rafiee, H.; Badi, H.N.; Mehrafarin, A. Application of plant biostimulants as new approach to improve the biological responses of medicinal plants—A critical review. J. Med. Plants 2016, 15, 6–39. [Google Scholar]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop J. 2016, 4, 367–376. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Taofiq, O.; Fernandes, Â.; Tzortzakis, N.; Ciric, A.; Sokovic, M.; Barros, L.; Ferreira, I.C. Bioactive properties of greenhouse-cultivated green beans Phaseolus vulgaris L.) under biostimulants and water-stress effect. J. Sci. Food Agric. 2019, 99, 6049–6059. [Google Scholar] [CrossRef]
- Rowell, D. Soil Science: Methods and Applications; Routledge: London, UK, 1994; ISBN 9780582087842. [Google Scholar]
- FAO. Standard Operating Procedure for Soil Calcium Carbonate Equivalent: Volumetric Calcimeter Method; FAO: Rome, Italy, 2020. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Agronomy Monographs; Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983; pp. 539–579. ISBN 9780891189770. [Google Scholar]
- Bremner, J.M.; Muvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G., Eds.; MD: AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011. Off. J. Eur. Union 2011, 54, 1–46. [Google Scholar]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the analysis of organic acids in thirty-five species of food and medicinal plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Obodai, M.; Mensah, D.L.N.; Fernandes, Â.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical characterization and antioxidant potential of wild Ganoderma species from Ghana. Molecules 2017, 22, 196. [Google Scholar] [CrossRef] [PubMed]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef]
- Sokovic, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Van Griensven, L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress. A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Singh, S. Enhancing phytochemical levels, enzymatic and antioxidant activity of spinach leaves by chitosan treatment and an insight into the metabolic pathway using DART-MS technique. Food Chem. 2016, 199, 176–184. [Google Scholar] [CrossRef]
- Rathinasabapathi, B. Metabolic engineering for stress tolerance: Installing osmoprotectant synthesis pathways. Ann. Bot. 2000, 86, 709–716. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Zushi, K.; Matsuzoe, N.; Kitano, M. Developmental and tissue-specific changes in oxidative parameters and antioxidant systems in tomato fruits grown under salt stress. Sci. Hortic. 2009, 122, 362–368. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Jiménez-Becker, S.; de Bélair, G. Fatty acid profiles and sn-2 fatty acid distribution of γ-linolenic acid-rich Borago species. J. Food Compos. Anal. 2018, 66, 74–80. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Adeleye, A.S.; Keller, A.A. Metabolomics Reveals Cu(OH)2 Nanopesticide-activated anti-oxidative pathways and decreased beneficial antioxidants in spinach leaves. Environ. Sci. Technol. 2017, 51, 10184–10194. [Google Scholar] [CrossRef]
- Kellős, T.; Tímár, I.; Szilágyi, V.; Szalai, G.; Galiba, G.; Kocsy, G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol. 2008, 10, 563–572. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.S.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? South African J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Liu, Z.; Xie, M. Antioxidant activities and anthocyanins composition of seed coats from twenty-six kidney bean cultivars. J. Funct. Foods 2016, 26, 622–631. [Google Scholar] [CrossRef]
- Arribas, C.; Pereira, E.; Barros, L.; Alves, M.J.; Calhelha, R.C.; Guillamón, E.; Pedrosa, M.M.; Ferreira, I.C.F.R. Healthy novel gluten-free formulations based on beans, carob fruit and rice: Extrusion effect on organic acids, tocopherols, phenolic compounds and bioactivity. Food Chem. 2019, 292, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Chen, P.X.; Zhang, H.; Marcone, M.F.; Pauls, K.P.; Liu, R.; Tang, Y.; Zhang, B.; Renaud, J.B.; Tsao, R. Anti-inflammatory effects of phenolic-rich cranberry bean (Phaseolus vulgaris L.) extracts and enhanced cellular antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2017, 38, 675–685. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S. Development and validation of ultrasound assisted extraction (UAE) and HPLC-DAD method for determination of polyphenols in dry beans (Phaseolus vulgaris). J. Food Compos. Anal. 2020, 85, 103334. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Gąsecka, M.; Spiżewski, T. Influence of biostimulants on phenolic content in broccoli heads directly after harvest and after storage. Folia Hortic. 2017, 29, 221–230. [Google Scholar] [CrossRef]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards sustainable agriculture-agronomic and economic effects of biostimulant use in common bean cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Fatemi, H.; Carvajal, M.; Rios, J.J. Foliar application of Zn alleviates salt stress symptoms of pak choi plants by activating water relations and glucosinolate synthesis. Agronomy 2020, 10, 1528. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111 Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Deng, Z.Q.; Yan, A.X.; Shah, N.P.; Lui, W.Y.; Chan, C.L.; Corke, H. Pigmented edible bean coats as natural sources of polyphenols with antioxidant and antibacterial effects. Lwt 2016, 73, 168–177. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Morelock, T. Flavonoid content and antioxidant capacity of spinach genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2008, 88, 1099–1106. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of Flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef]
- Mello, É.D.O.; Santos, I.S.; Carvalho, A.D.O.; De Souza, L.S.; Souza-filho, G.A. De Functional expression and activity of the recombinant antifungal defensin PvD1r from Phaseolus vulgaris L. (common bean) seeds. BMC Biochem. 2014, 15, 1–13. [Google Scholar]
- Mello, E.O.; Ribeiro, S.F.F.; Carvalho, A.; Santos, I.; da Cunha, M.; Santa-Catarina, C.; Gomes, V. Antifungal activity of PvD1 defensin involves plasma membrane permeabilization, inhibition of medium acidification, and induction of ROS in fungi cells. Curr. Microbiol. 2011, 62, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, B.; Fu, H.; Rao, P. Isolation of a thermostable legume chitinase and study on the antifungal activity. Appl. Microbiol. Biotechnol. 2009, 85, 313–321. [Google Scholar] [CrossRef]
- Pereira, E.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and phytochemical characterization of Arenaria Montana L. Food Funct. 2014, 5, 1848–1855. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Petropoulos, S.A.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Colla, G.; Dario Troise, A.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. The bioactive profile of lettuce produced in a closed soilless system as configured by combinatorial effects of genotype and macrocation supply composition. Food Chem. 2020, 309, 125713. [Google Scholar] [CrossRef]
- Perrino, E.V.; Musarella, C.M.; Magazzini, P. Management of grazing Italian river buffalo to preserve habitats defined by Directive 92/43/EEC in a protected wetland area on the Mediterranean coast: Palude Frattarolo, Apulia, Italy. Euro-Mediterranean J. Environ. Integr. 2021, 6. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Plants of the same place do not have the same metabolic pace: Soil properties affect differently essential oil yields of plants growing wild in semiarid Mediterranean lands. Arab. J. Geosci. 2020, 13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).