Applying Remotely Sensed Environmental Information to Model Mosquito Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Description of the Dataset
2.2.1. Experimental Setting
Mosquito Sampling and Identification
Mosquito DNA Barcoding
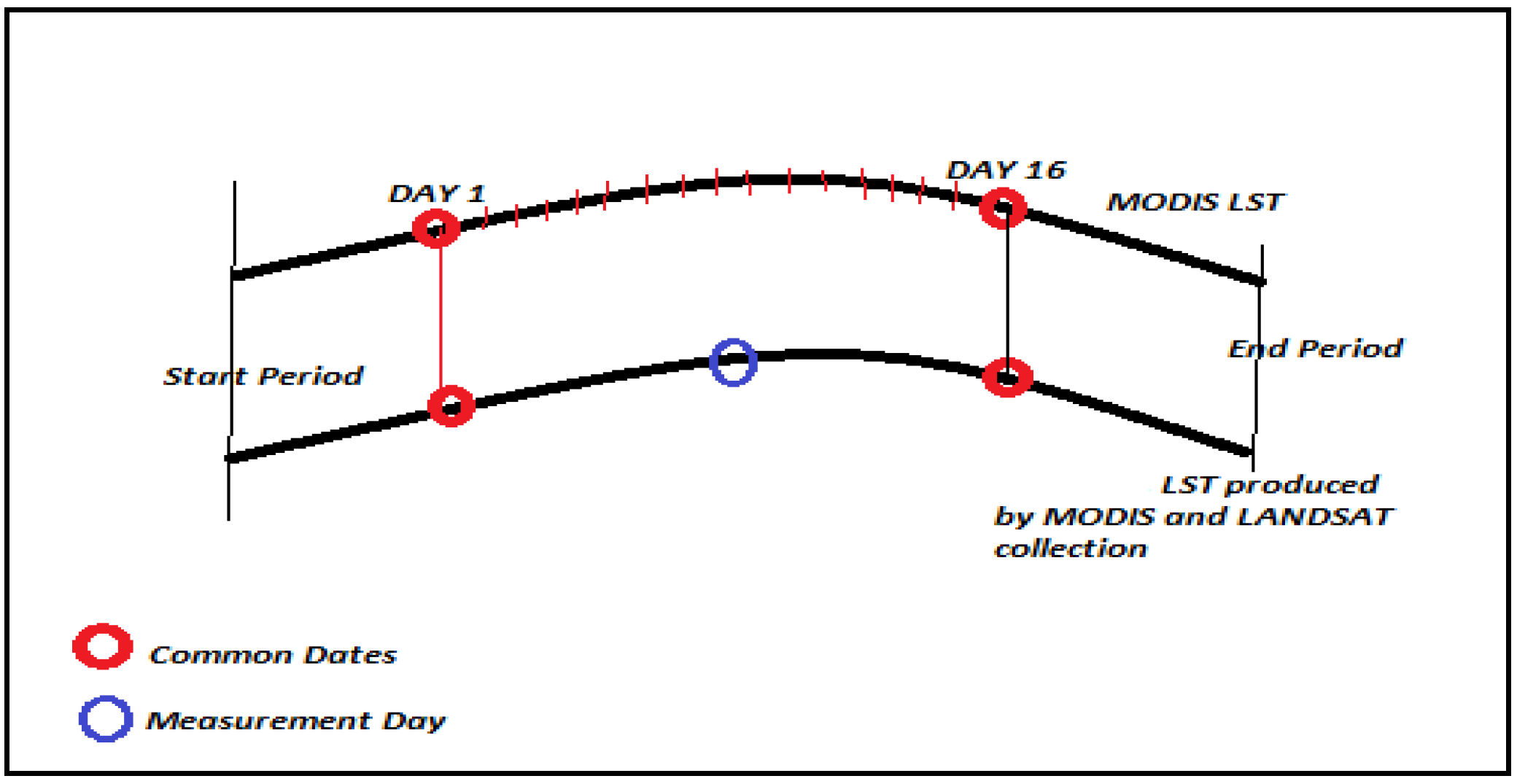
2.2.2. Remotely Sensed Data and Environmental Datasets
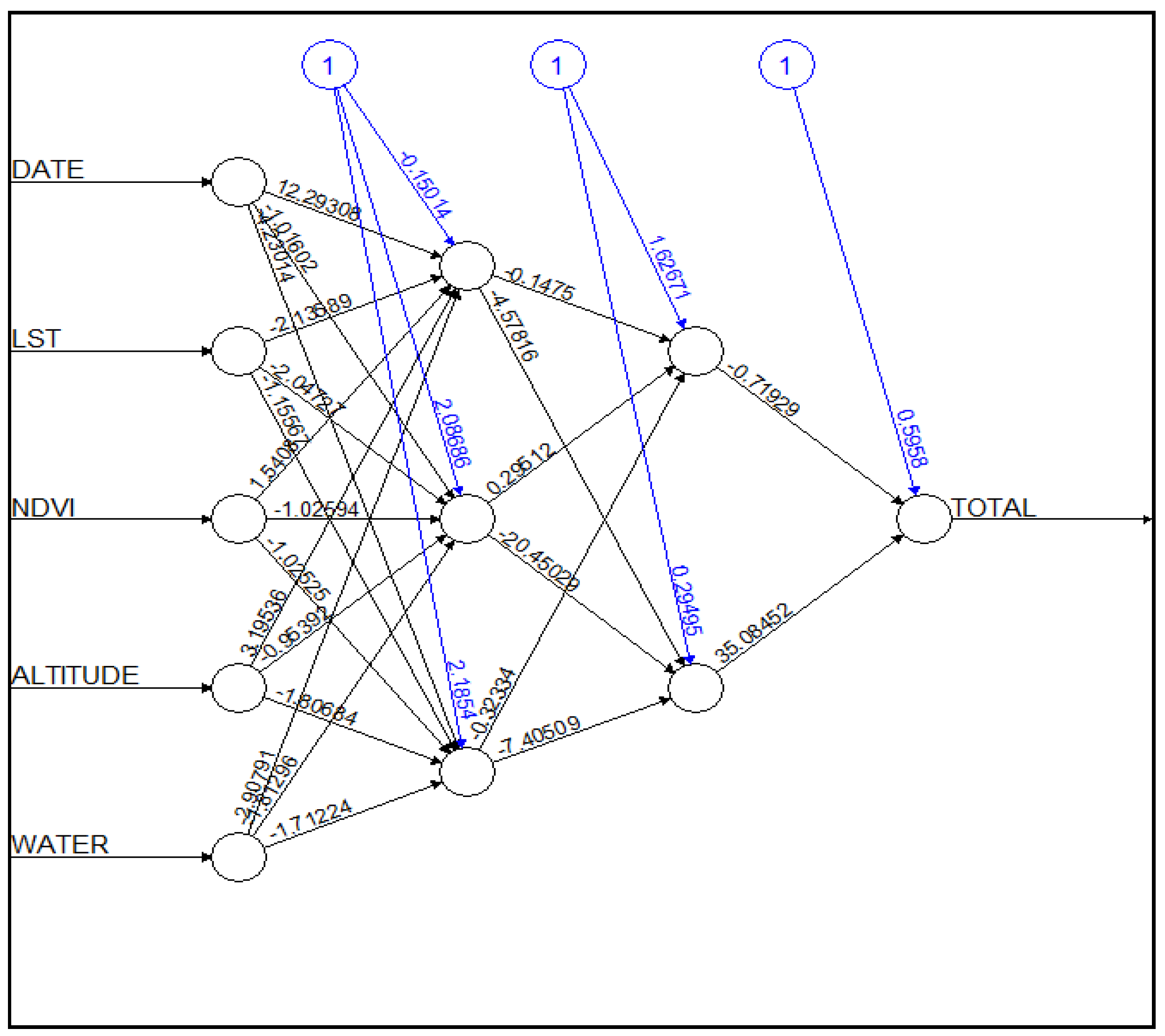
2.3. Model Development
3. Results
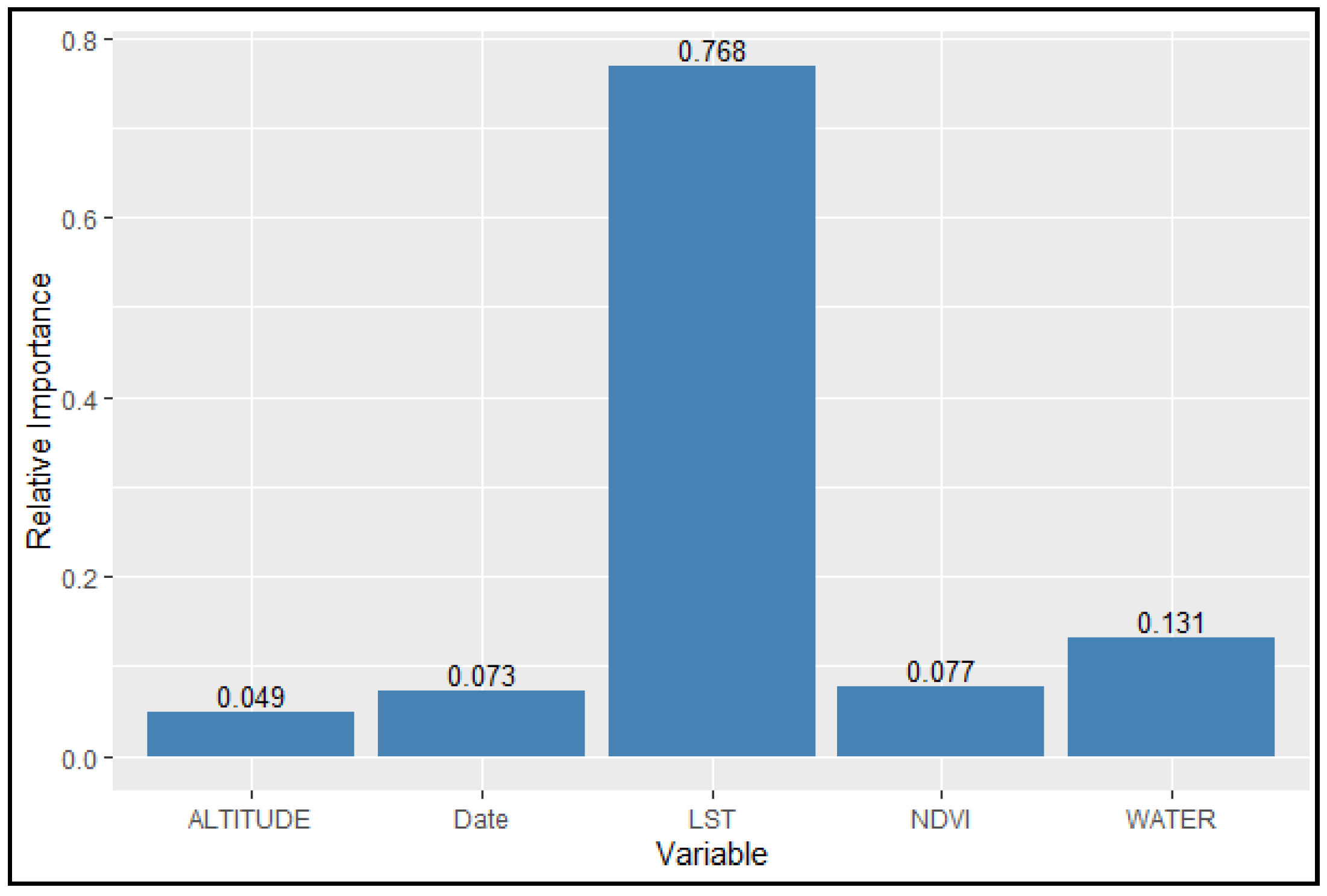
3.1. Parameter Estimation
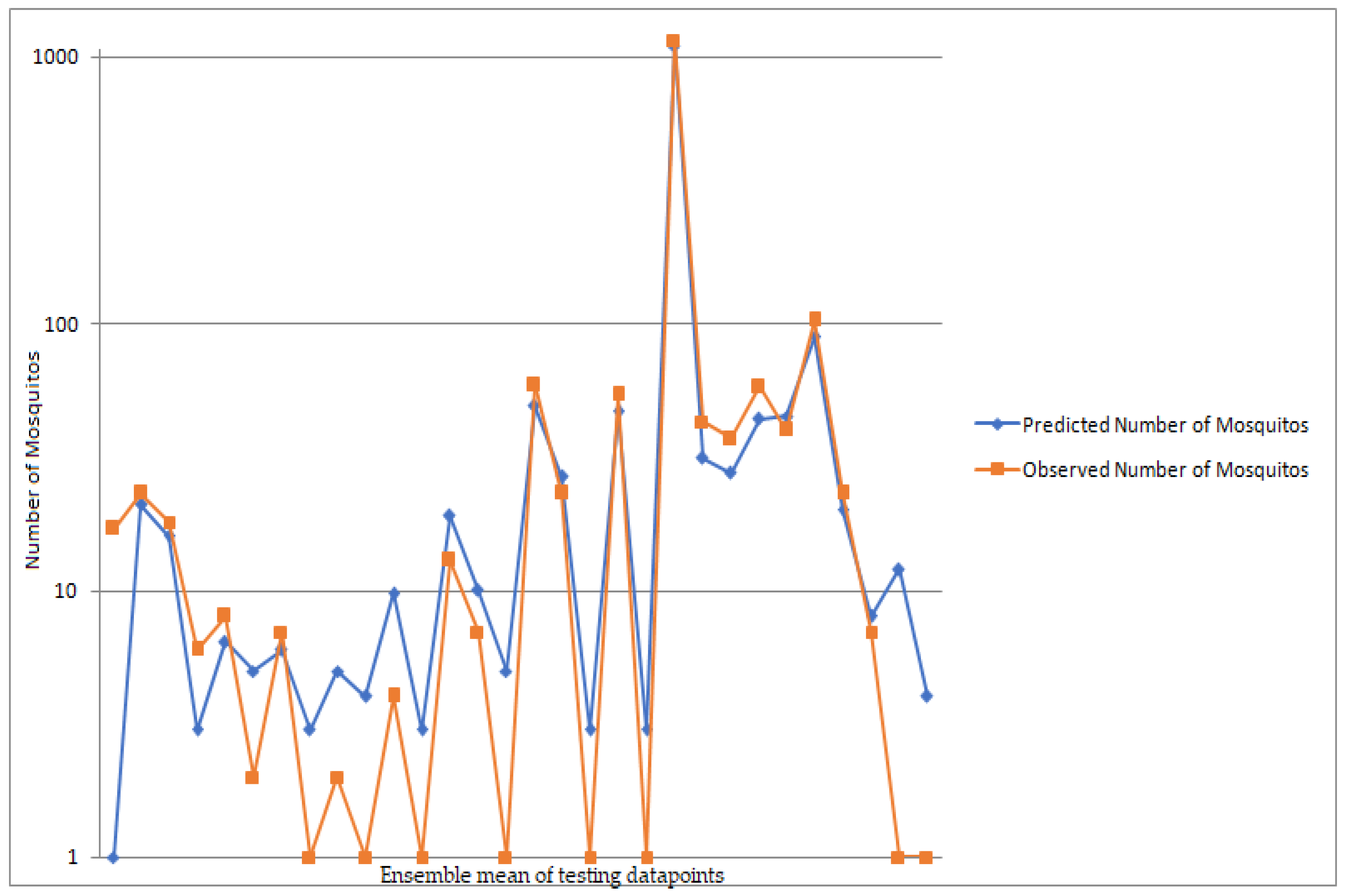
3.2. Model Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourenço, P.M.; Sousa, C.A.; Seixas, J.; Lopes, P.; Novo, M.T.; Paulo, A.; Almeida, G. Anopheles atroparvus density modeling using MODIS NDVI in a former malarious area in Portugal. J. Vector Ecol. 2011, 36, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.S.M.; Bergo, E.S.; Conn, J.E.; Laporta, G.Z.; Prist, P.R.; Sallum, M.A.M. Anthropogenic landscape decreases mosquito biodiversity and drives malaria vector proliferation in the Amazon rainforest. PLoS ONE 2021, 16, e0245087. [Google Scholar] [CrossRef]
