Effect of Mangrove Biochar Residue Amended Shrimp Pond Sediment on Nitrogen Adsorption and Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar and Sediment
2.2. Characterization of Biochar and Sediment
2.3. Batch Adsorption Experiments
2.3.1. Effect of Contact Time
2.3.2. Effect of Solution pH
2.3.3. Effect of Biochar Dosage
2.3.4. Effect of Initial NH4+-N and NO3−-N Concentrations
2.3.5. Adsorption Isotherm Studies
2.4. Column Leaching Experiment
2.5. Statistical Analysis
3. Results
3.1. Physical and Chemical Properties of Biochar and Sediment
3.2. Adsorption
3.2.1. Effect of Contact Time
3.2.2. Effect of Solution pH
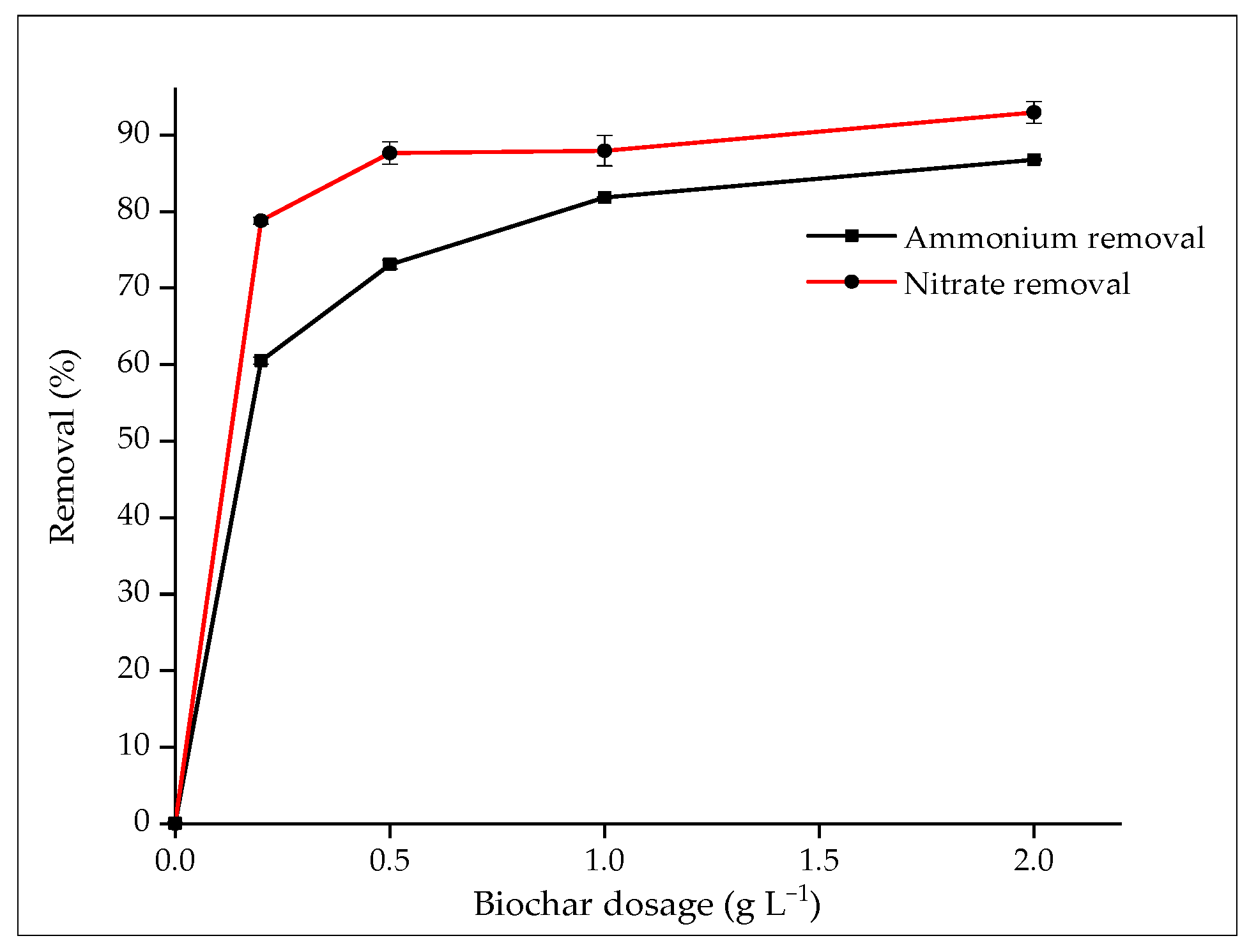
3.2.3. Effect of Biochar Dosage
3.2.4. Effect of Initial NH4+-N and NO3−-N Concentrations
3.2.5. Adsorption Isotherm
3.3. Nitrogen Leaching
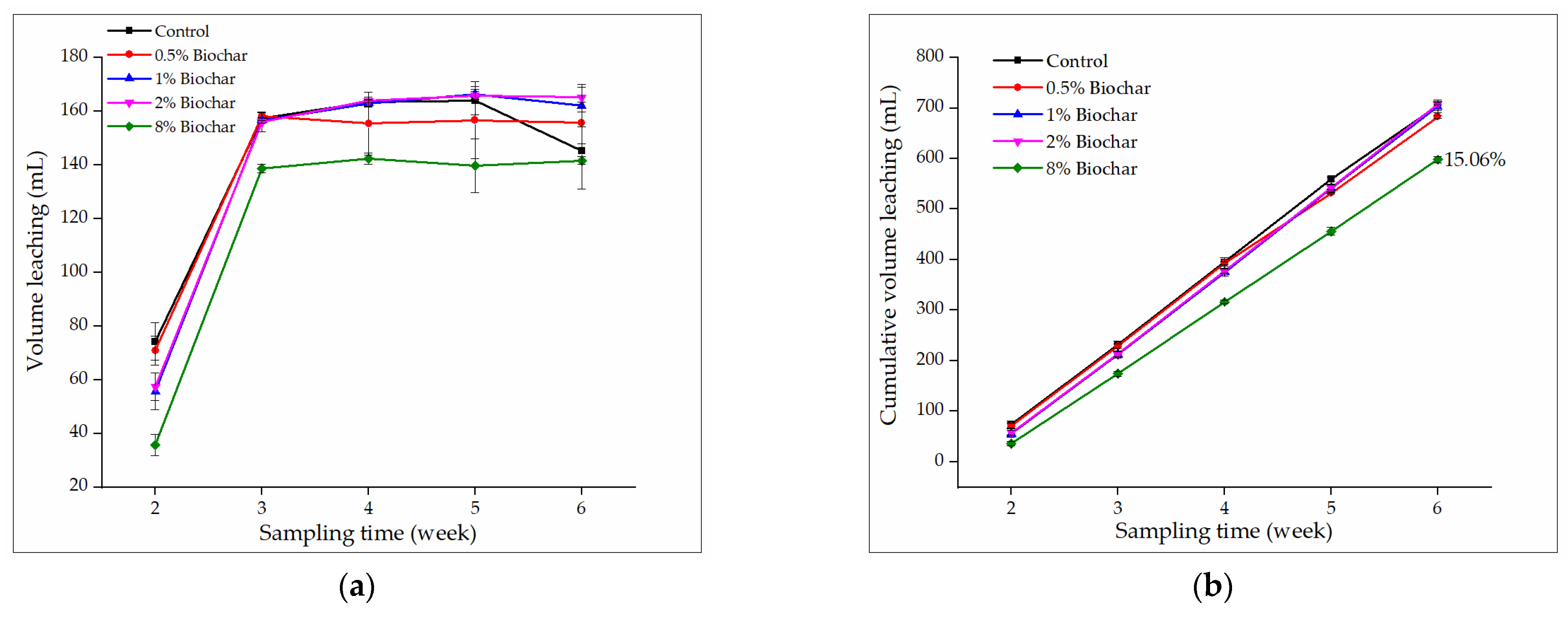
3.3.1. Effect of Biochar on Volume Leaching
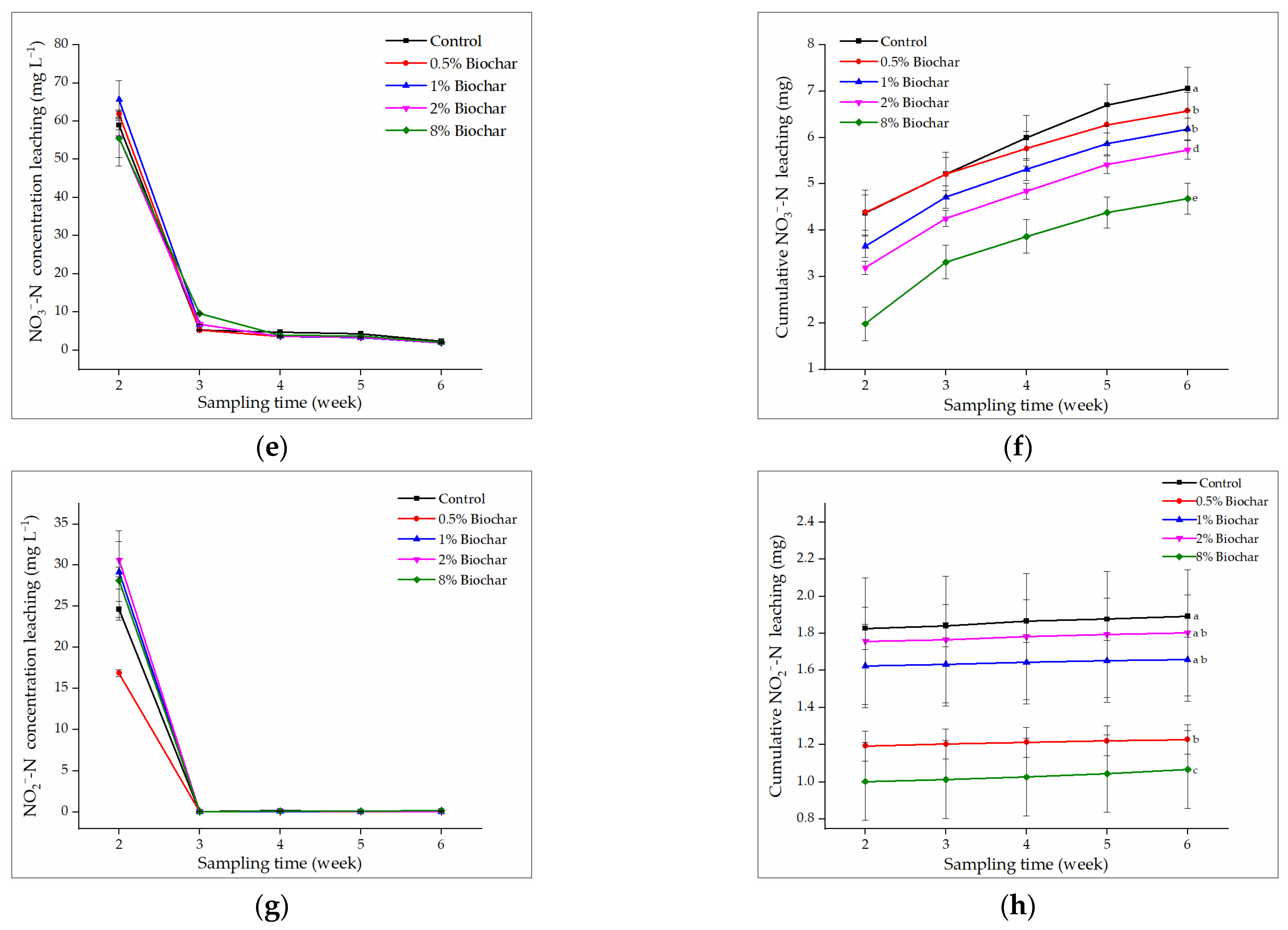
3.3.2. Effect of Biochar on TKN, NH4+-N, NO3−-N, and NO2−-N Leaching
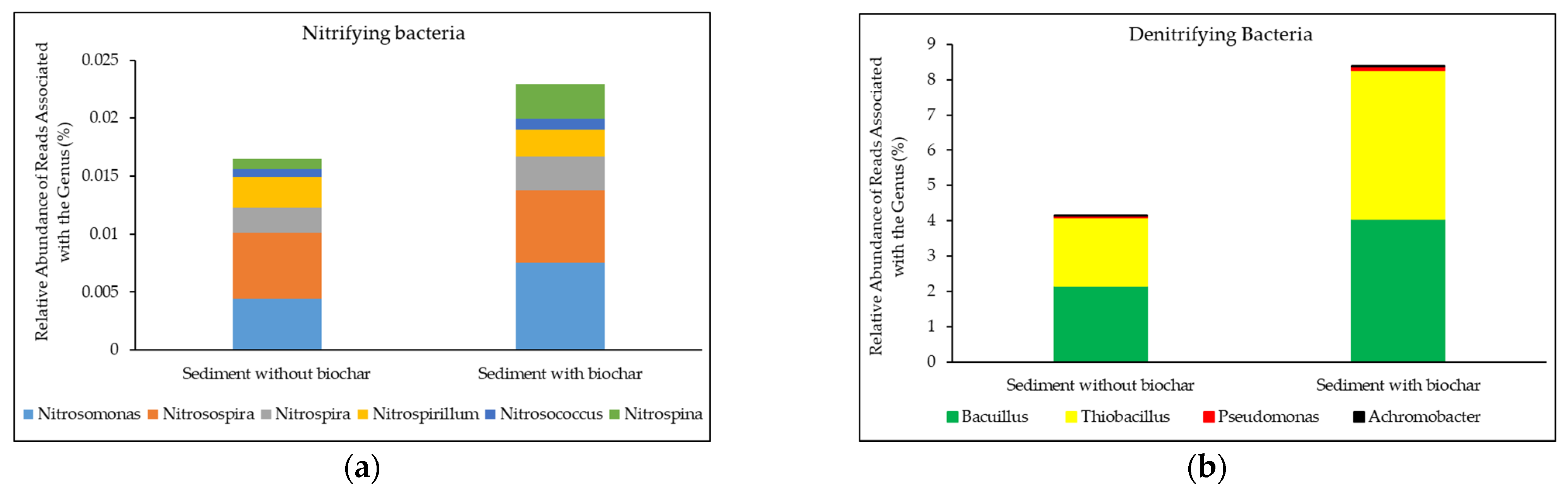
3.3.3. Effect of Biochar on Nitrifying and Denitrifying Microorganisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawlak, K.; Kołodziejczak, M. The Role of Agriculture in Ensuring Food Security in Developing Countries: Considerations in the Context of the Problem of Sustainable Food Production. Sustainability 2020, 12, 5488. [Google Scholar] [CrossRef]
- Popp, J.; Pető, K.; Nagy, J. Pesticide productivity and food security. A review. Agron. Sustain. Dev. 2013, 33, 243–255. [Google Scholar] [CrossRef]
- Campos, E.V.R.; De Oliveira, J.L.; Fraceto, L.F. Applications of Controlled Release Systems for Fungicides, Herbicides, Acaricides, Nutrients, and Plant Growth Hormones: A Review. Adv. Sci. Eng. Med. 2014, 6, 373–387. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- Thakur, I.S.; Medhi, K. Nitrification and denitrification processes for mitigation of nitrous oxide from waste water treatment plants for biovalorization: Challenges and opportunities. Bioresour. Technol. 2019, 282, 502–513. [Google Scholar] [CrossRef]
