Critical Leaf Water Content for Maize Photosynthesis under Drought Stress and Its Response to Rewatering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Climate and Soil Characteristics
2.2. Experimental Design
2.3. Field Management
2.4. Sampling and Measurements
2.4.1. Leaf Gas Exchange
2.4.2. Leaf Water Content
2.4.3. Maximum Carboxylation Rate at 25 °C (Vcmax25)
2.5. Statistical Analyses
3. Results
3.1. Response of Maize Leaf Water Content to Drought and Rewatering
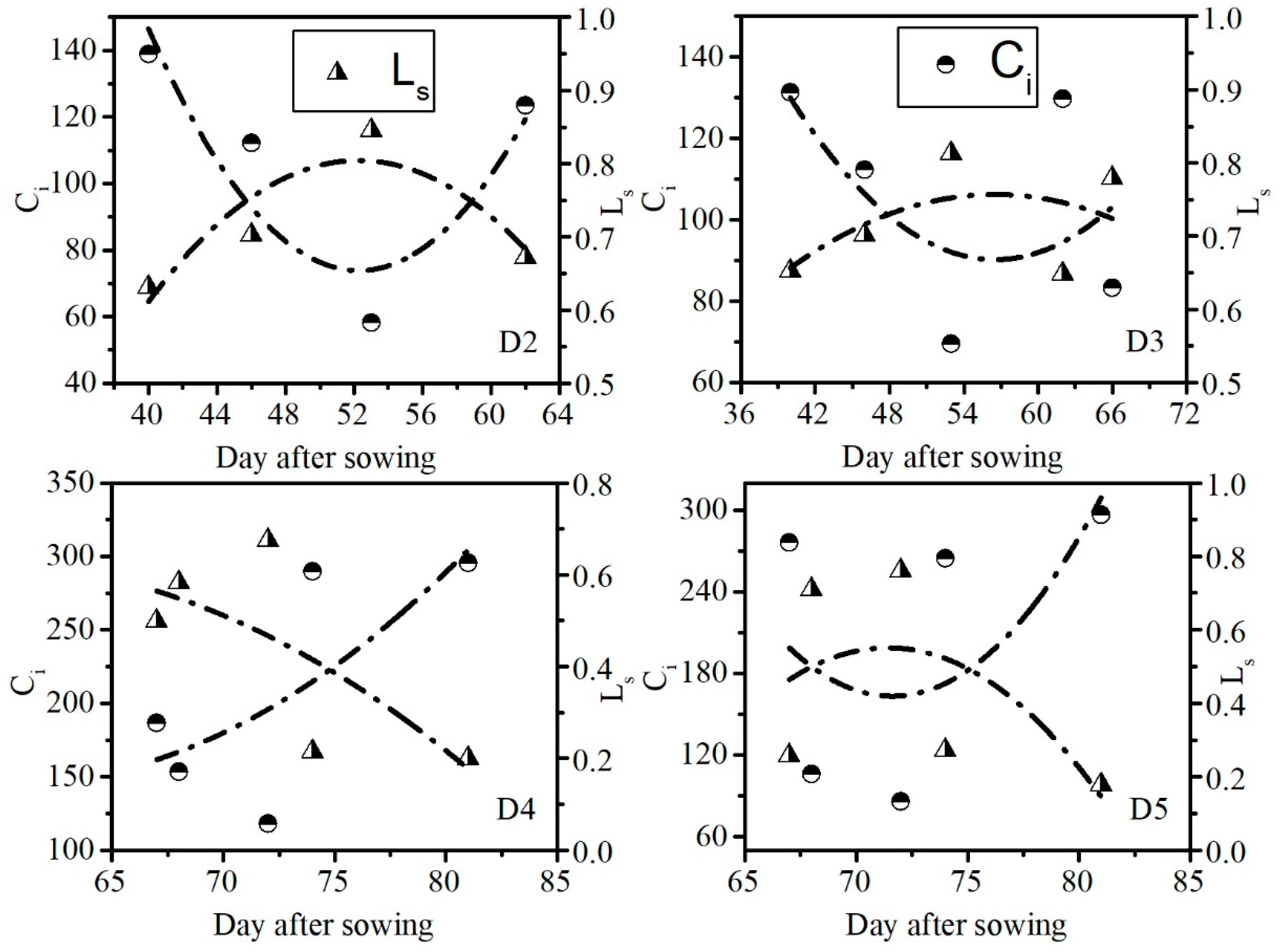
3.2. Leaf Water Content at Which Maize Photosynthesis Changed from Stomatal Limitation to Non-Stomatal Limitation
3.3. Response of Maize Vcmax25 to Drought and Rewatering
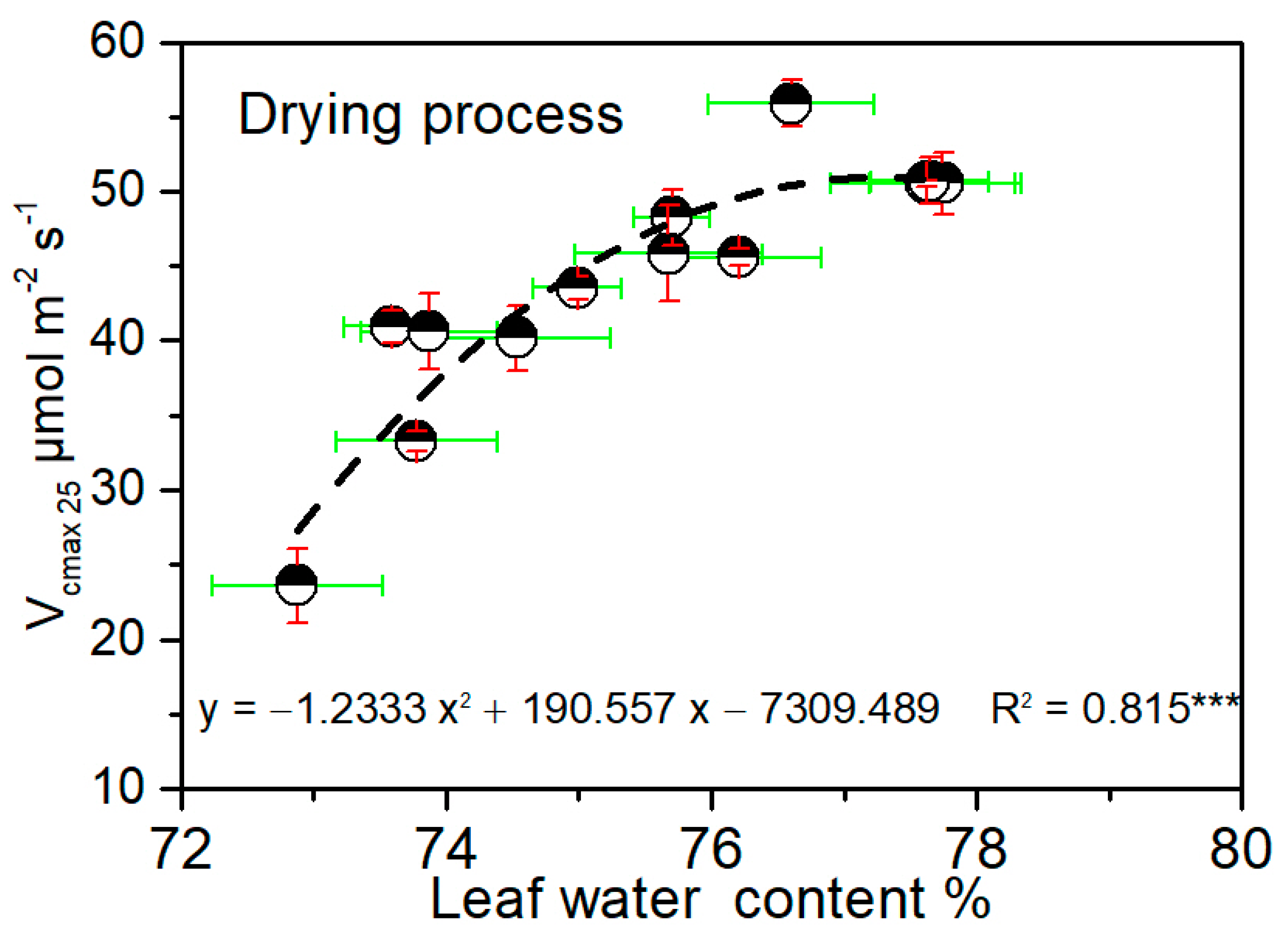
3.4. Critical Water Conditions for Maize Photosynthetic Capacity during Drought
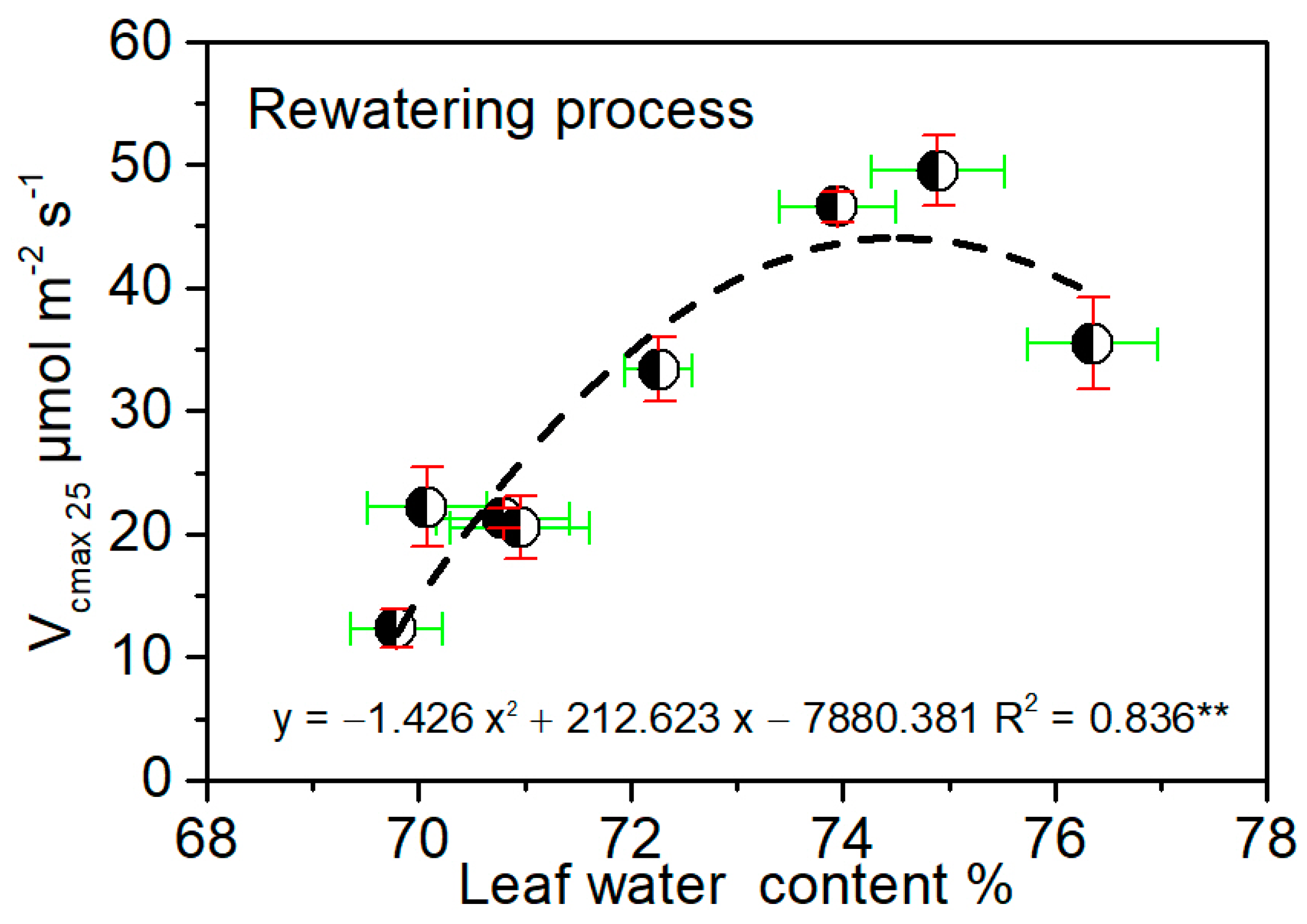
3.5. Critical Water Conditions for Maize Photosynthetic Capacity during Rewatering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, A.K.; Singh, V.P. A review of drought concepts. J. Hydrol. 2010, 391, 202–216. [Google Scholar] [CrossRef]
- Shao, D.; Chen, S.; Tan, X.; Gu, W. Drought characteristics over China during 1980-2015. Int. J. Climatol. 2018, 38, 3532–3545. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, L.; Jia, J.; Song, L.; Wang, J. Management of drought risk under global warming. Theor. Appl. Climatol. 2016, 125, 187–196. [Google Scholar] [CrossRef]
- Stringer, L.C.; Dyer, J.C.; Reed, M.S.; Dougill, A.J.; Twyman, C.; Mkwambisi, D. Adaptations to climate change, drought and desertification: Local insights to enhance policy in southern Africa. Environ. Sci. Policy 2009, 12, 748–765. [Google Scholar] [CrossRef]
- Li, Y.; Ye, W.; Wang, M.; Yan, X. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Qian, W.; Hu, Q.; Zhu, Y.; Lee, D.K. Centennial-scale dry-wet in East Asia. Clim. Dyn. 2003, 21, 77–89. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Q.; Ma, P.; Jia, J.; Wang, J. The spatial distribution characteristics of a comprehensive drought risk index in southwestern China and underlying causes. Theor. Appl. Climatol. 2016, 124, 517–528. [Google Scholar] [CrossRef]
