Investigation of the Electrochemical Breakdown Response in Sensitised AA5083 Aluminium Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
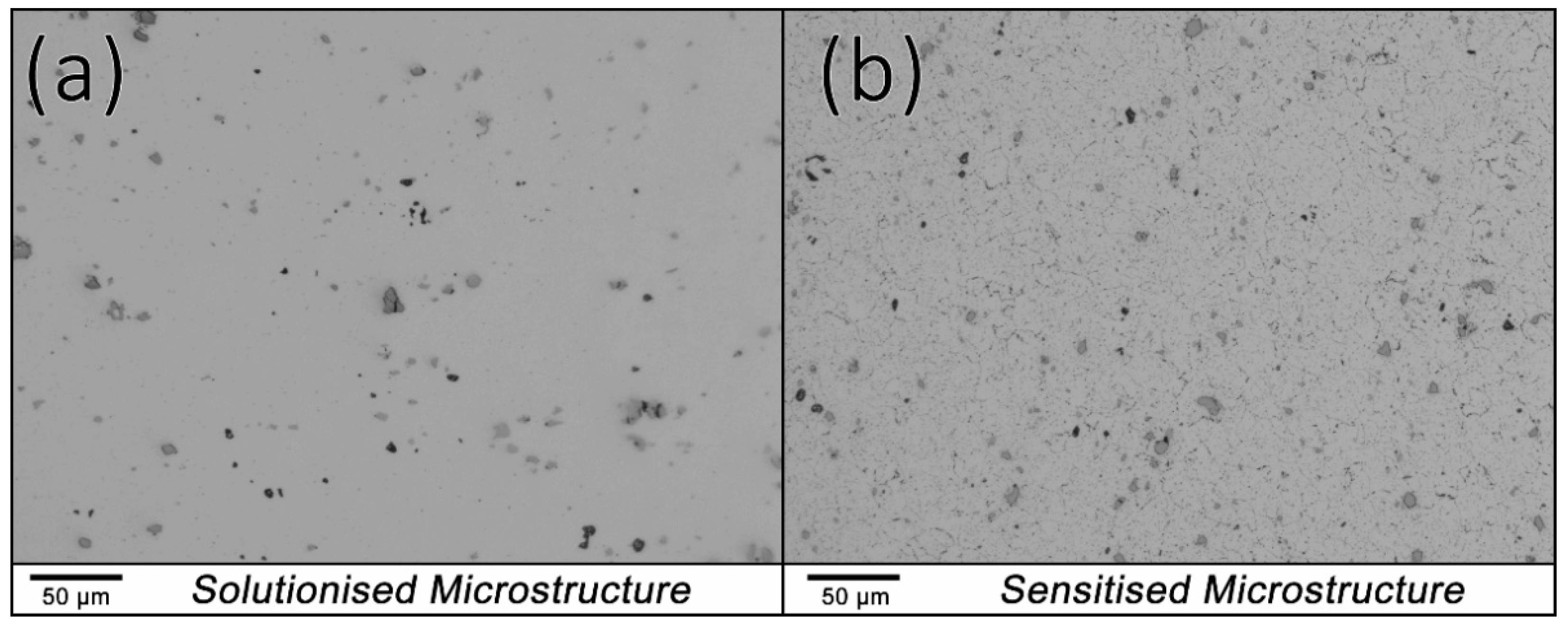
3.1. Microstructural Analysis—Degree of Sensitisation
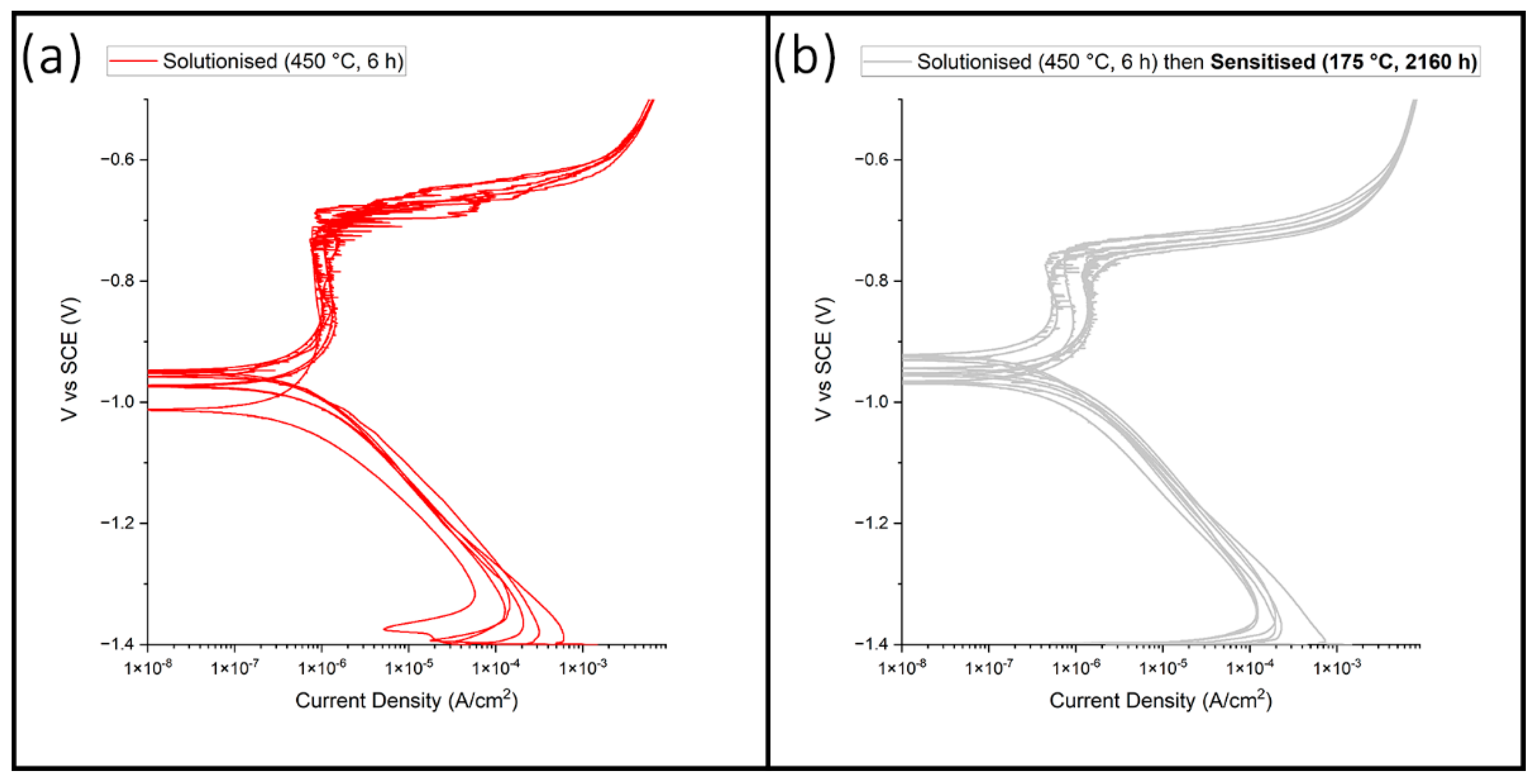
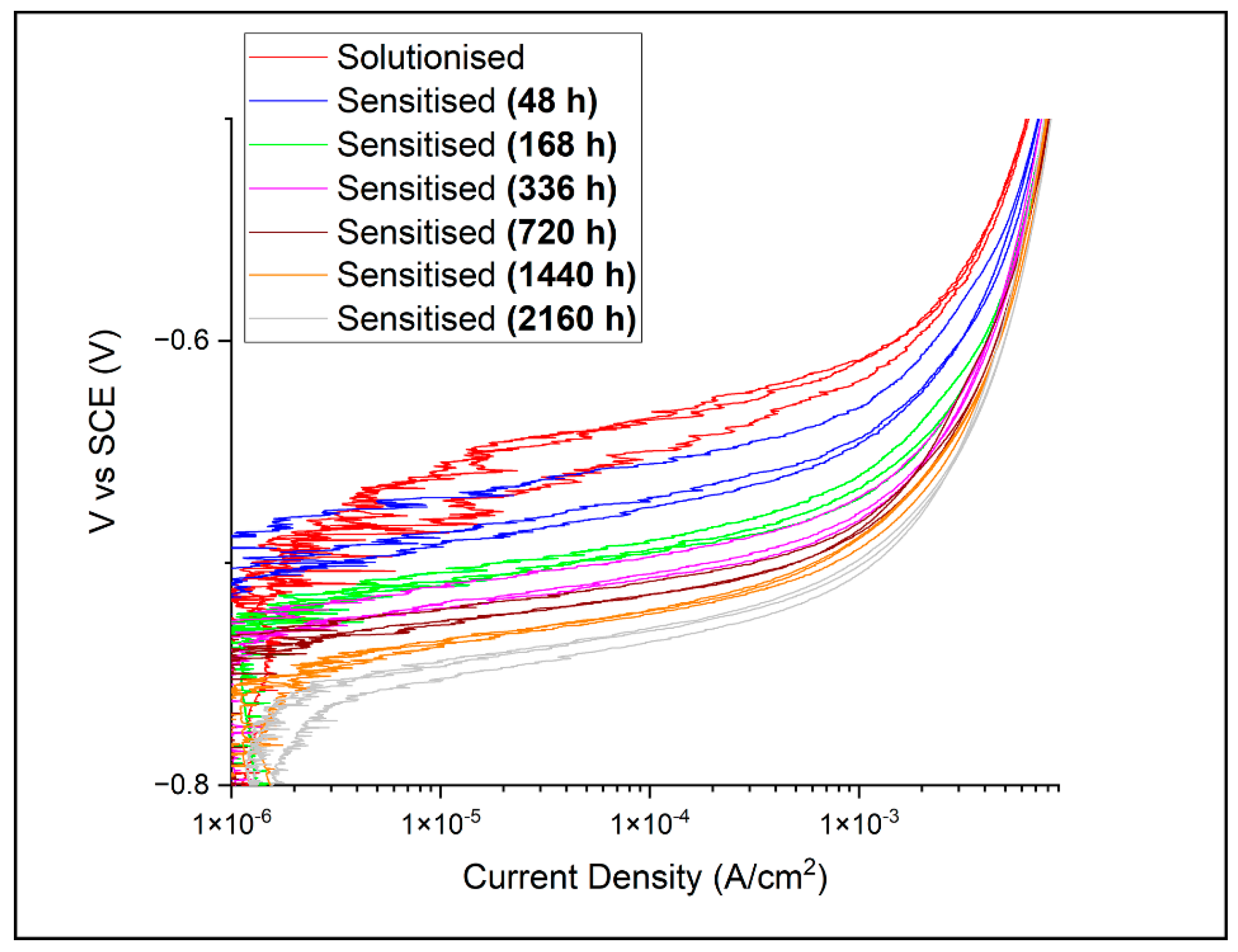
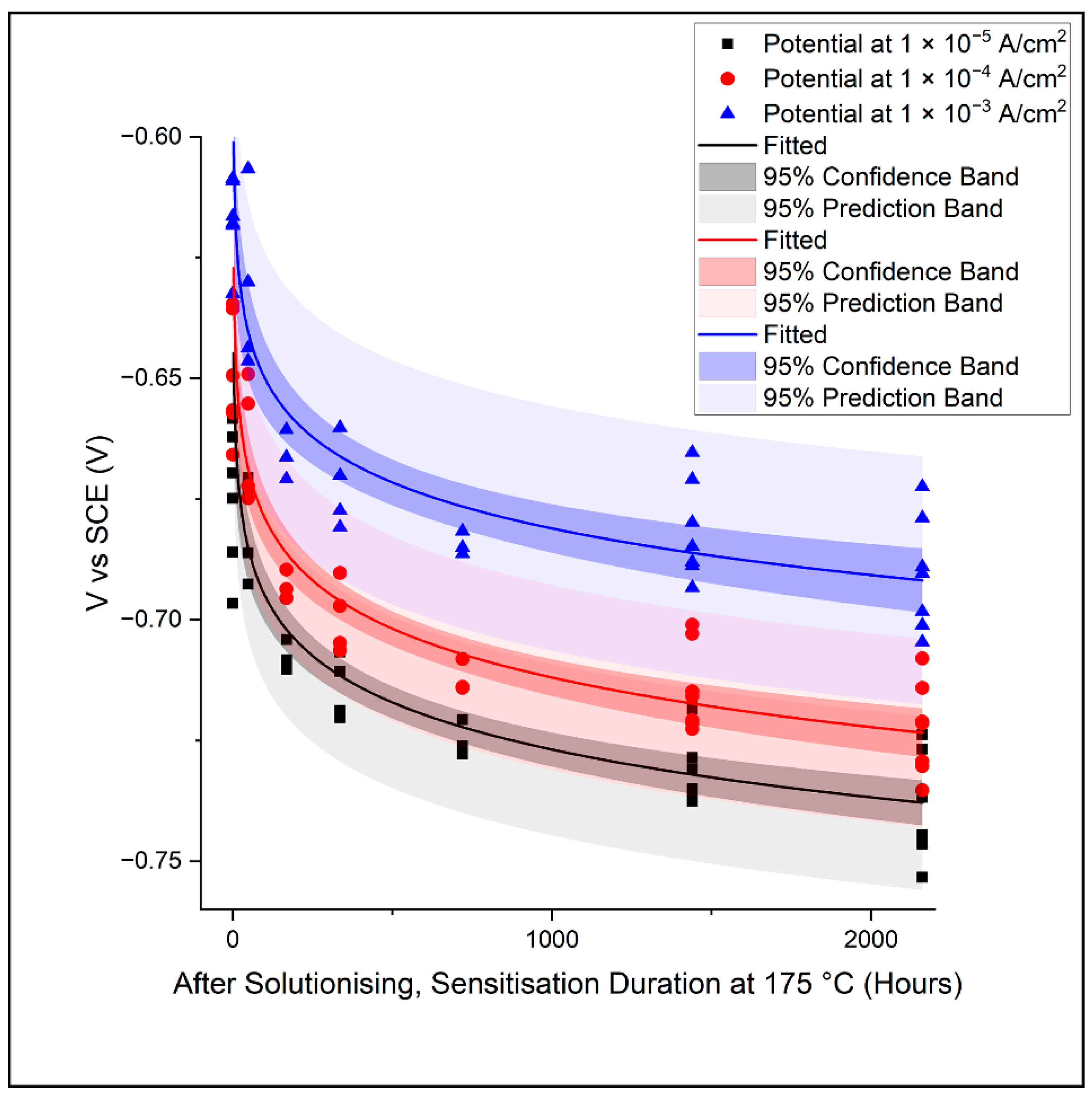
3.2. Electrochemical Potentiodynamic Polarisation Scans
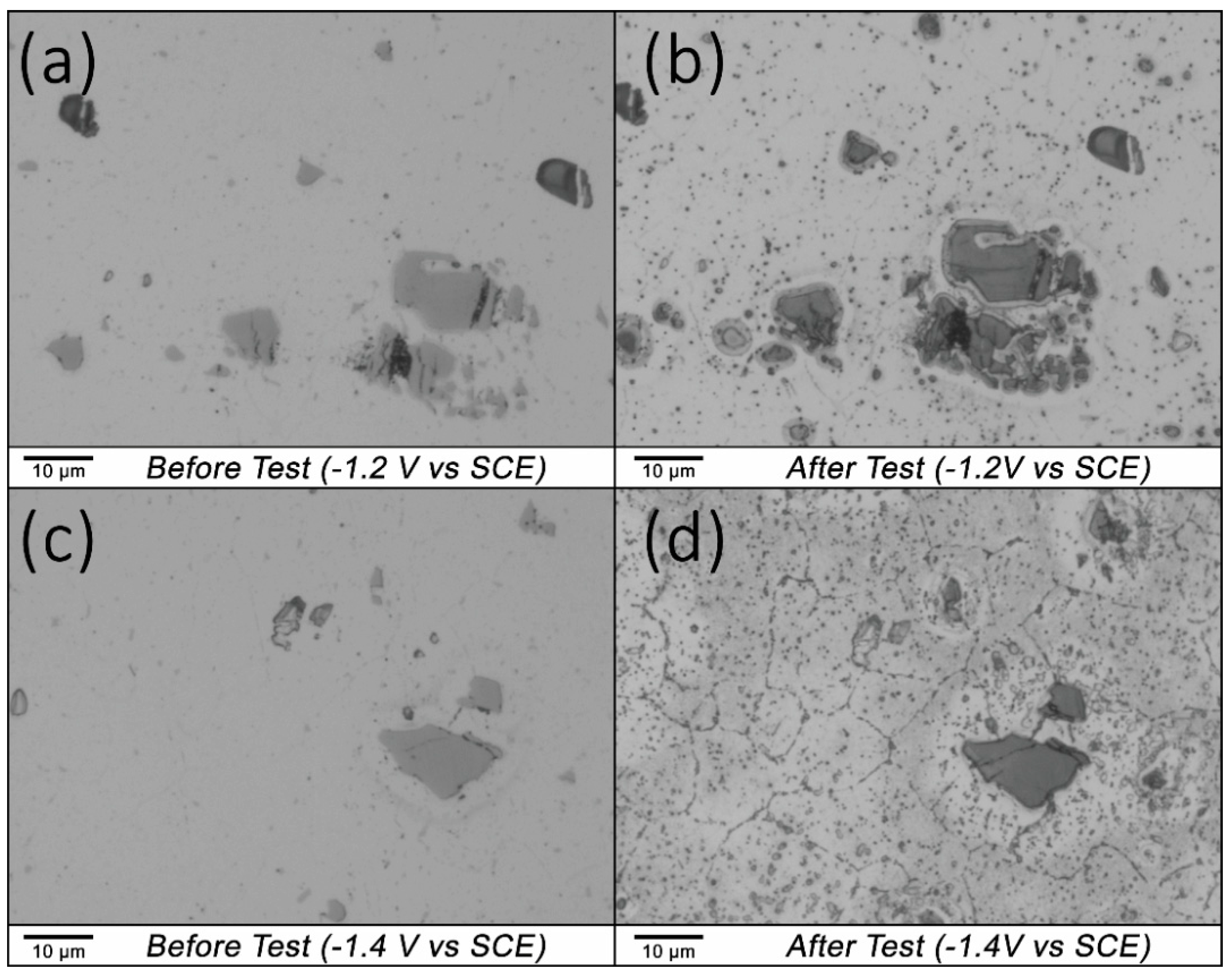
3.3. Microstructural Analysis—Electrochemical Potentiostatic Polarisation
4. Discussion
4.1. Possible Factors Contributing to Breakdown Response
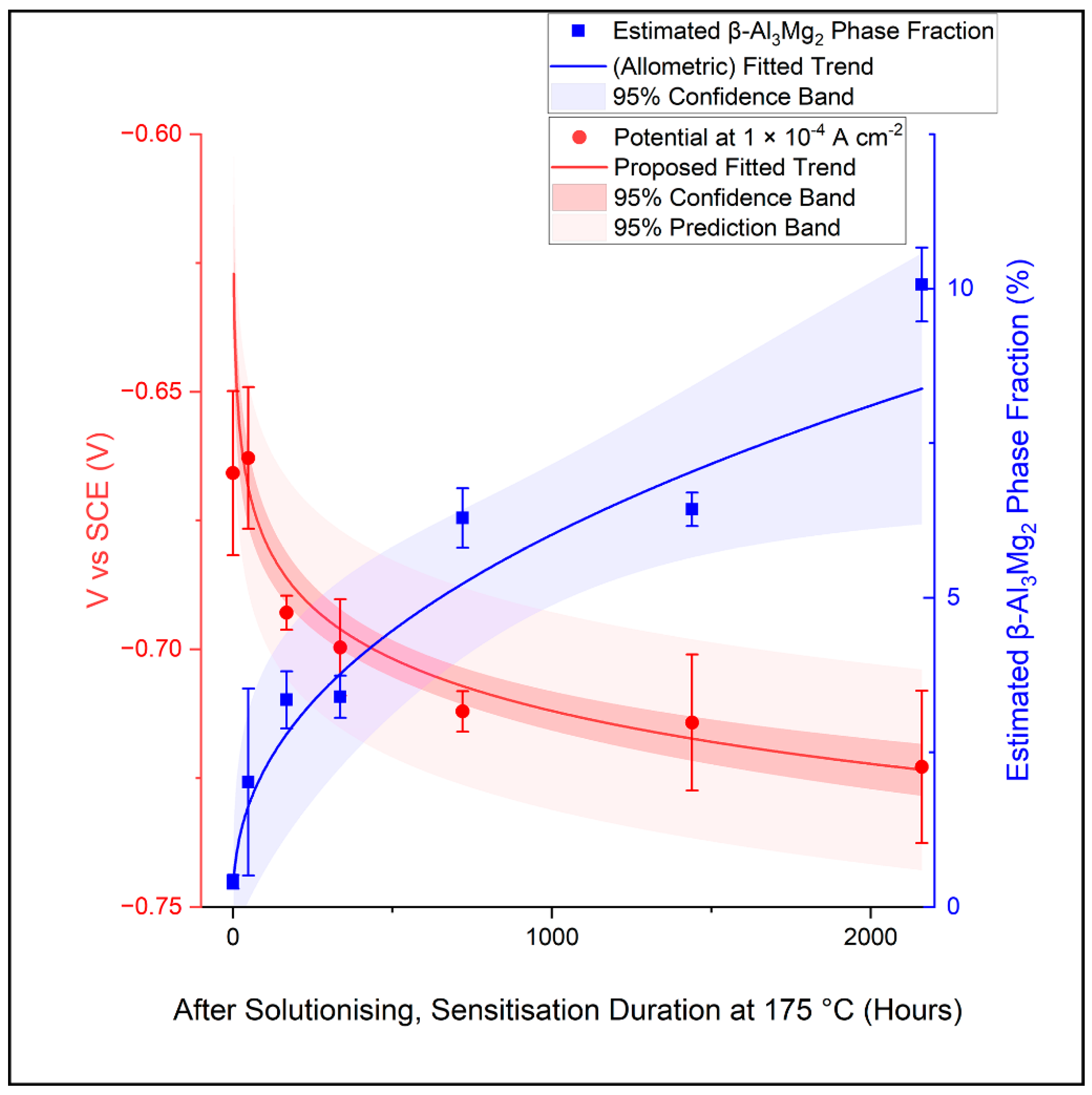
4.2. Proposed Trend of Sensitised AA5083 Microstructure
4.3. Final Remarks
5. Conclusions
- Starting the scan at −1.4 V (vs. SCE) results in an increase in surface pH due to the rapid evolution of hydrogen, which in turn causes active dissolution of part of the AA5083 microstructure, which is likely to be predominately the vulnerable β-Al3Mg2 phase;
- Under the test conditions employed in this study, AA5083 (sensitised) microstructures with a more extensive β-Al3Mg2 growth (from ca. 0.4% to 8.4%) recorded a more rapid increase in the measured current density after passive film breakdown (reaching 1 × 10−4 A cm−2 at ca. −0.63 V to −0.72 V vs. SCE);