- Plaimart, J.; Acharya, K.; Mrozik, W.; Davenport, R.J.; Vinitnantharat, S.; Werner, D. Coconut husk biochar amendment enhances nutrient retention by suppressing nitrification in agricultural soil following anaerobic digestate application. Environ. Pollut. 2021, 268, 115684. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Ye, C.; Zhu, P.; Ba, Q.; Pang, J.; Shu, L. Effects of biochar application on the abundance and community composition of denitrifying bacteria in a reclaimed soil from coal mining subsidence area. Sci. Total Environ. 2018, 625, 1218–1224. [Google Scholar] [CrossRef]
- Yin, Q.; Ren, H.; Wang, R.; Zhao, Z. Evaluation of nitrate and phosphate adsorption on Al-modified biochar: Influence of Al content. Sci. Total Environ. 2018, 631–632, 895–903. [Google Scholar] [CrossRef]
- Viglašová, E.; Galamboš, M.; Diviš, D.; Danková, Z.; Daňo, M.; Krivosudský, L.; Lengauer, C.L.; Matik, M.; Briančin, J.; Soja, G. Engineered biochar as a tool for nitrogen pollutants removal: Preparation, characterization and sorption study. Desalination Water Treat. 2020, 191, 318–331. [Google Scholar] [CrossRef]
- Hu, L.; Li, S.; Li, K.; Huang, H.; Wan, W.; Huang, Q.; Li, Q.; Li, Y.; Deng, H.; He, T. Effects of Two Types of Straw Biochar on the Mineralization of Soil Organic Carbon in Farmland. Sustainability 2020, 12, 10586. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, R.; Zhao, Z. Application of Mg–Al-modified biochar for simultaneous removal of ammonium, nitrate, and phosphate from eutrophic water. J. Clean. Prod. 2018, 176, 230–240. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Iwata, Y.; Shiono, T. Influences of feedstock and pyrolysis temperature on the nitrate adsorption of biochar. Soil Sci. Plant Nutr. 2016, 62, 180–184. [Google Scholar] [CrossRef]
- Pratiwi, E.P.A.; Hillary, A.K.; Fukuda, T.; Shinogi, Y. The effects of rice husk char on ammonium, nitrate and phosphate retention and leaching in loamy soil. Geoderma 2016, 277, 61–68. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.-X.; Wu, W.-X.; Shi, D.-Z.; Yang, M.; Zhong, Z.-K. Evaluation of Biochar Effects on Nitrogen Retention and Leaching in Multi-Layered Soil Columns. Water Air Soil Pollut. 2010, 213, 47–55. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Shen, F.; Yang, G.; Zhang, Y.; Zeng, Y.; Wang, L.; Xiao, H.; Deng, S. Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere 2015, 119, 646–653. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Husson, O.; Brunet, A.; Babre, D.; Charpentier, H.; Durand, M.; Sarthou, J.-P. Conservation Agriculture systems alter the electrical characteristics (Eh, pH and EC) of four soil types in France. Soil Tillage Res. 2018, 176, 57–68. [Google Scholar] [CrossRef]
