Foliar Urea with N-(n-butyl) Thiophosphoric Triamide for Sustainable Yield and Quality of Pineapple in a Controlled Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geographical Position, Climate, and Soil Condition
2.2. Plant Growth Conditions and Experimental Setup
2.3. Observations
2.3.1. Determination of Plant Growth Traits
2.3.2. Chlorophyll
2.3.3. Physical Attributes of the Fruit
2.3.4. Quality Attributes of the Fruit
Total Soluble Solids (TSS)
Titratable Acidity (TA)
Determination of Sugars and Organic Acids
Nitrogen Use
2.4. Statistical Analysis
3. Results
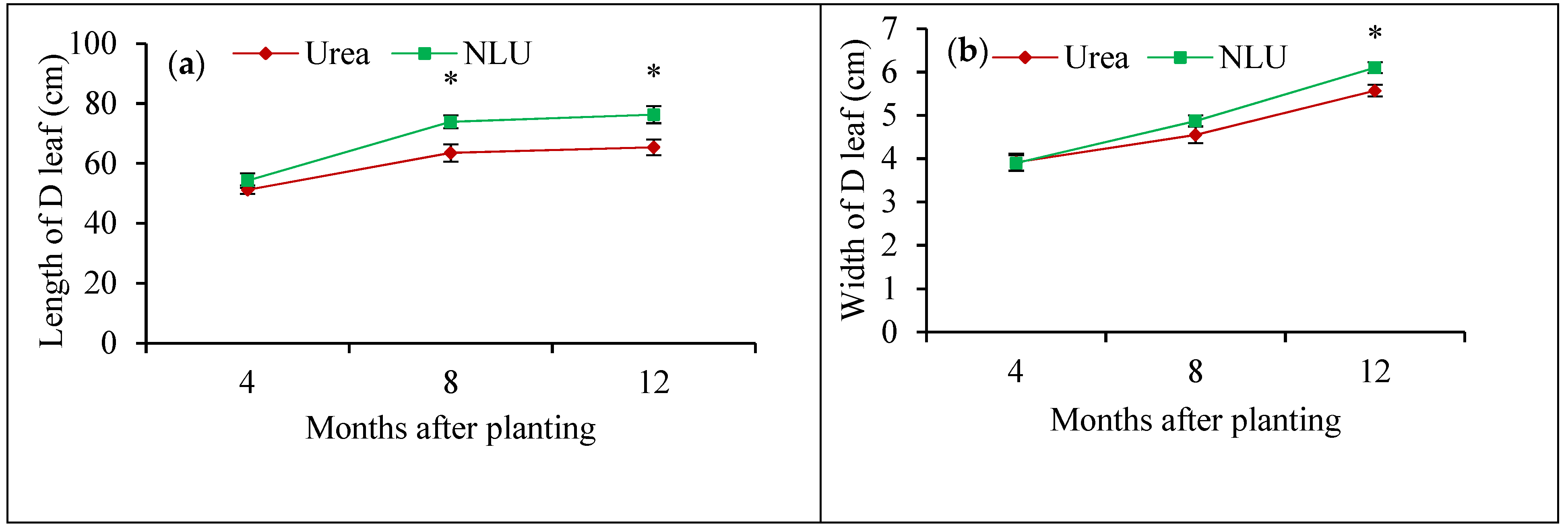
3.1. Plant Growth and N Accumulation
3.2. Nitrogen Use and Fruit Attributes
4. Discussion
4.1. Plant Growth and N Accumulation
4.2. Nitrogen Use and Fruit Attribute
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinhardt, D.H.R.C.; Bartholomew, D.P.; Souza, F.V.D.; de Carvalho, A.C.P.P.; de Pádua, T.R.P.; Junghans, D.T.; de Matos, A.P. Advances in pineapple plant propagation. Rev. Bras. Frutic. 2018, 40, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Sossa, E.L.; Agbangba, C.E.; Accalogoun, S.G.G.S.; Amadji, G.L.; Agbossou, K.E.; Hounhouigan, D.J. Residues management practices and nitrogen-potassium fertilization influence on the quality of pineapple (Ananas comosus L. Merrill) sugarloaf fruit for exportation and local consumption. Agronomy 2017, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT. Crop Statistics, Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2020. [Google Scholar]
- Herrera, J.M.; Rubio, G.; Häner, L.L.; Delgado, J.A.; Lucho-Constantino, C.A.; Islas-Valdez, S.; Pellet, D. Emerging and established technologies to increase nitrogen use efficiency of cereals. Agronomy 2016, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.E.; Chen, H.Y.; Tseng, C.S.; Tsay, Y.F. Improving nitrogen use efficiency by manipulating nitrate remobilization in plants. Nat. Plants 2020, 2. [Google Scholar] [CrossRef] [PubMed]
- Van Ut, L.; Mai, V.T.B. Effect of gibberellic acid and urea on the growth of pineapple fruit (Ananas comosus L.) Merr. Sci. Technol. Dev. J. Nat. Sci. 2019, 3. [Google Scholar] [CrossRef]
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.K.; Bayat, A.R.; Yáñez-Ruiz, D.R.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcallister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Hefley, C.; Rhoades, M.; Blaser, B.; Parker, D. Nutrient content of wheat and corn in response to the application of urea and the urease inhibitor NPBT. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, DC, USA, 16–19 July 2017; American Society of Agricultural and Biological Engineers: St. Joseph, Michigan, 2017; p. 1. [Google Scholar]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Grant, C.A.; Moulin, A.P.; Tremblay, N. Nitrogen management effects on spring wheat yield and protein concentration vary with seeding date and slope position. Agron. J. 2016, 108, 1246–1256. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; de Brito Silva, A.G. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef]
