The Influence of Tilia tomentosa Moench on Plant Species Diversity and Composition in Mesophilic Forests of Western Romania–A Potential Tree Species for Warming Forests in Central Europe?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Data Collection
2.3. Data Assessment
2.3.1. Assessment of the Silver Lime-Dataset
2.3.2. Assessment of the Beech/Oak/Lime-Dataset
3. Results
3.1. Influencing Factors on the Canopy Cover of T. tomentosa
3.2. Influencing Factors on T. tomentosa Cover in the Understorey
3.3. Effect of T. tomentosa Tree Cover on Understorey Species Richness across Regions
3.4. Comparing Species Richness and Composition of Beech, Oak and Lime Forests
3.5. Indicator Species of Beech, Oak and Lime Forests
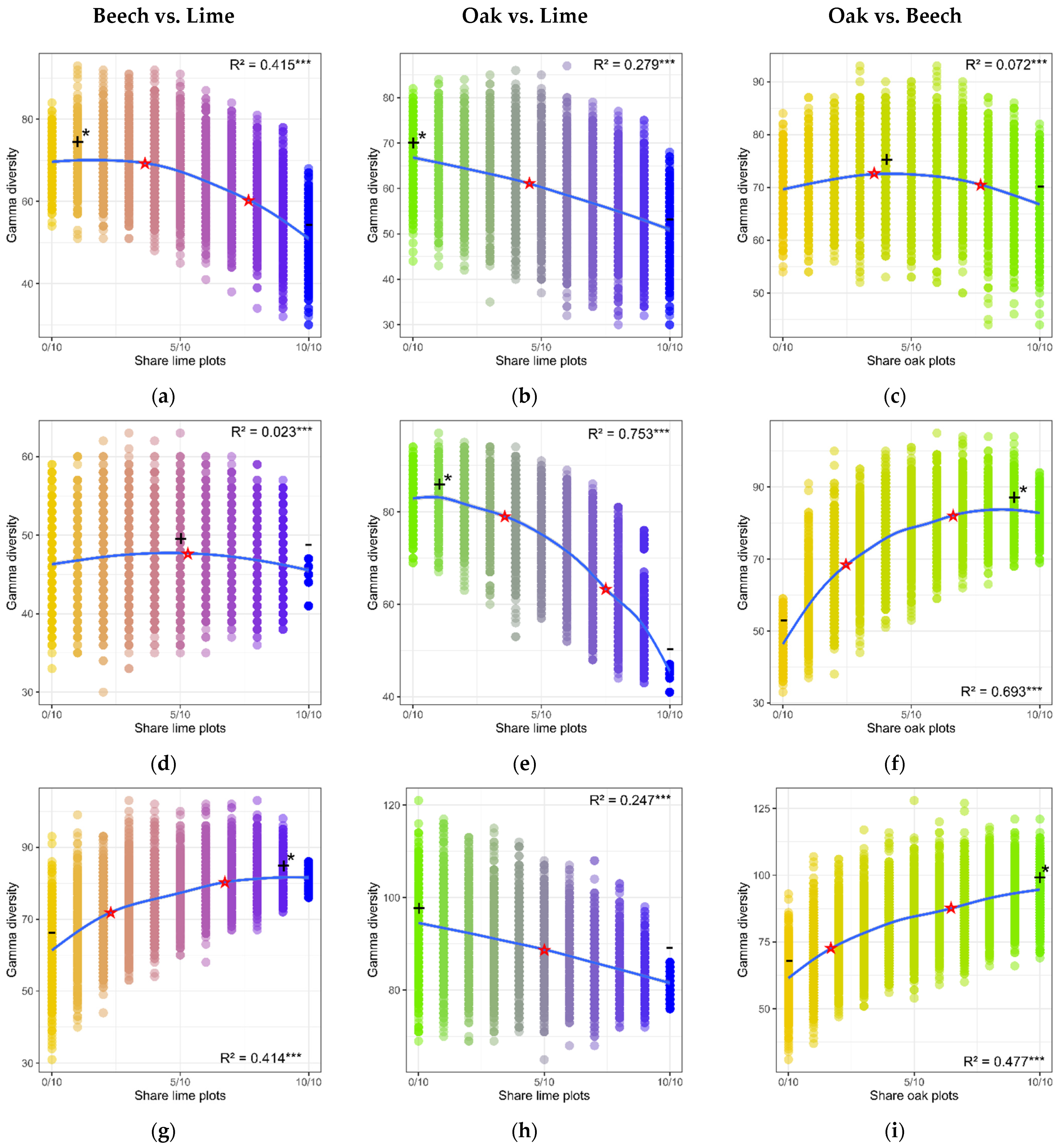
3.6. Gamma-Diversity of Lime, Oak and Beech Forests and Their Combinations
4. Discussion
4.1. Local Site Factors Determine the Abundance of T. tomentosa
4.2. The Effect of Lime on Plant Species Diversity and Composition
4.3. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Milova | Maciova | Eşelniţa | L | T | C | M | R | N | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Beech | ||||||||||
| Acer platanoides_sl | Beech | 4 | 6 | 4 | 5 | x | x | |||
| Acer pseudoplatanus | Beech | 3 | x | 4 | 6 | x | 7 | |||
| Acer pseudoplatanus_sl | Beech | |||||||||
| Circaea lutetiana | Beech | 4 | 5 | 3 | 7 | 7 | 7 | |||
| Dentaria glandulosa | Beech | 2 | 4 | 6 | 6 | 7 | 8 | |||
| Lathyrus hallersteinii | Beech | 5 | 5 | 6 | 5 | 6 | x | |||
| Sambucus nigra | Beech | 6 | 5 | 3 | 6 | x | 9 | |||
| Populus tremula | Beech | 6 | 5 | 5 | 5 | x | x | |||
| Tilia cordata | Beech | 5 | 5 | 4 | 4 | x | 5 | |||
| Urtica dioica | Beech | x | x | x | 6 | 7 | 9 | |||
| ∅ | 4.4 | 5.0 | 4.4 | 5.6 | 6.8 | 7.5 | ||||
| Beech & oak | ||||||||||
| Dentaria bulbifera | Beech&Oak | Beech | 3 | 5 | 4 | 5 | 7 | 6 | ||
| Beech & Lime | ||||||||||
| Fagus sylvatica | Beech | Beech&Lime | Beech | 2 | 5 | 3 | 6 | x | x | |
| Fagus sylvatica_sl | Beech | Beech&Lime | ||||||||