- Hollister, C.C.; Bisogni, J.J.; Lehmann, J. Ammonium, Nitrate, and Phosphate Sorption to and Solute Leaching from Biochars Prepared from Corn Stover (Zea mays L.) and Oak Wood (Quercus spp.). J. Environ. Qual. 2013, 42, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and Nitrogen Adsorption Capacities of Biochars Derived from Feedstocks at Different Pyrolysis Temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Si, L.; Xie, Y.; Ma, Q.; Wu, L. The Short-Term Effects of Rice Straw Biochar, Nitrogen and Phosphorus Fertilizer on Rice Yield and Soil Properties in a Cold Waterlogged Paddy Field. Sustainability 2018, 10, 537. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yan, W.; Shangguan, Z. Effect of biochar application method on nitrogen leaching and hydraulic conductivity in a silty clay soil. Soil Tillage Res. 2018, 183, 100–108. [Google Scholar] [CrossRef]
- Saiya, H.G.; Katoppo, D.R. Journal of Degraded and Mining Lands Management. J. Degrad. Min. Lands Manag. 2015, 3, 423–432. [Google Scholar] [CrossRef]
- Nimrat, S.; Suksawat, S.; Maleeweach, P.; Vuthiphandchai, V. Effect of different shrimp pond bottom soil treatments on the change of physical characteristics and pathogenic bacteria in pond bottom soil. Aquaculture 2008, 285, 123–129. [Google Scholar] [CrossRef]
- Adesuyi, A.; Ngwoke, M.O.; Akinola, M.O.; Njoku, K.L.; Jolaoso, A.O. Assessment of Physicochemical Characteristics of Sediment from Nwaja Creek, Niger Delta, Nigeria. J. Geosci. Environ. Prot. 2016, 4, 16–27. [Google Scholar] [CrossRef]
- Ockenden, M.C.; Deasy, C.; Quinton, J.N.; Surridge, B.; Stoate, C. Keeping agricultural soil out of rivers: Evidence of sediment and nutrient accumulation within field wetlands in the UK. J. Environ. Manag. 2014, 135, 54–62. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, B.; Hao, H.; Zhou, H.; Lu, J. Nitrogen and phosphorus in sediments in China: A national-scale assessment and review. Sci. Total Environ. 2017, 576, 840–849. [Google Scholar] [CrossRef]
- Avnimelech, Y. Shrimp and fish pond soils: Processes and management. Aquaculture 2003, 220, 549–567. [Google Scholar] [CrossRef]