- Zuki, M.M.M.; Jaafar, N.M.; Sakimin, S.Z.; Yusop, M.K. N-(n-Butyl) thiophosphoric triamide (NBPT)-coated urea (NCU) improved maize growth and nitrogen use efficiency (NUE) in highly weathered tropical soil. Sustainability 2020, 12, 8780. [Google Scholar] [CrossRef]
- Rawluk, C.D.L.; Grant, C.A.; Racz, G.J. Ammonia volatilization from soils fertilized with urea and varying rates of urease inhibitor NBPT. Can. J. Soil Sci. 2001, 81, 239–246. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent Developments of Fertilizer Production and Use to Improve Nutrient Efficiency and Minimize Environmental Impacts, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 102, ISBN 9780123748188. [Google Scholar]
- Silva, D.R.G.; Pereira, A.F.; Dourado, R.L.; Silva, F.P.D.; Ávila, F.W.; Faquin, V. Productivity and efficiency of nitrogen fertilization in maize under different levels of urea and NBPT-treated urea. Ciência Agrotecnologia 2011, 35, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Dawar, K.; Zaman, M.; Rowarth, J.S.; Blennerhassett, J.; Turnbull, M.H. The impact of urease inhibitor on the bioavailability of nitrogen in urea and in comparison with other nitrogen sources in ryegrass (Lolium perenne L.). Crop Pasture Sci. 2010, 61, 214–221. [Google Scholar] [CrossRef]
- Chuan, L.; Zhao, T.; An, Z.; Du, L.; Li, S. Effects of a urease inhibitor NBPT on the growth and quality of rape. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 50–52. [Google Scholar]
- Silva, A.G.B.; Sequeira, C.H.; Sermarini, R.A.; Otto, R. Urease inhibitor NBPT on ammonia volatilization and crop productivity: A meta-analysis. Agron. J. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Blennerhassett, J.D.; Quin, B.F.; Zaman, M.; Ramakrishnan, C. The potential for increasing nitrogen responses using Agrotain treated urea. In Proceedings of the New Zealand Grassland Association, Wellington, New Zealand, 10 June 2006; pp. 297–301. [Google Scholar]
- Martin, R.J.; van der Weerden, T.J.; Riddle, M.U.; Butler, R.C. Comparison of Agrotain-treated and standard urea on an irrigated dairy pasture. In Proceedings of the New Zealand Grassland Association, Wellington, New Zealand, 1 January 2008; pp. 91–94. [Google Scholar]
- Zaman, M.; Nguyen, M.L.; Blennerhassett, J.D.; Quin, B.F. Reducing NH3,N2O and NO3–-N losses from a pasture soil with urease or nitrification inhibitors and elemental S-amended nitrogenous fertilizers. Biol. Fertil. Soils 2008, 44, 693–705. [Google Scholar] [CrossRef]
- Mathialagan, R.; Mansor, N.; Shamsuddin, M.R. Kinetic properties of soil urease inhibited by allicin and NBPT (N-(n-butyl) thiophosphoric triamide). AIP Conf. Proc. 2019, 2124. [Google Scholar] [CrossRef]
- Curitiba Espindula, M.; Soares Rocha, V.; Alves de Souza, M.; Campanharo, M.; de Sousa Paula, G. Rates of urea with or without urease inhibitor for topdressing wheat. Chil. J. Agric. Res. 2013, 73, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, E.M.; Oosterhuis, D.M.; Snider, J.L.; Mozaffari, M. Physiological and yield responses of field-grown cotton to application of urea with the urease inhibitor NBPT and the nitrification inhibitor DCD. Eur. J. Agron. 2012, 43, 147–154. [Google Scholar] [CrossRef]