| Galium odoratum | Beech | Beech&Lime | Beech | 2 | 5 | 2 | 5 | 6 | 5 | |
| Lamiastrum galeobdolon agg. | Beech&Lime | 2 | 5 | 4 | 5 | 7 | 5 | |||
| Mercurialis perennis | Beech | Beech&Lime | 3 | x | 3 | 5 | 8 | 7 | ||
| Carex digitata | Lime | Beech&Oak | 3 | x | 4 | 5 | x | 4 | ||
| Ulmus glabra | Beech&Lime | 4 | 5 | 3 | 5 | 7 | 7 | |||
| ∅ | 2.7 | 5.0 | 3.2 | 5.2 | 7.0 | 5.6 | ||||
| Oak | ||||||||||
| Ajuga reptans | Oak | 6 | x | 2 | 5 | 6 | 6 | |||
| Buglossoides purpurocaerulea | Oak | 5 | 7 | 4 | 4 | 7 | 4 | |||
| Calamagrostis arundinacea | Oak | 6 | 5 | 4 | 5 | 4 | 5 | |||
| Campanula persicifolia | Oak | 6 | 5 | 4 | 4 | 8 | 3 | |||
| Carex caryophyllea | Oak | 7 | x | 3 | 4 | x | 2 | |||
| Carpinus betulus_sl | Oak | 3 | 5 | 4 | 5 | x | x | |||
| Cephalanthera longifolia | Oak | 5 | 5 | 3 | 4 | 6 | 4 | |||
| Chamaecytisus leiocarpus | Oak | 8 | 5 | 6 | 3 | 8 | x | |||
| Crataegus monogyna | Oak | 7 | 5 | 3 | 4 | 8 | 4 | |||
| Crataegus monogyna_sl | Oak | |||||||||
| Cruciata glabra | Oak | 6 | 5 | 4 | 5 | 7 | 5 | |||
| Fragaria vesca | Oak | 6 | x | 5 | 5 | x | 6 | |||
| Galium schultesii | Oak | Oak | 5 | 5 | 5 | 4 | 7 | 4 | ||
| Genista tinctoria | Oak | 7 | 5 | 5 | 4 | 5 | 2 | |||
| Hieracium sabaudum | Oak | 5 | 6 | 3 | 4 | 4 | 2 | |||
| Lapsana communis | Oak | 5 | 6 | 3 | 5 | x | 7 | |||
| Lunaria annua | Oak | 4 | 6 | 6 | 6 | 7 | 8 | |||
| Melica nutans | Oak | 3 | x | 3 | 4 | x | 3 | |||
| Melittis melissophyllum | Oak | 5 | 7 | 2 | 4 | 6 | 3 | |||
| Mycelis muralis | Oak | 3 | 5 | 2 | 5 | x | 6 | |||
| Quercus cerris | Oak | 7 | 8 | 4 | 3 | 6 | x | |||
| Quercus frainetto | Oak | 7 | 8 | 6 | 3 | 7 | x | |||
| Rosa arvensis | Oak | 5 | 6 | 2 | 5 | 7 | 5 | |||
| Rosa canina | Oak | 7 | 5 | 3 | 4 | x | x | |||
| Solidago virgaurea | Oak | 6 | x | x | 5 | x | 4 | |||
| Sorbus torminalis_sl | Oak | 5 | 7 | 4 | 3 | 7 | 4 | |||
| Symphytum tuberosum | Oak | 4 | x | 4 | 5 | 6 | 5 | |||
| Trifolium medium | Oak | Oak | 7 | 6 | 4 | 4 | 6 | 3 | ||
| Veronica chamaedrys | Oak | 6 | x | x | 4 | 7 | x | |||