- Campos, H.; Cooper, A.; Habben, J.E.; Edmeades, G.O.; Schussler, J.R. Improving drought tolerance in maize: A view from industry. Field Crop. Res. 2004, 90, 19–34. [Google Scholar] [CrossRef]
- Jin, Y.; Zhou, D.; Jiang, S. Comparison of soil water content and corn yield in furrow and conventional ridge sown systems in a semiarid region of China. Agric. Water Manag. 2010, 97, 326–332. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Zhou, L.; Xu, Z.; Zhou, G. Maize leaf functional responses to drought episode and rewatering. Agric. For. Meteorol. 2018, 249, 57–70. [Google Scholar] [CrossRef]
- Cheng, Z.; Meng, J.; Wang, Y. Improving spring maize yield estimation at field scale by assimilating timeseries HJ-1 CCD data into the WOFOST model using a new method with fast algorithms. Remote Sens. 2016, 8, 303. [Google Scholar] [CrossRef] [Green Version]
- Ghannoum, O. C4 photosynthesis and water stress. Ann. Bot. Lond. 2009, 103, 635–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lei, L.; Lai, J.; Zhao, H.; Song, W. Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biol. 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Oukarroum, A.; Schansker, G.; Strasser, R.J. Drought stress effects on photosystem I content and photosystem II thermo tolerance analyzed using Chla fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol. Plant. 2009, 137, 188–199. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.; Wu, X.; Zhang, H.; Chen, Y.; Wang, W. Making better maize plants for sustainable grain production in a changing climate. Front. Plant Sci. 2015, 6, 835. [Google Scholar] [CrossRef] [Green Version]
- Flexas, J.; Medrano, H. Drought-inhibition of Photosynthesis in C3 Plants: Stomatal and Non-stomatal Limitations Revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Grassi, G.; Magnani, F. Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ. 2005, 28, 834–849. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.; Luo, X.; Barlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal Behav. 