- Espindula, M.C.; Rocha, V.S.; de Souza, M.A.; Campanharo, M.; Badaró Pimentel, A.J. Urease inhibitor (NBPT) and efficiency of single or split application of urea in wheat crop. Rev. Ceres. 2014, 61, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Shah, Z.; Rab, A.; Arif, M.; Shah, T. Effect of urease and nitrification inhibitors on wheat yield. Sarhad J. Agric. 2013, 29, 371–378. [Google Scholar]
- Rekowski, A.; Wimmer, M.A.; Hitzmann, B.; Hermannseder, B.; Hahn, H.; Zörb, C. Application of urease inhibitor improves protein composition and bread-baking quality of urea fertilized winter wheat. J. Plant Nutr. Soil Sci. 2020, 183, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yang, A.; Wang, Z.; Roelcke, M.; Chen, X.; Zhang, F.; Pasda, G.; Zerulla, W.; Wissemeier, A.H.; Liu, X. Effect of a new urease inhibitor on ammonia volatilization and nitrogen utilization in wheat in north and northwest China. Field Crop. Res. 2015, 175, 96–105. [Google Scholar] [CrossRef]
- Selim, E.M.; Elsirafy, Z.M.; Taha, A.A. Effect of irrigation methods and N-applications on the utilization of nitrogen by sugar beet grown under arid condition. Aust. J. Basic Appl. Sci. 2010, 4, 2114–2124. [Google Scholar]
- Sakimin, S.Z.; Samah, M.N.G.A.; Juraimi, A.S.; Alam, M.A.; Aslani, F. Effects of different type of fertilizers on growth and physiology of MD2 pineapple. Bangladesh J. Bot. 2017, 46, 489–495. [Google Scholar]
- Omotoso, S.O.; Akinrinde, E.A. Effect of nitrogen fertilizer on some growth, yield and fruit quality parameters in pineapple (Ananas comosus) plant at Ado-Ekiti Southwestern, Nigeria. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 11–16. [Google Scholar]
- Tewodros, M.; Mesfin, S.; Getachew, W.; Ashenafi, A.; Neim, S. Effect of Inorganic N and P Fertilizers on Fruit Yield and Yield Components of Pineapple (Annanas comosus MERR L. Var. Smooth cayanne) at Jimma, Southwest Ethiopia. Agrotechnology 2018, 07, 1–6. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Chapman, H.D. Cation exchange capacity. Agron. J. 1965, 9, 891–901. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Wiley Online Library: Hoboken, NJ, USA, 1983; Volume 9, pp. 643–698. [Google Scholar]
- Dos Santos, M.P.; Maia, V.M.; Oliveira, F.S.; Pegoraro, R.F.; Dos Santos, S.R.; Aspiazú, I. Estimation of total leaf area and d leaf area of pineapple from biometric characteristics. Rev. Bras. Frutic. 2018, 40, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Aiman, M.; Sakimin, S.Z.; Juraimi, A.S.; Ramlan, F.; Jaafar, H.Z.E.; Ali, B.; Siti, N. Effect of different water regimes and plant growth regulators on growth, physiology and yield of banana (Musa acuminata cv. Berangan) in tropical climate. Fundam. Appl. Agric. 2018, 3, 505–514. [Google Scholar]
- Fernandes, A.G.; Santos, G.M.d.; Silva, D.S.d.; Sousa, P.H.M.d.; Maia, G.A.; Figueiredo, R.W. de Chemical and physicochemical characteristics changes during passion fruit juice processing. Ciência Tecnol. Aliment. 2011, 31, 747–751. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.H.; Sun, D.Q.; Mo, Y.W.; Xi, J.G.; Sun, G.M. Effects of post-harvest salicylic acid treatment on fruit quality and anti-oxidant metabolism in pineapple during cold storage. J. Hortic. Sci. Biotechnol. 2010, 85, 454–458. [Google Scholar] [CrossRef]