| ∅ | 5.6 | 5.8 | 3.8 | 4.3 | 6.5 | 4.3 | ||||
| Oak & Lime | ||||||||||
| Acer campestre | Oak&Lime | 5 | 6 | 4 | 4 | 7 | 6 | |||
| Ajuga genevensis | Oak&Lime | 7 | 6 | x | 4 | 7 | 2 | |||
| Brachypodium sylvaticum | Oak | Oak&Lime | 3 | 5 | 3 | 5 | 6 | 6 | ||
| Bromus benekenii | Oak&Lime | 5 | 5 | 4 | 4 | 7 | 5 | |||
| Campanula rapunculoides | Oak&Lime | 6 | 6 | 4 | 4 | 7 | 4 | |||
| Carex leersiana | Oak&Lime | 5 | 6 | 3 | 4 | x | 6 | |||
| Carpinus betulus | Oak&Lime | 3 | 5 | 4 | 5 | x | x | |||
| Carpinus orientalis_sl | Oak&Lime | 4 | 8 | 5 | 3 | 8 | x | |||
| Clinopodium vulgare | Oak | Oak&Lime | 7 | x | 3 | 4 | 7 | 3 | ||
| Cornus mas_sl | Oak&Lime | Oak&Lime | Oak&Lime | 6 | 7 | 4 | 4 | 8 | 4 | |
| Dactylis glomerata | Oak | Oak&Lime | 6 | x | 3 | 4 | x | 6 | ||
| Euphorbia amygdaloides | Oak&Lime | 3 | 5 | 3 | 5 | 8 | 5 | |||
| Festuca heterophylla | Oak | Oak&Lime | 5 | 6 | 4 | 4 | 5 | 5 | ||
| Fraxinus ornus_sl | Oak | Oak&Lime | 6 | 8 | 4 | 3 | 8 | 3 | ||
| Galium pseudaristatum | Oak&Lime | 5 | 6 | 6 | 3 | 5 | x | |||
| Geum urbanum | Oak&Lime | 5 | 5 | 5 | 5 | x | 7 | |||
| Lathyrus niger | Oak | Oak&Lime | 5 | 6 | 4 | 3 | 7 | 3 | ||
| Lathyrus venetus | Lime | Oak&Lime | 3 | 7 | 6 | 4 | 8 | 4 | ||
| Luzula luzuloides | Oak&Lime | 4 | x | 4 | 5 | 3 | 4 | |||
| Lychnis coronaria | Oak&Lime | 6 | 7 | 5 | 2 | 7 | 4 | |||
| Poa nemoralis | Oak&Lime | Oak&Lime | 5 | x | 5 | 5 | 5 | 4 | ||
| Potentilla micrantha | Oak&Lime | Oak | Oak&Lime | 5 | 7 | 4 | 3 | 7 | 4 | |
| Potentilla thuringiaca | Oak&Lime | 6 | 6 | 5 | 4 | 6 | 3 | |||
| Prunus avium | Oak&Lime | Oak&Lime | 5 | 5 | 4 | 5 | 7 | 5 | ||
| Quercus petraea | Oak&Lime | Oak&Lime | Oak&Lime | 6 | 5 | 2 | 4 | x | x | |
| Rubus canescens | Oak | Oak&Lime | 7 | 7 | 5 | 3 | x | 5 | ||
| Rubus hirtus | Oak&Lime | 5 | 4 | 5 | 5 | 5 | x | |||
| Sorbus torminalis | Oak | Oak&Lime | 5 | 7 | 4 | 3 | 7 | 4 | ||
| Stellaria holostea | Oak&Lime | 5 | 6 | 3 | 5 | 6 | 5 | |||
| Tanacetum corymbosum | Oak&Lime | 6 | 7 | 5 | 3 | 7 | 4 | |||
| Tilia tomentosa_sl | Oak&Lime | Oak&Lime | Oak&Lime | 6 | 7 | 5 | 3 | 7 | 4 | |