- Under the test conditions employed in this study, a fitted correlation was found between the potential at which the current density reaches a pre-determined value, i.e., 1 × 10−4 A cm−2 after breakdown, and the extent of β-Al3Mg2 precipitation in the AA5083 microstructure.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargel, C. 5XXX series alloys. In Corrosion of Aluminium; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 469–484. [Google Scholar]
- Yasakau, K.; Zheludkevich, M.; Lamaka, S.V.; Ferreira, M.G. Role of intermetallic phases in localized corrosion of AA5083. Electrochim. Acta 2007, 52, 7651–7659. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R.G. Electrochemical Characteristics of Intermetallic Phases in Aluminum Alloys. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef] [Green Version]
- Lim, M.L.; Kelly, R.; Scully, J. Overview of Intergranular Corrosion Mechanisms, Phenomenological Observations, and Modeling of AA5083. Corrosion 2015, 72, 1818. [Google Scholar] [CrossRef]
- Zhang, R.; Knight, S.; Holtz, R.; Goswami, R.; Davies, C.; Birbilis, N. A Survey of Sensitization in 5xxx Series Aluminum Alloys. Corrosion 2016, 72, 144–159. [Google Scholar] [CrossRef]
- Gupta, R.; Zhang, R.; Davies, C.; Birbilis, N. Influence of Mg Content on the Sensitization and Corrosion of Al-xMg(-Mn) Alloys. Corrosion 2013, 69, 1081–1087. [Google Scholar] [CrossRef]
- Oguocha, I.N.A.; Adigun, O.J.; Yannacopoulos, S. Effect of sensitization heat treatment on properties of Al–Mg alloy AA5083-H116. J. Mater. Sci. 2008, 43, 4208–4214. [Google Scholar] [CrossRef]
- Searles, J.L.; Gouma, P.I.; Buchheit, R.G. Stress corrosion cracking of sensitized AA5083 (Al-4.5Mg-1.0Mn). Met. Mater. Trans. A 2001, 32, 2859–2867. [Google Scholar] [CrossRef]
- Crane, C.B.; Gangloff, R.P. Stress Corrosion Cracking of Low Temperature Sensitized Al-Mg Alloy. Corrosion 2016, 72, 221–241. [Google Scholar]
- Lyndon, J.; Gupta, R.; Gibson, M.; Birbilis, N. Electrochemical behaviour of the β-phase intermetallic (Mg2Al3) as a function of pH as relevant to corrosion of aluminium–magnesium alloys. Corros. Sci. 2013, 70, 290–293. [Google Scholar] [CrossRef]