- Lu, X.H.; Sun, D.Q.; Wu, Q.S.; Liu, S.H.; Sun, G.M. Physico-chemical properties, antioxidant activity and mineral contents of pineapple genotypes grown in China. Molecules 2014, 19, 8518–8532. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Huang, S.; Tian, B.; Ren, J.; Meng, Q.; Wang, P. Manipulating planting density and nitrogen fertilizer application to improve yield and reduce environmental impact in Chinese Maize production. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.B. Groundwater Quality and Nitrogen Use Efficiency in Nebraska’s Central Platte River Valley. J. Environ. Qual. 2015, 44, 449–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Wang, Z.H.; Li, S.X. Nitrate N Loss by Leaching and Surface Runoff in Agricultural Land: A Global Issue (A Review), 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 156, ISBN 9780128175989. [Google Scholar]
- Mozaffari, M.; Slaton, N.A.; Long, J.; Kelley, J.; Chlapecka, R.; Wimberley, R. Effect of urea and urea treated with Agrotain on corn grain yield in Arkansas. In AAES Research Series 558: W.E. Sabbe Arkansas Soil Fertility Studies; University of Arkansas System: Fayetteville, NC, USA, April 2008; pp. 38–40. [Google Scholar]
- Luo, L.; Pan, S.; Liu, X.; Wang, H.; Xu, G. Nitrogen deficiency inhibits cell division-determined elongation, but not initiation, of rice tiller buds. Isr. J. Plant Sci. 2017, 64, 32–40. [Google Scholar] [CrossRef]
- Dawar, K.; Zaman, M.; Rowarth, J.S.; Blennerhassett, J.; Turnbull, M.H. Urease inhibitor reduces N losses and improves plant-bioavailability of urea applied in fine particle and granular forms under field conditions. Agric. Ecosyst. Environ. 2011, 144, 41–50. [Google Scholar] [CrossRef]
- Galindo, F.S.; Filho, M.C.M.T.; Buzetti, S.; Pagliari, P.H.; Santini, J.M.K. Can NBPT urease inhibitor in combination with Azospirillum brasilense inoculation improve wheat development? Nutr. Cycl. Agroecosystems 2020, 117, 131–143. [Google Scholar] [CrossRef]
- Khan, A.; Najeeb, U.; Wang, L.; Tan, D.K.Y.; Yang, G.; Munsif, F.; Ali, S.; Hafeez, A. Planting density and sowing date strongly influence growth and lint yield of cotton crops. Field Crop. Res. 2017, 209, 129–135. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, X.; Khan, A.; Tan, D.K.Y.; Luo, H. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruchaga, S.; Artola, E.; Lasa, B.; Ariz, I.; Irigoyen, I.; Moran, J.F.; Aparicio-Tejo, P.M. Short term physiological implications of NBPT application on the N metabolism of Pisum sativum and Spinacea oleracea. J. Plant Physiol. 2011, 168, 329–336. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Saxena, P. Auxin driven indoleamine biosynthesis and the role of tryptophan as an inductive signal in Hypericum perforatum (L.). PLoS ONE 2019, 14, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Soni, K.V.; Korgaokar, S.S. Effect of nickel and zinc metal on biomass and growth rate of some pulses. Int. J. Adv. Res. 2016, 4, 1678–1689. [Google Scholar] [CrossRef]