| Tilia tomentosa | Oak&Lime | |||||||||
| Verbascum glabratum | Lime | Oak&Lime | 7 | 7 | 7 | 3 | 7 | x | ||
| Viola alba | Oak&Lime | 6 | 7 | 4 | 4 | 7 | 6 | |||
| ∅ | 5.2 | 6.2 | 4.3 | 3.9 | 6.6 | 4.5 | ||||
| Lime | ||||||||||
| Alliaria petiolata | Lime | 5 | 6 | 3 | 5 | 7 | 9 | |||
| Cornus mas | Lime | 6 | 7 | 4 | 4 | 8 | 4 | |||
| Scrophularia nodosa | Lime | 3 | 5 | 3 | 6 | 6 | 7 | |||
| Scutellaria altissima | Lime | 5 | 7 | 6 | 4 | 7 | 6 | |||
| Lamium maculatum | Lime | 5 | x | 4 | 6 | 7 | 8 | |||
| Pteridium aquilinum | Lime | x | 5 | 3 | 4 | 3 | 3 | |||
| ∅ | 4.8 | 6.0 | 3.8 | 4.8 | 6.3 | 6.2 | ||||
| Summary of groups | ||||||||||
| Beech | 4.4 | 5.0 | 4.4 | 5.6 | 6.8 | 7.5 | ||||
| Beech/Oak | 3 | 5 | 4 | 5 | 7 | 6 | ||||
| Beech/Lime | 2.7 | 5.0 | 3.2 | 5.2 | 7.0 | 5.6 | ||||
| Oak | 5.6 | 5.8 | 3.8 | 4.3 | 6.5 | 4.3 | ||||
| Oak/Lime | 5.2 | 6.2 | 4.3 | 3.9 | 6.6 | 4.5 | ||||
| Lime | 4.8 | 6.0 | 3.8 | 4.8 | 6.3 | 6.2 |
References
- Machar, I.; Vlckova, V.; Salek, L.; Pechanec, V.; Nowak, A.; Nowak, S.; Plasek, V.; Svajda, J.; Oprsal, Z.; Topacoglu, O. Environmental Modelling of Forest Vegetation Zones as A Support Tool for Sustainable Management of Central European Spruce Forests. J. Landsc. Ecol. 2018, 11, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Buras, A.; Menzel, A. Projecting Tree Species Composition Changes of European Forests for 2061–2090 Under RCP 4.5 and RCP 8.5 Scenarios. Front. Plant Sci. 2019, 9, 1986. [Google Scholar] [CrossRef] [Green Version]
- Schuldt, B.; Buras, A.; Arend, M.; Vitasse, Y.; Beierkuhnlein, C.; Damm, A.; Gharun, M.; Grams, T.E.; Hauck, M.; Hajek, P.; et al. A first assessment of the impact of the extreme 2018 summer drought on Central European forests. Basic Appl. Ecol. 2020, 45, 86–103. [Google Scholar] [CrossRef]
- Gessler, A.; Keitel, C.; Kreuzwieser, J.; Matyssek, R.; Seiler, W.; Rennenberg, H. Potential risks for European beech (Fagus sylvatica L.) in a changing climate. Trees 2006, 21, 1–11. [Google Scholar] [CrossRef]