- Bu, X.; Xue, J.; Zhao, C.; Wu, Y.; Han, F. Nutrient Leaching and Retention in Riparian Soils as Influenced by Rice Husk Biochar Addition. Soil Sci. 2017, 182, 241–247. [Google Scholar] [CrossRef]
- Wasis, B.; Ghaida, S.; Winata, B. Application of coconut shell charcoal and NPK fertilizer toward Acacia mangium growth on the soil of ex-limestone mining in Bogor, Indonesia. Arch. Agric. Environ. Sci. 2019, 4, 75–82. [Google Scholar] [CrossRef]
- Sun, H.; Feng, Y.; Ji, Y.; Shi, W.; Yang, L.; Xing, B. N2O and CH4 emissions from N-fertilized rice paddy soil can be mitigated by wood vinegar application at an appropriate rate. Atmos. Environ. 2018, 185, 153–158. [Google Scholar] [CrossRef]
- Kridiborworn, P.; Chidthaisong, A.; Yuttitham, M.; Tripetchkul, S. Carbon sequestration by mangrove forest planted specifically for charcoal production in Yeesarn, Samut Songkram. J. Sustain. Energy Environ. 2012, 3, 87–92. [Google Scholar]
- Sivakumarana, S.S.; Sivakumaranc, S.; Jeyakumarb, P.; Mellisho, C.D.; Salasd, M.D.; McIvora, I.; Clothiera, B. Effect of charcoal (biochar) amendments in Manawatu sandy-loam soil (New Zealand) on white clover growth and nodulation. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Sahu, S.C.; Kumar, M.; Ravindranath, N.H. Carbon Stocks in Natural and Planted Mangrove forests of Mahanadi Mangrove Wetland, East Coast of India. Curr. Sci. 2016, 110, 2253–2260. [Google Scholar] [CrossRef]
- Topp, G.C.; Parkin, G.W.; Ferre, P.A. Soil water content. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; Canadian Society of Soil Science: Boca Raton, FL, USA, 2008; pp. 939–947. [Google Scholar]
- Mclean, E.O. Soil pH (hydrogen ion activity). In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 200–209. [Google Scholar]
- Rhoades, J.D. Soluble salts. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 167–173. [Google Scholar]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 149–157. [Google Scholar]
- Walkey, A. A critical examination of a rapid method for determining organic carbon in soil—Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1947, 63, 64–257. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis Part 2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall Inc.: New York, NY, USA, 1958; 498p. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Anderson, J.; Ingram, J. A Handbook of Methods; CAB International: Wallingford, UK, 1993; 221p. [Google Scholar]