- Trájer, A.J. The changing risk patterns of Plasmodium vivax malaria in Greece due to climate change. Int. J. Environ. Health Res. 2020, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Parselia, E.; Kontoes, C.; Tsouni, A.; Hadjichristodoulou, C.; Kioutsioukis, I.; Magiorkinis, G.; Stilianakis, N.I. Satellite Earth Observation data in epidemiological modeling of malaria, dengue and West Nile Virus: A scoping review. Remote Sens. 2019, 11, 1862. [Google Scholar] [CrossRef] [Green Version]
- Kazansky, Y.; Wood, D.; Sutherlun, J. The current and potential role of satellite remote sensing in the campaign against malaria. Acta Astronaut. 2016, 121, 292–305. [Google Scholar] [CrossRef] [Green Version]
- Dantur Juri, M.J.; Estallo, E.; Almirón, W.; Santana, M.; Sartor, P.; Lamfri, M.; Zaidenberg, M. Satellite-derived NDVI, LST, and climatic factors driving the distribution and abundance of Anopheles mosquitoes in a former malarious area in northwest Argentina. J. Vector Ecol. 2015, 40, 36–45. [Google Scholar] [CrossRef]
- Chuang, T.W.; Henebry, G.M.; Kimball, J.S.; VanRoekel-Patton, D.L.; Hildreth, M.B.; Wimberly, M.C. Satellite microwave remote sensing for environmental modeling of mosquito population dynamics. Remote Sens. Environ. 2012, 125, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Pergantas, P.; Tsatsaris, A.; Malesios, C.; Kriparakou, G.; Demiris, N.; Tselentis, Y. A spatial predictive model for malaria resurgence in central Greece integrating entomological, environmental and social data. PLoS ONE 2017, 12, e0178836. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.; Schellenberg, J.A.; Kofoed, P.E.; Aaby, P.; Greenwood, B. Changing pattern of malaria in Bissau, Guinea Bissau. Trop. Med. Int. Health 2008, 13, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.A.; Abruzzo, N.O.; Taylor, C.E.; Stevens, B.R.; Cuda, J.P.; Weeks, E.N.I. Methionine as an Effective Mosquito Larvicide in Natural Water Sources. Florida Entomol. 2020, 103, 479–483. [Google Scholar] [CrossRef]
- Scavuzzo, J.M.; Trucco, F.; Espinosa, M.; Tauro, C.B.; Abril, M.; Scavuzzo, C.M.; Frery, A.C. Modeling Dengue vector population using remotely sensed data and machine learning. Acta Trop. 2018, 185, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Climate Atlas of Greece, Hellenic National Meteorological Service. Available online: https://web.archive.org/web/20170921184739/http://www.hnms.gr:80/hnms/greek/pdf/Climate_Atlas_Of_Greece.pdf (accessed on 20 February 2021).
- McNelly, J.R. The CDC Trap As a special monitoring tool. In Proceedings of the Seventy-Sixth Annual Meeting of the New Jersey Mosquito Control Association; 1989; pp. 26–33. Available online: http://vectorbio.rutgers.edu/outreach/cdctrap.htm (accessed on 19 May 2021).