- Seong, J.; Yang, Z.-S.; Scheltens, F.; Frankel, G.S.; Sridhar, N. Influence of the Altered Surface Layer on the Corrosion of AA5083. J. Electrochem. Soc. 2015, 162, C209–C218. [Google Scholar] [CrossRef]
- Jain, S.; Lim, M.; Hudson, J.; Scully, J. Spreading of intergranular corrosion on the surface of sensitized Al-4.4Mg alloys: A general finding. Corros. Sci. 2012, 59, 136–147. [Google Scholar] [CrossRef]
- Jain, S.; Hudson, J.; Scully, J. Effects of constituent particles and sensitization on surface spreading of intergranular corrosion on a sensitized AA5083 alloy. Electrochim. Acta 2013, 108, 253–264. [Google Scholar] [CrossRef]
- Jones, R.H.; Baer, D.R.; Danielson, M.J.; Vetrano, J.S. Role of Mg in the stress corrosion cracking of an Al-Mg alloy. Met. Mater. Trans. A 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- De De Micheli, S.M. The electrochemical study of pitting corrosion of aluminium in chloride solutions. Corros. Sci. 1978, 18, 605–616. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S. The repassivation response from single cycle anodic polarization: The case study of a sensitized Al-Mg alloy. Electrochim. Acta 2018, 259, 492–499. [Google Scholar] [CrossRef]