- Tahir, H.; Sultan, M.; Qadir, Z. Physiochemical Modification and Characterization of Bentonite Clay and Its Application for the Removal of Reactive Dyes. Int. J. Chem. 2013, 5, 19. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’Ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Xu, S.; Yu, W.; Liu, S.; Xu, C.; Li, J.; Zhang, Y. Adsorption of Hexavalent Chromium Using Banana Pseudostem Biochar and Its Mechanism. Sustainability 2018, 10, 4250. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Sun, L.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.Q.; Chen, S.; Wang, X.L.; Guo, S.; Qiu, Y.F.; Di Liu, Y.; Duan, X.L.; Yu, Y.J. Wheat straw biochar-supported nanoscale zerovalent iron for removal of trichloroethylene from groundwater. PLoS ONE 2017, 12, e0172337. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.; Lee, C.W.; Lee, W.M.; Woo, S.H. The Cell Viability on Kelp and Fir Biochar and the Effect on the Field Cultivation of Corn. Clean Technol. 2016, 22, 29–34. [Google Scholar] [CrossRef][Green Version]
- Viglašová, E.; Galamboš, M.; Danková, Z.; Krivosudský, L.; Lengauer, C.L.; Hood-Nowotny, R.; Soja, G.; Rompel, A.; Matík, M.; Briančin, J. Production, characterization and adsorption studies of bamboo-based biochar/montmorillonite composite for nitrate removal. Waste Manag. 2018, 79, 385–394. [Google Scholar] [CrossRef]
- Naik, D.K.; Monika, K.; Prabhakar, S.; Parthasarathy, R.; Satyavathi, B. Pyrolysis of sorghum bagasse biomass into bio-char and bio-oil products. J. Therm. Anal. Calorim. 2017, 127, 1277–1289. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A. Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol. Eng. 2016, 95, 101–111. [Google Scholar] [CrossRef]
- Halim, A.A.; Latif, M.T.; Ithnin, A. Ammonia removal from aqueous solution using organic acid modified activated carbon. World Appl. Sci. J. 2013, 24, 1–6. [Google Scholar] [CrossRef]
- Zhu, K.; Fu, H.; Zhang, J.; Lv, X.; Tang, J.; Xu, X. Studies on removal of NH4+-N from aqueous solution by using the activated carbons derived from rice husk. Biomass Bioenergy 2012, 43, 18–25. [Google Scholar] [CrossRef]
- Kucić, D.; Cosić, I.; Vuković, M.; Briski, F. Sorption kinetic studies of ammonium from aqueous solution on different inorganic and organic media. Acta Chim. Slov. 2013, 60, 109–119. [Google Scholar]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef]