- Gad, N.; El-Sherif, M.H.; El-Gereedly, N.H.M. Influence of Nickel on Some Physiological Aspects of Tomato Plants. Aust. J. Basic Appl. Sci. 2007, 1, 286–293. [Google Scholar]
- Uruç Parlak, K. Effect of nickel on growth and biochemical characteristics of wheat (Triticum aestivum L.) seedlings. NJAS Wageningen J. Life Sci. 2016, 76, 1–5. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Buscaglia, H.J.; Varco, J.J. Early detection of cotton leaf nitrogen status using leaf reflectance. J. Plant Nutr. 2002, 25, 2067. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Galmes, J.; Medrano, H.; Ribas-Carbó, M. Keeping a positive carbon balance under adverse conditions: Responses of photosynthesis and respiration to water stress. Physiol. Plant. 2006, 127, 343–352. [Google Scholar] [CrossRef]
- Ladjal, M.; Epron, D.; Ducrey, M. Effects of drought preconditioning on thermotolerance of photosystem II and susceptibility of photosynthesis to heat stress in cedar seedlings. Tree Physiol. 2000, 20, 1235–1241. [Google Scholar] [CrossRef] [Green Version]
- Dhir, B.; Sharmila, P.; Pardha Saradhi, P.; Nasim, S.A. Physiological and antioxidant responses of Salvinia natans exposed to chromium-rich wastewater. Ecotoxicol. Environ. Saf. 2009, 72, 1790–1797. [Google Scholar] [CrossRef]
- Zaman, M.; Zaman, S.; Adhinarayanan, C.; Nguyen, M.L.; Nawaz, S.; Dawar, K.M. Effects of urease and nitrification inhibitors on the efficient use of urea for pastoral systems. Soil Sci. Plant Nutr. 2013, 59, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.Z.; Ali, B.; Afzal, M.; Wahab, S.; Khalil, S.K.; Amin, N.; Ping, Q.; Qiaojing, T.; Zhou, W. Effects of sulfur and urease coated controlled release urea on dry matter yield, n uptake and grain quality of rice. J. Anim. Plant Sci. 2015, 25, 679–685. [Google Scholar]
- Cui, Z.; Zhang, F.; Mi, G.; Chen, F.; Li, F.; Chen, X.; Li, J.; Shi, L. Interaction between genotypic difference and nitrogen management strategy in determining nitrogen use efficiency of summer maize. Plant Soil 2009, 317, 267–276. [Google Scholar] [CrossRef]
- Khan, M.J.; Malik, A.; Zaman, M.; Khan, Q. Nitrogen use efficiency and yield of maize crop as affected by agrotain coated urea in arid calcareous soils. Soil Environ. 2014, 33. [Google Scholar]
- Venterea, R.T.; Maharjan, B.; Dolan, M.S. Fertilizer source and tillage effects on yield-scaled nitrous oxide emissions in a corn cropping system. J. Environ. Qual. 2011, 40, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Li, Y.; Hu, H. Estimating the effect of urease inhibitor on rice yield based on NDVI at key growth stages. Front. Agric. Sci. Eng. 2014, 1, 150–157. [Google Scholar] [CrossRef]
- Henning, S.W.; Branham, B.E.; Mulvaney, R.L. Response of turfgrass to urea-based fertilizers formulated to reduce ammonia volatilization and nitrate conversion. Biol. Fertil. Soils 2013, 49, 51–60. [Google Scholar] [CrossRef]