2010, 5–6, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Shi, Y.; Guo, Z.; Zhang, Y.; Yu, Z. Water use and soil nitrate nitrogen changes under supplemental irrigation with nitrogen application rate in wheat field. Field Crop Res. 2015, 183, 117–125. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Wang, F.; Liu, Y.; Zahoor, R.; Jiang, D.; Dai, T. Adaptation to and recovery from drought stress at vegetative stages in wheat (Triticum aestivum) cultivars. Funct. Plant Biol. 2016, 43, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.; Passarinho, J.; Ricardo, C.P. Effect of drought and rewatering on the metabolism of Lupinus albus organs. J. Plant Physiol. 2004, 161, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, T.; Ding, S. Effect of drought and rewatering on photosynthetic physioecological characteristics of soybean. Acta Ecol. Sin. 2006, 26, 2073–2078. [Google Scholar] [CrossRef]
- Galle, A.; Florez-Sarasa, I.; Thameur, A.; De Paepe, R.; Flexas, J.; Ribas-Carbo, M. Effects of drought stress and subsequent rewatering on photosynthetic and respiratory pathways in Nicotiana sylvestris wild type and the mitochondrial complex I-deficient CMSII mutant. J. Exp. Bot. 2010, 61, 765–775. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, H.; Zhou, L.; Xu, Z.; Zhou, G. Vertical distributions of chlorophyll and nitrogen and their associations with photosynthesis under drought and rewatering regimes in a maize field. Agric. For. Meteorol. 2019, 272–273, 40–54. [Google Scholar] [CrossRef]
- Junttila, S.; Sugano, J.; Vastaranta, M.; Linnakoski, R.; Kaartinen, H.; Kukko, A.; Holopainen, M.; Hyyppa, H.; Hyyppa, J. Can leaf water content be estimated using multispectral terrestrial laser scanning? A case study with Norway spruce Seedlings. Front. Plant Sci. 2018, 9, 299. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Elsayed, S.; Hassan, W.; Dewir, Y.; Refay, Y.; Abdella, K. Potential of the existing and novel spectral reflectance indices for estimating the leaf water status and grain yield of spring wheat exposed to different irrigation rates. Agric. Water Manag. 