- G01 Committee. Test Method for Determining the Susceptibility to Intergranular Corrosion of 5XXX Series Aluminum Alloys by Mass Loss after Exposure to Nitric Acid (NAMLT Test); ASTM B Stand; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar] [CrossRef]
- Liew, Y.; Örnek, C.; Pan, J.; Thierry, D.; Wijesinghe, S.; Blackwood, D.J. In-Situ Time-Lapse SKPFM Investigation of Sensitized AA5083 Aluminum Alloy to Understand Localized Corrosion. J. Electrochem. Soc. 2020, 167, 141502. [Google Scholar] [CrossRef]
- Yi, G.; Sun, B.; Poplawsky, J.D.; Zhu, Y.; Free, M.L. Investigation of pre-existing particles in Al 5083 alloys. J. Alloys Compd. 2018, 740, 461–469. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sukiman, N.L.; Fleming, K.M.; Gibson, M.A.; Birbilis, N. Electrochemical Behavior and Localized Corrosion Associated with Mg2Si Particles in Al and Mg Alloys. ECS Electrochem. Lett. 2012, 1, C1–C3. [Google Scholar] [CrossRef]
- Yang, Y.-K.; Allen, T. Direct visualization of β phase causing intergranular forms of corrosion in Al–Mg alloys. Mater. Charact. 2013, 80, 76–85. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Garves, J.; Bland, L.G.; Locke, J.; Allison, J.; Frankel, G. Micro- and nano-scale intermetallic phases in AA2070-T8 and their corrosion behavior. Electrochim. Acta 2019, 319, 634–648. [Google Scholar] [CrossRef]