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Papiernik, S.K.; Malo, D.D.; Clay, D.E.; Kumar, S.; Gulbrandson, D.W. Nitrate sorption and desorption in biochars from fast pyrolysis. Microporous Mesoporous Mater. 2013, 179, 250–257. [Google Scholar] [CrossRef]
- Olgun, A.; Atar, N.; Wang, S. Batch and column studies of phosphate and nitrate adsorption on waste solids containing boron impurity. Chem. Eng. J. 2013, 222, 108–119. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Nasab, S.B.; Gholami, R.M.; Moradzadeh, M.; Izadpanah, Z.; Hafshejani, S.B.; Bhatnagar, A. Removal of zinc and lead from aqueous solution by nanostructured cedar leaf ash as biosorbent. J. Mol. Liq. 2015, 211, 448–456. [Google Scholar] [CrossRef]
- Ahmadi, S.; Igwegbe, C.A. Adsorptive removal of phenol and aniline by modified bentonite: Adsorption isotherm and kinetics study. Appl. Water Sci. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, J.; Hu, M. Adsorption of ammonium from aqueous solutions on environmentally friendly barbecue bamboo charcoal: Characteristics and kinetic and thermodynamic studies. Environ. Prog. Sustain. Energy 2014, 34, 655–662. [Google Scholar] [CrossRef]
- Panda, H.; Tiadi, N.; Mohanty, M.; Mohanty, C. Studies on adsorption behavior of an industrial waste for removal of chromium from aqueous solution. S. Afr. J. Chem. Eng. 2017, 23, 132–138. [Google Scholar] [CrossRef]
- Inam, E.; Etim, U.; Akpabio, E.; Umoren, S. Process optimization for the application of carbon from plantain peels in dye abstraction. J. Taibah Univ. Sci. 2017, 11, 173–185. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, Y.; Long, L.; Hu, X. Adsorption of nitrate onto biochar derived from agricultural residuals. Water Sci. Technol. 2018, 77, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, F.G.; Zhuravel, A.; Silva, F.; Amaro, A.; Ben-Hur, M.; Keizer, J.J. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma 2019, 347, 194–202. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Lee, X.; Chen, Y.; Gao, W.; Pan, W.; Tang, Y. Effects of biochar-based fertilizers on nutrient leaching in a tobacco-planting soil. Acta Geochim. 2018, 38, 1–7. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, X.; Wang, S.; Xing, G.; Zhou, Y. Effects of the addition of rice-straw-based biochar on leaching and retention of fertilizer N in highly fertilized cropland soils. Soil Sci. Plant. Nutr. 2013, 59, 771–782. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Bi, Q.-F.; Chen, Q.-H.; Yang, X.-R.; Li, H.; Zheng, B.-X.; Zhou, W.-W.; Liu, X.-X.; Dai, P.-B.; Li, K.-J.; Lin, X.-Y. Effects of combined application of nitrogen fertilizer and biochar on the nitrification and ammonia oxidizers in an intensive vegetable soil. AMB Express 2017, 7, 198. [Google Scholar] [CrossRef]
- Prommer, J.; Wanek, W.; Hofhansl, F.; Trojan, D.; Offre, P.; Urich, T.; Schleper, C.; Sassmann, S.; Kitzler, B.; Soja, G.; et al. Biochar Decelerates Soil Organic Nitrogen Cycling but Stimulates Soil Nitrification in a Temperate Arable Field Trial. PLoS ONE 2014, 9, e86388. [Google Scholar] [CrossRef]
- Luo, S.; He, B.; Song, D.; Li, T.; Wu, Y.; Yang, L. Response of Bacterial Community Structure to Different Biochar Addition Dosages in Karst Yellow Soil Planted with Ryegrass and Daylily. Sustainability 2020, 12, 2124. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Dippold, M.; Gao, Y.; Blagodatskaya, E.; Kuzyakov, Y. Biochar affects soil organic matter cycling and microbial functions but does not alter microbial community structure in a paddy soil. Sci. Total Environ. 2016, 556, 89–97. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]







| Properties | Biochar | Sediment |
|---|---|---|
| Moisture (%) | 8.51 ± 0.40 | 56.66 ± 0.82 |
| pH (1:1) | 6.95 ± 0.07 | 7.97 ± 0.01 |
| EC (1:5) (dS m−1) | 1.26 ± 0.01 | 1.53 ± 0.01 |
| OM (%) | 16.55 ± 0.35 | 1.53 ± 0.02 |
| TKN (%) | 0.58 ± 0.02 | 0.10 ± 0.01 |
| CEC (cmol kg−1) | 17.77 ± 2.63 | 16.85 ± 1.37 |
| Available K (mg kg−1) | 1286.51 ± 2.42 | 761.67 ± 1.27 |
| Available p (mg kg−1) | 1719.15 ± 3.66 | 39.85 ± 0.44 |
| NH4+-N (mg kg−1) | 5.76 ± 0.34 | 40.05 ± 0.75 |
| NO3−-N (mg kg−1) | 8.29 ± 0.16 | 9.25 ± 0.21 |
| Data | This Study | [48] | [50] | [51] | [52] | [52] |
|---|---|---|---|---|---|---|
| BET surface area (m2 g−1) | 11.74 | 4.98 | 2.46 | 155.7 | 34.9 | 26.6 |
| Cumulative volume (cm3 g−1) | 0.0065 | 0.00959 | 0.0029 | 0.12 | 0.0158 | 0.0174 |
| Average pore width (nm) | 5.5 | 10.39 | 4.47 | 8.4 | 1.81 | 2.62 |
| Absorbent | Mangrove biochar residue | Banana pseudostem biochar | Palm bark biochar | Wheat straw biochar | Fir wood pellet biochar | Kelp seaweed biochar |