2019, 217, 356–373. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; He, Q.; Zhou, H. Stomatal limitations to photosynthesis and their critical Water conditions in different growth stages of maize under water stress. Agric. Water Manag. 2020, 241, 106330. [Google Scholar] [CrossRef]
- Ullah, S.; Skidmore, A.K.; Naeem, M.; Schlerf, M. An accurate retrieval of leaf water content from mid to thermal infrared spectra using continuous wavelet analysis. Sci. Total Environ. 2012, 437, 145–152. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Q.; Yao, Y.; Du, S.; Zhang, C.; Li, J.; Zhao, J. Estimating and Validating Wheat Leaf Water Content with Three MODIS Spectral Indexes: A Case Study in Ningxia Plain, China. J. Agric. Sci. Technol. 2016, 18, 387–398. [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; CSIRO Publishing: Clayton, Australia, 2000. [Google Scholar]
- Massad, R.S.; Andrée, T.; Bethenod, O. The effect of temperature on C4-type leaf photosynthesis parameters. Plant Cell Environ. 2007, 30, 1191–1204. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Powers, S.J.; Keys, A.J.; Arrabaça, M.C.; Parry, M. Photorespiration in c4 grasses remains slow under drought conditions. Plant Cell Environ. 2010, 31, 925–940. [Google Scholar] [CrossRef]
- Acevedo, E.; Hsiao, T.C.; Henderson, D.W. Immediate and subsequent growth responses of Maize leaves to changes in water status. Plant Physiol. 1971, 48, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Zhao, X.; Zhou, R.; Zuo, X.; Zhang, J.; Li, Y. Physiological acclimation of two psammophytes to repeated soil drought and rewatering. Acta Physiol. Plant 2011, 33, 79–91. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Photosynthetic recovery of a perennial grass Leymus chinensis after different periods of soil drought. Plant Prod. Sci. 2007, 10, 277–285. [Google Scholar] [CrossRef]