- Darsie, R.F.; Samanidou-Voyadjoglou, A. Keys for the identification of the mosquitoes of Greece. J. Am. Mosq. Control Assoc. 1997, 13, 247–254. [Google Scholar]
- Samanidou-Voyadjoglou, A.; Harbach, R.E. Keys to the adult female mosquitoes (Culicidae) of Greece. Eur. Mosq. Bull. 2001, 10, 13–20. [Google Scholar]
- Gunay, F.; Picard, M.; Robert, V. Interactive Identification Key for Female Mosquitoes (Diptera: Culicidae) of Euro-Mediterranean and Black Sea Regions. Int. J. Infect. Dis. 2016, 53, 110–111. [Google Scholar] [CrossRef] [Green Version]
- Harbach, R.E. Culicipedia: Species-Group, Genus-Group and Family-Group Names in Culicidae (Diptera); Cabi: Wallingford, UK, 2018; ISBN 9781786399052. [Google Scholar]
- Wilkerson, R.C.; Linton, Y.M.; Fonseca, D.M.; Schultz, T.R.; Price, D.C.; Strickman, D.A. Making mosquito taxonomy useful: A stable classification of tribe Aedini that balances utility with current knowledge of evolutionary relationships. PLoS ONE 2015, 10, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nieman, C.C.; Yamasaki, Y.; Collier, T.C. A DNA extraction protocol for improved DNA yield from individual mosquitoes. F1000Research 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System: Barcoding. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.L.; Tang, B.H.; Wu, H.; Ren, H.; Yan, G.; Wan, Z.; Trigo, I.F.; Sobrino, J.A. Satellite-derived land surface temperature: Current status and perspectives. Remote Sens. Environ. 2013, 131, 14–37. [Google Scholar] [CrossRef] [Green Version]
- Fahri, S.; Ariyani, S.; Yamistada, G.; Manulang, E.S. Hatchability of the Eggs Aedesspp in Clean and Polluted Water. KnE Life Sci. 2019, 2019, 134–140. [Google Scholar] [CrossRef]
- Roux, O.; Renault, D.; Mouline, K.; Diabaté, A.; Simard, F. Living with predators at the larval stage has differential long-lasting effects on adult life history and physiological traits in two anopheline mosquito species. J. Insect Physiol. 2021, 131, 104234. [Google Scholar] [CrossRef]
- Department of Epidemiological Surveillance and Intervention, Center for Disease Control and Prevention of Greece (KEELPNO). Available online: https://eody.gov.gr/ (accessed on 19 March 2021).
- Dantur Juri, M.J.; Galante, G.B.; Zaidenberg, M.; Almirón, W.R.; Claps, G.L.; Santana, M. Longitudinal study of the species composition and spatio-temporal abundance of Anopheles larvae in a malaria risk area in Argentina. Fla. Entomol. 2014, 97, 1167–1181. [Google Scholar] [CrossRef]