- Zhu, Y.; Frankel, G. Effect of Major Intermetallic Particles on Localized Corrosion of AA2060-T8. Corrosion 2019, 75, 29–41. [Google Scholar] [CrossRef]
- Kosari, A.; Zandbergen, H.; Tichelaar, F.; Visser, P.; Taheri, P.; Terryn, H.; Mol, J. In-situ nanoscopic observations of dealloying-driven local corrosion from surface initiation to in-depth propagation. Corros. Sci. 2020, 177, 108912. [Google Scholar] [CrossRef]
- Kosari, A.; Tichelaar, F.; Visser, P.; Zandbergen, H.; Terryn, H.; Mol, J. Dealloying-driven local corrosion by intermetallic constituent particles and dispersoids in aerospace aluminium alloys. Corros. Sci. 2020, 177, 108947. [Google Scholar] [CrossRef]
- Gimenez, P.; Rameau, J.J.; Reboul, M.C. Experimental pH Potential Diagram of Aluminum for Sea Water. Corrosion 1981, 37, 673–682. [Google Scholar] [CrossRef]
- Vargel, C. The corrosion of aluminium. In Corrosion of Aluminium; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 41–61. [Google Scholar]
- Watkins, K.G.; Davie, D.E. Cathodic protection of 6351 aluminium alloy in sea water: Protection potential and surface pH effects. Br. Corros. J. 1987, 22, 157–161. [Google Scholar] [CrossRef]
- Číhal, V.; Štefec, R. On the development of the electrochemical potentiokinetic method. Electrochim. Acta 2001, 46, 3867–3877. [Google Scholar] [CrossRef]
- ISO 12732:2008 ISO 12732:2008 Corrosion of Metals and Alloys—Electrochemical Potentiokinetic Reactivation Measurement Using the Double Loop Method (Based on Cihal’s Method). 2008. Available online: https://shop.bsigroup.com/ProductDetail?pid=000000000030172111 (accessed on 29 June 2021).
- ASTM G108-94(Reapproved 2015) ASTM G108-94 (Reapproved 2015): Standard Test Method for Electrochemical Reactivation (EPR) for Detecting Sensitization of AISI Type 304 and 304L Stainless Steels 2015. Available online: https://www.astm.org/Standards/G108.htm (accessed on 29 June 2021).

| Al | Mg | Mn | Cr | Fe | Si | Cu | Zn |
|---|---|---|---|---|---|---|---|
| 94.607 ± 0.439 | 3.909 ± 0.451 | 0.661 ± 0.051 | 0.079 ± 0.030 | 0.424 ± 0.034 | 0.147 ± 0.039 | 0.032 ± 0.006 | 0.021 ± 0.004 |
| Isothermal Heat Treatment History | Duration (Hours) | Approximate Fraction of β-Al3Mg2 (and Dispersoid) (%) |
|---|---|---|