- Asseng, S.; Ritchie, J.T.; Smucker, A.J.M.; Robertson, M.J. Root growth and water uptake during water deficit and recovering in wheat. Plant Soil 1998, 201, 265–273. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barón, M.; Bota, J.; Ducruet, J.-M.; Gallé, A.; Galmés, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandierixV. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wullschleger, S.D. Biochemical limitations to carbon assimilation in C3 plants-A retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Ju, W.; Wang, S.; Yu, G.; Zhou, Y.; Wang, H. Modeling the impact of drought on canopy carbon and water fluxes for a subtropical evergreen coniferous plantation in southern China through parameter optimization using an ensemble Kalman filter. Biogeosciences 2010, 7, 845–857. [Google Scholar] [CrossRef] [Green Version]
- Vaz, M.; Pereira, J.S.; Gazarini, L.C.; David, T.S.; David, J.S.; Rodrigues, A.; Maroco, J.; Chaves, M.M. Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiol. 2010, 30, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; He, Q.; Zhou, G. Sequence of Changes in Maize Responding to Soil Water Deficit and Related Critical Thresholds. Front. Plant Sci. 2018, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, C.; Huang, W.; Guo, X.; Li, H. Effect of soil water content on the wheat leaf water content and the physiological function. J. Triticeae Crop. 2001, 21, 42–47, (In Chinese with English Abstract). [Google Scholar]
- Ruiz-Sánchez, M.C.; Domingo, R.; Torrecillas, A.; Pérez-Pastor, A. Water stress preconditioning to improve drought resistance in young apricot plants. Plant Sci. 2000, 156, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.F.; González, M. Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nature 2012, 3, 740. [Google Scholar] [CrossRef]
- Lloret, F.; Siscart, D.; Dalmases, C. Canopy recovery after drought dieback in holm-oak Mediterranean forests of Catalonia (NE Spain). Glob. Chang. Biol. 2004, 10, 2092–2099. [Google Scholar] [CrossRef]
- Zavalloni, C.; Gielen, B.; Lemmens, C.M.H.M.; Boeck, H.D.; Blasi, S.; den Bergh, S.V.; Nijs, I.; Ceulemans, R. Does a warmer climate with frequent mild water shortages protect grassland communities against a prolonged drought? Plant Soil 2008, 308, 119–130. [Google Scholar] [CrossRef]