- Dambach, P.; Machault, V.; Lacaux, J.P.; Vignolles, C.; Sié, A.; Sauerborn, R. Utilization of combined remote sensing techniques to detect environmental variables influencing malaria vector densities in rural West Africa. Int. J. Health Geogr. 2012, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Avdan, U.; Jovanovska, G. Algorithm for automated mapping of land surface temperature using LANDSAT 8 satellite data. J. Sensors 2016, 2016, 1480307. [Google Scholar] [CrossRef] [Green Version]
- Gemitzi, A.; Dalampakis, P.; Falalakis, G. Detecting geothermal anomalies using Landsat 8 thermal infrared remotely sensed data. Int. J. Appl. Earth Obs. Geoinf. 2021, 96, 102283. [Google Scholar] [CrossRef]
- Morawitz, D.F.; Blewett, T.M.; Cohen, A.; Alberti, M. Using NDVI to assess vegetative land cover change in Central Puget Sound. Environ. Monit. Assess. 2006, 114, 85–106. [Google Scholar] [CrossRef]
- Jeevalakshmi, D.; Reddy, S.N.; Manikiam, B. Land cover classification based on NDVI using LANDSAT8 time series: A case study Tirupati region. Int. Conf. Commun. Signal Process. ICCSP 2016 2016, 560056, 1332–1335. [Google Scholar] [CrossRef]
- Pekel, J.F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-resolution mapping of global surface water and its long-term changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Kimball, J.S.; Jones, L.A.; Kim, Y.; Glassy, J.; Watts, J.D. A global satellite environmental data record derived from AMSR-E and AMSR2 microwave Earth observations. Earth Syst. Sci. Data 2017, 9, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Ambikairajah, E.; Lennon, S. Neural Networks for Speech Recognition. In Proceedings of the AI and Cognitive Science ’90, University of Ulster, Jordanstown, UK, 20–21 September 1990; McTear, M.F., Creaney, N., Eds.; Springer: London, UK, 1990; pp. 163–177. [Google Scholar]
- Cristea, P.D. Application of Neural Networks In Image Processing and Visualization. In Proceedings of the GeoSpatial Visual Analytics; Amicis, R.D., Stojanovic, R., Conti, G., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 59–71. [Google Scholar]
- Gemitzi, A.; Lakshmi, V. Estimating Groundwater Abstractions at the Aquifer Scale Using GRACE Observations. Geosciences 2018, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Keller, P.E.; Kangas, L.J.; Hashem, S.; Kouzes, R.T. Applications of Neural Networks in Environment, Energy and Health; World Scientific: Singapore, 1996; Volume 5, ISBN 978-981-02-2758-6. [Google Scholar]
- Nguyen, T.A.; Ly, H.B.; Pham, B.T. Backpropagation Neural Network-Based Machine Learning Model for Prediction of Soil Friction Angle. Math. Probl. Eng. 2020, 2020, 8845768. [Google Scholar] [CrossRef]