| Model | Constant | Ammonium | Nitrate | |||||
|---|---|---|---|---|---|---|---|---|
| This Experiment | [59] | [66] | This Experiment | [10] | [55] | [69] | ||
| Langmuir | qmax (mg g−1) | 7.96 | 71.94 | 20 | 7.32 | 90.74 | 28.21 | 14.46 |
| KL (L mg−1) | 0.64 | 5.895 × 10−3 | 0.5 | 0.14 | 0.003 | 0.13 | 0.0014 | |
| R2 | 0.9773 | 0.994 | 0.3414 | 0.9806 | 0.967 | 0.99 | 0.968 | |
| Freundlich | KF (mg g−1) | 3.14 | 0.6604 | 12.9152 | 1.75 | 4.285 | 5.82 | 0.249 |
| n (g L−1) | 1.75 | 0.6725 | 1.18 | 1.59 | 2.553 | 2.59 | 0.503 | |
| R2 | 0.9971 | 0.985 | 0.9346 | 0.9958 | 0.935 | 0.98 | 0.917 | |
| Temkin | A (L g−1) | 5.09 × 10−3 | – | 0.0121 | 1.89 | – | – | – |
| b (kJ mol−1) | 2.15 | – | 2.5943 | 14.06 | – | – | – | |
| R2 | 0.9238 | – | 0.8474 | 0.9688 | – | – | – | |
| Adsorbent | Mangrove biochar residue | Rice husks biochar | Barbecue bamboo biochar | Mangrove biochar residue | Poplar chips biochar modified with AlCl3 | Modified sugarcane bagasse biochar | Corncobs biochar | |
| Condition | ||||||||
| Temperature (°C) | 25 | 25 | 25 | 25 | 25 | 22 | 30 | |
| Biochar dosage (g L−1) | 2 | 1 | 0.4 | 2 | 0.1 | 2 | 0.1 | |
| Concentration (mg L−1) | 2–10 | 250–1400 | 2–80 | 2–10 | 50 | 1–100 | 0–2000 | |
| pH | 5.5 | 7 | 9 | 5 | 6 | 4.64 | – | |
| Contact time (min) | 240 | 1200 | 2880 | 240 | 1440 | 1440 | 1440 | |
| Speed (rpm) | 180 | 120 | 120 | 180 | 120 | 120 | 150 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Be, S.; Vinitnantharat, S.; Pinisakul, A. Effect of Mangrove Biochar Residue Amended Shrimp Pond Sediment on Nitrogen Adsorption and Leaching. Sustainability 2021, 13, 7230. https://doi.org/10.3390/su13137230
Be S, Vinitnantharat S, Pinisakul A. Effect of Mangrove Biochar Residue Amended Shrimp Pond Sediment on Nitrogen Adsorption and Leaching. Sustainability. 2021; 13(13):7230. https://doi.org/10.3390/su13137230
Chicago/Turabian StyleBe, Sokkeang, Soydoa Vinitnantharat, and Anawat Pinisakul. 2021. "Effect of Mangrove Biochar Residue Amended Shrimp Pond Sediment on Nitrogen Adsorption and Leaching" Sustainability 13, no. 13: 7230. https://doi.org/10.3390/su13137230
APA StyleBe, S., Vinitnantharat, S., & Pinisakul, A. (2021). Effect of Mangrove Biochar Residue Amended Shrimp Pond Sediment on Nitrogen Adsorption and Leaching. Sustainability, 13(13), 7230. https://doi.org/10.3390/su13137230