- Walter, J.; Nagy, L.; Hein, R.; Rascher, U.; Beierkuhnlein, C.; Willner, E.; Jentsch, A. Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 2011, 71, 34–40. [Google Scholar] [CrossRef]
- Yordanov, I.; Velikova, V.; Tsonev, T. Plant Responses to drought, acclimation, and stress tolerance. Photosynthetica 2000, 38, 171–186. [Google Scholar] [CrossRef]
- Chou, S.; Chen, B.; Chen, J.; Wang, M.; Wang, S.; Croft, H.; Shi, Q. Estimation of leaf photosynthetic capacity from the photochemical reflectance index and leaf pigments. Ecol. Indic. 2020, 110, 105867. [Google Scholar] [CrossRef]



| Treatment | DAS (Day after Sowing) | Total | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 8 | 17 | 24 | 31 | 38 | 45 | 52 | 59 | 66 | 73 | 80 | 87 | 94 | ||
| D1 | 8.7 | 0.8 | 10 | 10 | 10 | 24 | 24 | 24 | 25 | 25 | 25 | 25 | 25 | 24 | 260.5 |
| D2 | 8.7 | 0.8 | 10 | 10 | 10 | 24 | Drought | 25 | 25 | 25 | 25 | 24 | 187.5 | ||
| D3 | 8.7 | 0.8 | 10 | 10 | 10 | 24 | Drought | 25 | 25 | 24 | 137.5 | ||||
| D4 | 8.7 | 0.8 | 10 | 10 | 10 | 24 | 24 | 24 | Drought | 24 | 135.5 | ||||
| D5 | 8.7 | 0.8 | 10 | 10 | 10 | 24 | 24 | 24 | 25 | Drought | 24 | 160.5 | |||
| Parameter | Description | Value |
|---|---|---|
| (µmol m−2 s−1) | Net photosynthetic rate | Measured |
| (µmol m−2 s−1) | Leaf mitochondrial respiration | 0.01 Vcmax |
| C (µbar) | CO2 concentration in the bundle sheath | CO2 concertation in reference room |
| O (mbar) | Partial pressure of O2 | 210 |
| (bar/bar) | Half the reciprocal of Rubisco specificity | 0.000193 |
| (µmol m−2 s−1) | Maximum carboxylation rate | / |
| (µbar) | Michaelis–Menten constant of Rubisco for CO2 | 650 |
| (mbar) | Michaelis–Menten constant of Rubisco for O2 | 450 |
| Temperature correction function | / | |
| (K) | Leaf temperature | Measured |
| (kJ mol−1) | Activation energy | 58.52 |
| (JK−1 mol−1) | Gas constant | 8.31 |
| (JK−1 mol−1) | Entropy term | 710 |
| (KJ mol−1) | Deactivation energy | 220 |