- Suter, H.; Sultana, H.; Turner, D.; Davies, R.; Walker, C.; Chen, D. Influence of urea fertiliser formulation, urease inhibitor and season on ammonia loss from ryegrass. Nutr. Cycl. Agroecosystems 2013, 95, 175–185. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, X.; Miao, Y.; Li, F.; Zhang, F.; Li, J.; Ye, Y.; Yang, Z.; Zhang, Q.; Liu, C. On-farm evaluation of winter wheat yield response to residual soil nitrate-N in North China Plain. Agron. J. 2008, 100, 1527–1534. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Darnaudery, M.; Fournier, P.; Léchaudel, M. Low-Input pineapple crops with high quality fruit: Promising impacts of locally integrated and organic fertilization compared to chemical fertilizers. Exp. Agric. 2018, 54, 286–302. [Google Scholar] [CrossRef] [Green Version]
- Alva, A.K.; Mattos Jr, D.; Quaggio, J.A. Advances in nitrogen fertigation of citrus. J. Crop Improv. 2008, 22, 121–146. [Google Scholar] [CrossRef]
- Wenqing, L.; Min, Z.; Huairui, S. The physiological effects of nitrogen on fruit trees. Shandong Nongye Daxue Xuebao 2002, 33, 96–100. [Google Scholar]
- Agbangba, C.E.; Dagbenonbakin, G.D.; Djogbenou, C.P.; Houssou, P.; Assea, E.D.; Sossa, E.L.; Kotomale, U.A.; Ahotonou, P.; Ndiaga, C.; Akpo, L.E. Influence de la fertilisation minérale sur la qualité physico-chimique et organoleptique du jus d’ananas transformé de Cayenne lisse au Bénin. Int. J. Biol. Chem. Sci. 2015, 9, 1277–1288. [Google Scholar] [CrossRef]
- Serna, M.D.; Legaz, F.; Primo-Millo, E. Improvement of the N fertilizer efficiency with dicyandiamide (DCD) in citrus trees. In Fertilizers and Environment; Springer: Berlin/Heidelberg, Germany, 1996; pp. 205–210. [Google Scholar]
- Pistón, F.; Pérez, A.G.; Sanz, C.; Refoyo, A. Relationship between sugar content and brix as influenced by cultivar and ripening stages of strawberry. Acta Hortic. 2017, 1156, 491–495. [Google Scholar] [CrossRef]
- Hajar, N.; Zainal, S.; Nadzirah, K.Z.; Roha, A.M.S.; Atikah, O.; Elida, T.Z.M.T. Physicochemical Properties Analysis of Three Indexes Pineapple (Ananas Comosus) Peel Extract Variety N36. APCBEE Procedia 2012, 4, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Chuenboonngarm, N.; Juntawong, N.; Engkagul, A.; Arirob, W.; Peyachoknakul, S. Changing in TSS, TA and sugar contents and sucrose synthase activity in ethephon-treated ‘Pattavia’ pineapple fruit. Agric. Nat. Resour. 2007, 41, 205–212. [Google Scholar]
- Zhang, Y.; Hu, C.X.; Tan, Q.L.; Zheng, C.S.; Gui, H.P.; Zeng, W.N.; Sun, X.C.; Zhao, X.H. Plant nutrition status, yield and quality of satsuma mandarin (Citrus unshiu Marc.) under soil application of Fe-EDDHA and combination with zinc and manganese in calcareous soil. Sci. Hortic. 2014, 174, 46–53. [Google Scholar] [CrossRef]
- Schaller, R.G.; Schnitzler, W.H. Nitrogen nutrition and flavour compounds of carrots (Daucus carota L) cultivated in Mitscherlich pots. J. Sci. Food Agric. 2000, 80, 49–56. [Google Scholar] [CrossRef]
- Liao, L.; Dong, T.; Qiu, X.; Rong, Y.; Wang, Z.; Zhu, J. Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit. PLoS ONE 2019, 14, e0223356. [Google Scholar] [CrossRef]
- Jakopic, J.; Veberic, R.; Zupancic, K.; Štampar, F. Influence of nitrogen on the contents of carbohydrates and organic acids in apples (Malus domestica Borkh.) cv. “Golden Delicious.”. Eur. J. Hortic. Sci. 2007, 72, 66–72. [Google Scholar]