| Solutionised (450 °C) | 6 | 0.4 ± 0.1 |
| Solutionised (450 °C, 6 h), then Sensitised (175 °C) | 48 | 2.0 ± 1.5 |
| 168 | 3.4 ± 0.5 | |
| 336 | 3.4 ± 0.3 | |
| 720 | 6.3 ± 0.5 | |
| 1440 | 6.4 ± 0.3 | |
| 2160 | 10.1 ± 0.6 | |
| As-Received | - | 1.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, Y.; Wijesinghe, S.; Blackwood, D.J. Investigation of the Electrochemical Breakdown Response in Sensitised AA5083 Aluminium Alloy. Sustainability 2021, 13, 7342. https://doi.org/10.3390/su13137342
Liew Y, Wijesinghe S, Blackwood DJ. Investigation of the Electrochemical Breakdown Response in Sensitised AA5083 Aluminium Alloy. Sustainability. 2021; 13(13):7342. https://doi.org/10.3390/su13137342
Chicago/Turabian StyleLiew, YanHan, Sudesh Wijesinghe, and Daniel J. Blackwood. 2021. "Investigation of the Electrochemical Breakdown Response in Sensitised AA5083 Aluminium Alloy" Sustainability 13, no. 13: 7342. https://doi.org/10.3390/su13137342
APA StyleLiew, Y., Wijesinghe, S., & Blackwood, D. J. (2021). Investigation of the Electrochemical Breakdown Response in Sensitised AA5083 Aluminium Alloy. Sustainability, 13(13), 7342. https://doi.org/10.3390/su13137342