| Treatments | DAS (Day after Sowing) | ||||
|---|---|---|---|---|---|
| 53 | 67 | 74 | 80 | 95 | |
| D1 | 76.5a | 76.7a | 73.5ab | 72.5a | 71.4a |
| D2 | 75.7ab | 76.4a | 74.9a | 72.3a | 69.8a |
| D3 | 75.0b | 73.7b | 72.1b | 73.9a | 70.1a |
| D4 | 75.2b | 75.8a | 73.8ab | 72.9a | 71.0a |
| D5 | 75.2b | 76.2a | 74.5a | 73.6a | 70.8a |
| Treatment | Parameter | Regression Equation | R2 |
|---|---|---|---|
| D2 | Ci | y = 0.4817 x2 – 50.367 x + 1390.4 | 0.810 |
| Ls | y = −0.0013 x2 + 0.1338 x − 2.6914 | 0.811 | |
| D3 | Ci | y = 0.145 x2 – 16.405 x + 554.14 | 0.294 |
| Ls | y = −0.0004 x2 + 0.0415 x − 0.4152 | 0.260 | |
| D4 | Ci | y = 0.3721 x2 – 44.882 x + 1498.2 | 0.514 |
| Ls | y = −0.0009 x2 + 0.1071 x − 2.5226 | 0.523 | |
| D5 | Ci | y = 1.6631 x2 – 238.25 x + 8696 | 0.344 |
| Ls | y = −0.0044 x2 + 0.6333 x − 22.067 | 0.360 |
| Treatments | DAS (Days after Sowing) | ||||
|---|---|---|---|---|---|
| 53 | 67 | 74 | 80 | 95 | |
| D1 | 58.6a | 37.7bc | 48.7a | 48.4a | 24.9a |
| D2 | 45.8c | 35.5c | 48.5a | 33.3c | 12.3c |
| D3 | 43.6c | 40.8b | 32.1c | 46.6a | 22.2ab |
| D4 | 58.4a | 48.4a | 33.3c | 23.6d | 20.5b |
| D5 | 57.3a | 45.6a | 40.2b | 40.9b | 21.3ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Zhou, G.; He, Q. Critical Leaf Water Content for Maize Photosynthesis under Drought Stress and Its Response to Rewatering. Sustainability 2021, 13, 7218. https://doi.org/10.3390/su13137218
Song X, Zhou G, He Q. Critical Leaf Water Content for Maize Photosynthesis under Drought Stress and Its Response to Rewatering. Sustainability. 2021; 13(13):7218. https://doi.org/10.3390/su13137218
Chicago/Turabian StyleSong, Xingyang, Guangsheng Zhou, and Qijin He. 2021. "Critical Leaf Water Content for Maize Photosynthesis under Drought Stress and Its Response to Rewatering" Sustainability 13, no. 13: 7218. https://doi.org/10.3390/su13137218
APA StyleSong, X., Zhou, G., & He, Q. (2021). Critical Leaf Water Content for Maize Photosynthesis under Drought Stress and Its Response to Rewatering. Sustainability, 13(13), 7218. https://doi.org/10.3390/su13137218