- Jiang, N.; Jin, L.F.; Teixeira da Silva, J.A.; Islam, M.Z.; Gao, H.W.; Liu, Y.Z.; Peng, S.A. Activities of enzymes directly related with sucrose and citric acid metabolism in citrus fruit in response to soil plastic film mulch. Sci. Hortic. 2014, 168, 73–80. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef]
- Chan, H.T.; Chenchin, E.; Vonnahme, P. Nonvolatile Acids in Pineapple Juice. J. Agric. Food Chem. 1973, 21, 208–211. [Google Scholar] [CrossRef]
- Saradhuldhat, P.; Paull, R.E. Pineapple organic acid metabolism and accumulation during fruit development. Sci. Hortic. 2007, 112, 297–303. [Google Scholar] [CrossRef]
- Cámara, M.M.; Díez, C.; Torija, M.E.; Cano, M.P. HPLC determination of organic acids in pineapple juices and nectars. Z. Lebensm. Unters. Forsch. 1994, 198, 52–56. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Cronje, P.J.R.; Landahl, S.; Terry, L.A. Canopy position affects rind biochemical profile of “Nules Clementine” mandarin fruit during postharvest storage. Postharvest Biol. Technol. 2013, 86, 300–308. [Google Scholar] [CrossRef]
- Wassel, A.H.; Ahmed, F.F.; Ragab, M.A.; Ragab, M.M. Response of balady mandarin trees to drip irrigation and nitrogen fertigation, I-Effect of nitrogen fertigation and drip irrigation on the vegetative growth and the yield of balady mandarin trees (Citrus reticulata). In Proceedings of the 8th African Crop Science Society Conference, El-Minia, Egypt, 27—31 October 2007; African Crop Science Society: El-Minia, Egypt; pp. 503–511. [Google Scholar]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Miceli, A.; Miceli, C. Effect of nitrogen fertilization on the quality of swiss chard at harvest and during storage as minimally processed produce. J. Food Qual. 2014, 37, 125–134. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Textural class | Sandy clay loam |
| pH | 4.59 |
| Organic C (%) | 0.72 |
| Organic matter (%) | 1.24 |
| Total N (%) | 0.013 |
| Available phosphorus (mg kg−1) | 3.65 |
| Available potassium (mg kg−1) | 1.82 |
| Available calcium (mg kg−1) | 22.06 |
| Available magnesium (mg kg−1) | 5.58 |
| Cation-exchange capacity (cmol kg−1) | 5.51 |
| NH4+ (mg kg−1) | 2.67 |
| NO3− (mg kg−1) | 1.25 |
| Treatment | Above-Ground Fresh Biomass (kg/plant) | Above-Ground Dry Biomass (g/plant) | ||||
|---|---|---|---|---|---|---|
| 4 MAP | 8 MAP | 12 MAP | 4 MAP | 8 MAP | 12 MAP | |
| Urea | 0.70 ± 0.049 | 1.25 ± 0.043 b | 2.70 ± 0.084 b | 108.47 ± 3.32 b | 180.47 ± 6.63 b | 378.36 ± 11.80 b |
| NLU | 0.75 ± 0.045 | 1.50 ± 0.064 a | 3.12 ± 0.129 a | 115.39 ± 4.16 a | 216.68 ± 8.47 a | 417.81 ± 9.41 a |
| Significance level | NS | * | * | NS | * | * |
| Treatment | Below-Ground Fresh Biomass (g/Plant) | Below-Ground Dry Biomass (g/Plant) | ||||
|---|---|---|---|---|---|---|
| 4 MAP | 8 MAP | 12 MAP | 4 MAP | 8 MAP | 12 MAP | |
| Urea | 65.95 ± 2.51 | 140.38 ± 4.99 | 288.32 ± 11.91 | 10.37 ± 0.39 | 22.50 ± 0.93 | 35.74 ± 1.78 |
| NLU | 78.86 ± 4.95 | 149.27 ± 4.58 | 326.61 ± 7.65 | 12.40 ± 0.77 | 25.39 ± 0.88 | 41.48 ± 1.14 |