- Spitz, F.; Sovan, L. Environmental impact prediction using neural network modelling. An example in wildlife damage. J. Appl. Ecol. 1999, 36, 317–326. [Google Scholar] [CrossRef]
- Sun, A.Y. Predicting groundwater level changes using GRACE data. Water Resour. Res. 2013, 49, 5900–5912. [Google Scholar] [CrossRef]
- Sheela, K.G.; Deepa, S.N. Selection of number of hidden neurons in neural networks in renewable energy systems. J. Sci. Ind. Res. 2014, 73, 686–688. [Google Scholar]
- Nash, J.E.; Sutcliffe, J.V. River Flow Forecasting Through Conceptual Models Part I—A Discussion of Principles. J. Hydrol. 1970, 10, 282–290. [Google Scholar] [CrossRef]
- Moriasi, D.N.; Arnold, J.G.; Van Liew, M.W.; Bingner, R.L.; Harmel, R.D.; Veith, T.L. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Trans. ASABE 2007, 50, 885–900. [Google Scholar] [CrossRef]
- Tachi, S.E.; Ouerdachi, L.; Remaoun, M.; Derdous, O.; Boutaghane, H. Forecasting suspended sediment load using regularized neural network: Case study of the Isser River (Algeria). J. Water L. Dev. 2016, 29, 75–81. [Google Scholar] [CrossRef] [Green Version]
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 February 2021).
- Fritsch, S.; Guenther, F.; Suling, M.; Mueller, S.M. Package ‘Neuralnet’ 2016. Available online: https://github.com/bips-hb/neuralnet (accessed on 12 February 2021).
- Olden, J.D.; Joy, M.K.; Death, R.G. An accurate comparison of methods for quantifying variable importance in artificial neural networks using simulated data. Ecol. Modell. 2004, 178, 389–397. [Google Scholar] [CrossRef]
- Marzban, C.; Illian, P.R.; Morison, D.; Mourad, P.D. Within-Group and between-Group Correlation: Illustration on Non-Invasive Estimation of Intracranial Pressure. 2013. Available online: http://faculty.washington.edu/marzban/within_between_simple.pdf (accessed on 15 March 2021).
- Wagner, C.H. Simpson’s paradox in real life. Am. Stat. 1982, 36, 46–48. [Google Scholar]
- Jani, C.; Walker, A.; Al Omari, O.; Patel, D.; Heffess, A.; Wolpow, E.; Page, S.; Bourque, D. Acute transverse myelitis in West Nile Virus, a rare neurological presentation. IDCases 2021, 24, e01104. [Google Scholar] [CrossRef]
- Giesen, C.; Roche, J.; Redondo-Bravo, L.; Ruiz-Huerta, C.; Gomez-Barroso, D.; Benito, A.; Herrador, Z. The impact of climate change on mosquito-borne diseases in Africa. Pathog. Glob. Health 2020, 114, 1–15. [Google Scholar] [CrossRef]

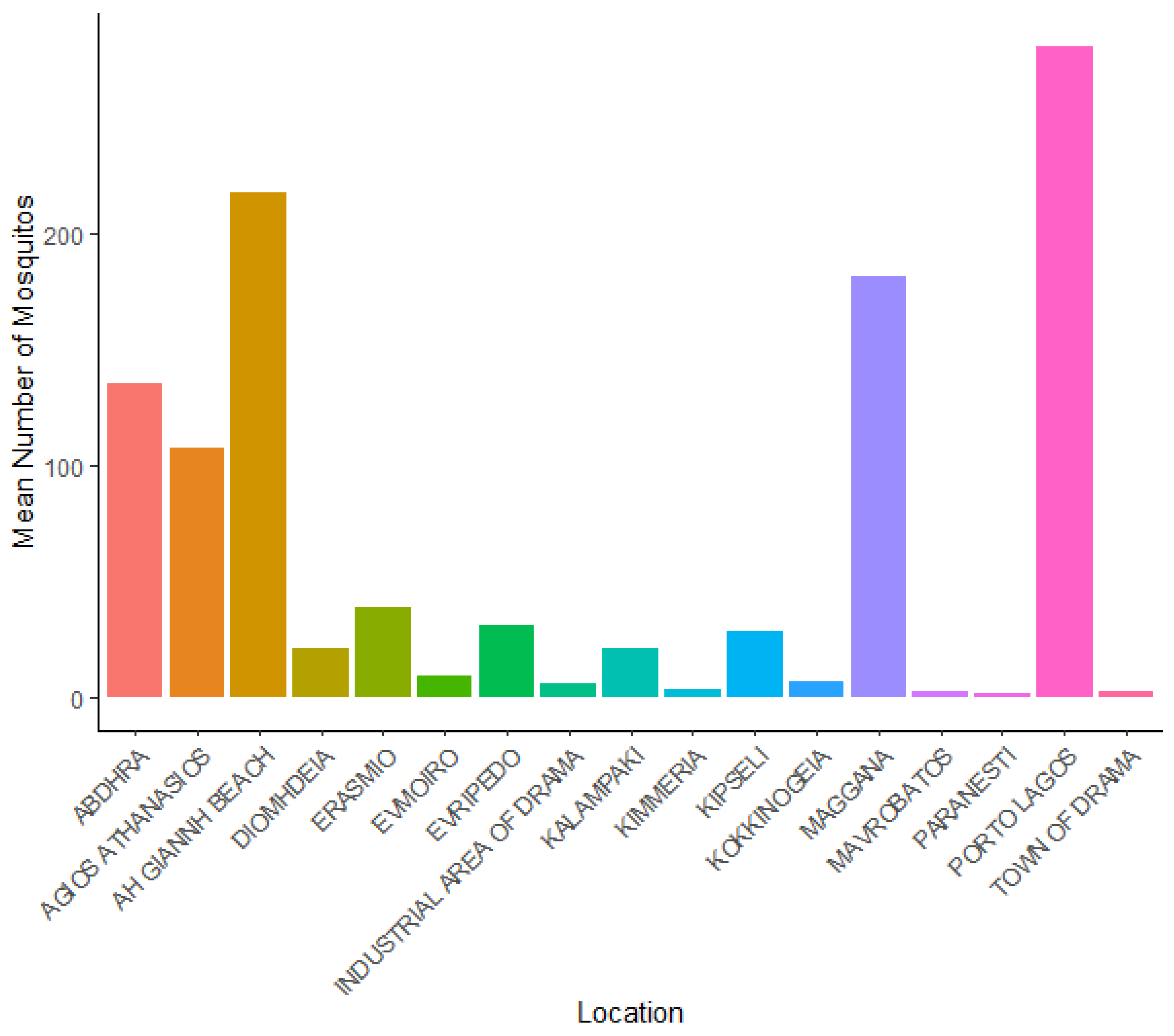