| Significance level | NS | NS | * | NS | NS | * |
| Treatment | Chlorophylla (mg cm−2) | Chlorophyllb (mg cm−2) | Chlorophyll(a+b) (mg cm−2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 MAP | 8 MAP | 12 MAP | 4 MAP | 8 MAP | 12 MAP | 4 MAP | 8 MAP | 12 MAP | |
| Urea | 3.49 ± 0.05 | 4.03 ± 0.05 | 4.52 ± 0.04 | 1.02 ± 0.80 | 1.15 ± 0.06 | 1.52 ± 0.11 | 3.94 ± 0.11 | 4.55 ± 0.09 | 5.21 ± 0.10 |
| NLU | 3.47 ± 0.06 | 4.45 ± 0.04 | 4.90 ± 0.05 | 1.05 ± 0.11 | 1.32 ± 0.08 | 1.78 ± 0.12 | 3.95 ± 0.15 | 5.04 ± 0.04 | 5.84 ± 0.11 |
| Significance level | NS | * | * | NS | NS | NS | NS | * | * |
| Treatment | Leaf Area/Plant (cm2) | Nitrogen Accumulation at 12 MAP (g/Plant) | ||
|---|---|---|---|---|
| 4 MAP | 8 MAP | 12 MAP | ||
| Urea | 780.50 ± 39.21 | 2087.78 ± 77.42 | 3255.11 ± 122.55 | 5.35 ± 0.20 |
| NLU | 846.25 ± 33.79 | 2576.89 ± 78.25 | 3745.55 ± 147.06 | 5.90 ± 0.16 |
| Significance level | NS | * | * | * |
| Parameters | Treatments | Level of Significance | |
|---|---|---|---|
| Urea | NLU | ||
| Nitrogen Use | |||
| PFPN (g g−1) | 244.08 ± 10.53 | 275.29 ± 6.27 | * |
| NUE (g g−1) | 308.97 ± 11.76 | 322.39 ± 9.66 | NS |
| Physical attributes of fruits | |||
| Fruit length (cm) | 15.95 ± 0.55 | 18.45 ± 0.53 | * |
| Fruit width (cm) | 11.46 ± 0.37 | 13.92 ± 0.61 | NS |
| Average fruit weight (cm) | 1.65 ± 0.08 | 1.90 ± 0.05 | * |
| Quality attributes of fruits | |||
| Total soluble solids (% brix) | 13.20 ± 0.44 | 15.77 ± 0.82 | * |
| Titratable acidity (% citric acid) | 0.44 ± 0.02 | 0.40 ± 0.01 | NS |
| Ascorbic acid (mg/100 g FW) | 46.24 ± 1.20 | 51.18 ± 1.49 | * |
| Juice pH | 3.78 ± 0.28 | 3.90 ± 0.24 | NS |
| Sugars | |||
| Sucrose (mg/100 g FW) | 34.12 ± 1.19 | 39.05 ± 1.14 | * |
| Glucose (mg/100 g FW) | 29.06 ± 1.04 | 29.50 ± 1.18 | NS |
| Fructose (mg/100 g FW) | 13.45 ± 0.43 | 14.84 ± 0.55 | NS |
| Total sugar content (mg/100 g FW) | 76.63 ± 2.50 | 83.39 ± 2.69 | NS |
| Organic acids | |||
| Citric acid (mg g−1 FW) | 3.24 ± 0.25 | 2.54 ± 0.15 | * |
| Malic acid (mg g−1 FW) | 1.01 ± 0.06 | 0.86 ± 0.05 | * |
| Quinic acid (mg g−1 FW) | 0.91 ± 0.05 | 0.84 ± 0.06 | NS |
| Total organic acid content (mg g−1 FW) | 5.17 ± 0.21 | 4.25 ± 0.20 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, M.A.; Sakimin, S.Z.; Ding, P.; Jaafar, N.M.; Yusop, M.K.; Sarker, B.C. Foliar Urea with N-(n-butyl) Thiophosphoric Triamide for Sustainable Yield and Quality of Pineapple in a Controlled Environment. Sustainability 2021, 13, 6880. https://doi.org/10.3390/su13126880
Haque MA, Sakimin SZ, Ding P, Jaafar NM, Yusop MK, Sarker BC. Foliar Urea with N-(n-butyl) Thiophosphoric Triamide for Sustainable Yield and Quality of Pineapple in a Controlled Environment. Sustainability. 2021; 13(12):6880. https://doi.org/10.3390/su13126880
Chicago/Turabian StyleHaque, Mohammad Amdadul, Siti Zaharah Sakimin, Phebe Ding, Noraini Md. Jaafar, Mohd Khanif Yusop, and Babul Chandra Sarker. 2021. "Foliar Urea with N-(n-butyl) Thiophosphoric Triamide for Sustainable Yield and Quality of Pineapple in a Controlled Environment" Sustainability 13, no. 12: 6880. https://doi.org/10.3390/su13126880