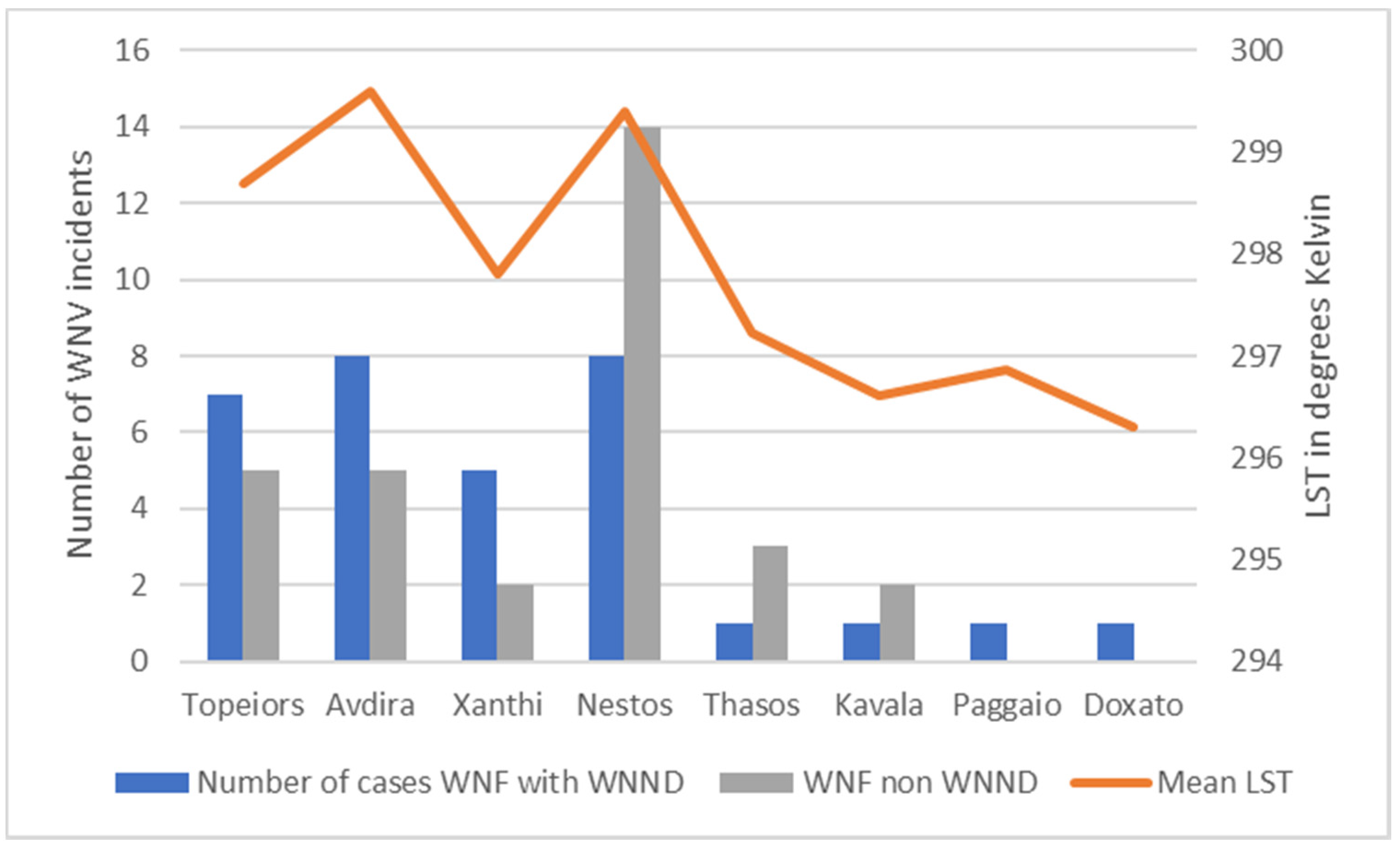
| Location | Total Mosquito Number | Mean LST | Mean NDVI | Altitude (m) | Water Area km2 |
|---|---|---|---|---|---|
| Kalampaki | 148 | 302.2 | 0.68 | 65 | 6.02 |
| Evripedo | 216 | 305.0 | 0.45 | 133 | 7.01 |
| Kokkinogeia | 47 | 304.0 | 0.57 | 101 | 3.02 |
| Town of Drama | 19 | 304.7 | 0.62 | 92 | 1.99 |
| Paranesti | 14 | 306.3 | 0.75 | 118 | 1.70 |
| Mavrobatos | 15 | 308.9 | 0.30 | 79 | 1.85 |
| Industrial area of Drama | 39 | 308.8 | 0.53 | 153 | 2.50 |
| Evmoiro | 62 | 308.2 | 0.31 | 95 | 4.03 |
| Kipseli | 199 | 309.8 | 0.26 | 62 | 6.89 |
| Erasmio | 271 | 309.7 | 0.27 | 8 | 7.32 |
| Maggana | 1268 | 312.2 | 0.31 | 7 | 9.88 |
| Abdhra | 947 | 311.9 | 0.21 | 42 | 9.02 |
| Ah Giannh Beach | 1523 | 309.6 | 0.56 | 4 | 10.01 |
| Porto Lagos | 1967 | 310.5 | 0.23 | 2 | 11.56 |
| Diomhdeia | 145 | 309.2 | 0.42 | 36 | 5.96 |
| Agios Athanasios | 751 | 308.5 | 0.39 | 33 | 8.46 |
| Kimmeria | 22 | 307.8 | 0.24 | 88 | 2.10 |
| Locations | LST | NDVI |
|---|---|---|
| Overall | 0.347 *** | −0.036 |
| Within-group correlation coefficients | ||
| Abdhra | 0.655 | 0.976 *** |
| Agios Athanasios | 0.773 * | 0.991 *** |
| Ah Giannh Beach | 0.843 * | 0.729 |
| Diomhdeia | 0.604 | 0.139 |
| Erasmio | 0.332 | 0.358 |
| Evmoiro | 0.531 | 0.647 |
| Evripedo | 0.571 | 0.581 |
| Industrial area of Drama | 0.830 * | 0.804 * |
| Kalampaki | 0.921 ** | 0.973 *** |
| Kimmeria | 0.943 ** | 0.871 * |
| Kipseli | 0.744 | 0.839 * |
| Kokkinogeia | 0.950 ** | 0.946 ** |
| Maggana | 0.866 * | 0.967 *** |
| Mavrobatos | 0.937 ** | 0.906 ** |
| Paranesti | 0.837 * | 0.990 *** |
| Porto Lagos | 0.704 | 0.899 ** |
| Town of Drama | 0.787 * | 0.901 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kofidou, M.; de Courcy Williams, M.; Nearchou, A.; Veletza, S.; Gemitzi, A.; Karakasiliotis, I. Applying Remotely Sensed Environmental Information to Model Mosquito Populations. Sustainability 2021, 13, 7655. https://doi.org/10.3390/su13147655
Kofidou M, de Courcy Williams M, Nearchou A, Veletza S, Gemitzi A, Karakasiliotis I. Applying Remotely Sensed Environmental Information to Model Mosquito Populations. Sustainability. 2021; 13(14):7655. https://doi.org/10.3390/su13147655
Chicago/Turabian StyleKofidou, Maria, Michael de Courcy Williams, Andreas Nearchou, Stavroula Veletza, Alexandra Gemitzi, and Ioannis Karakasiliotis. 2021. "Applying Remotely Sensed Environmental Information to Model Mosquito Populations" Sustainability 13, no. 14: 7655. https://doi.org/10.3390/su13147655