- Hohnwald, S.; Indreica, A.; Walentowski, H.; Leuschner, C. Microclimatic Tipping Points at the Beech–Oak Ecotone in the Western Romanian Carpathians. Forests 2020, 11, 919. [Google Scholar] [CrossRef]
- Leuschner, C. Drought response of European beech (Fagus sylvatica L.)—A review. Perspect. Plant Ecol. Evol. Syst. 2020, 47, 125576. [Google Scholar] [CrossRef]
- Hof, A.; Dymond, C.C.; Mladenoff, D.J. Climate change mitigation through adaptation: The effectiveness of forest diversification by novel tree planting regimes. Ecosphere 2017, 8, e01981. [Google Scholar] [CrossRef]
- Thom, D.; Keeton, W.S. Disturbance-based silviculture for habitat diversification: Effects on forest structure, dynamics, and carbon storage. For. Ecol. Manag. 2020, 469, 118132. [Google Scholar] [CrossRef]
- Pedro, M.S.; Rammer, W.; Seidl, R. Tree species diversity mitigates disturbance impacts on the forest carbon cycle. Oecologia 2015, 177, 619–630. [Google Scholar] [CrossRef]
- Ammer, C. Diversity and forest productivity in a changing climate. New Phytol. 2018, 221, 50–66. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.I.; Dumroese, R.K. Preparing for Climate Change: Forestry and Assisted Migration. J. For. 2013, 111, 287–297. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Wang, H.-H.; Grant, W.E.; Byram, T.D. Plants on the move: Assisted migration of forest trees in the face of climate change. For. Ecol. Manag. 2015, 344, 30–37. [Google Scholar] [CrossRef]
- Schmiedinger, A.; Bachmann, M.; Kölling, C.; Schirmer, R. Verfahren zur Auswahl von Baumarten für Anbauversuche vor dem Hintergrund des Klimawandels. Forstarchiv 2009, 80, 15–22. [Google Scholar]
- Mette, T.; Brandl, S.; Kölling, C. Climate Analogues for Temperate European Forests to Raise Silvicultural Evidence Using Twin Regions. Sustainability 2021, 13, 6522. [Google Scholar] [CrossRef]
- Frischbier, N.; Nikolova, P.S.; Brang, P.; Klumpp, R.; Aas, G.; Binder, F. Climate change adaptation with non-native tree species in Central European forests: Early tree survival in a multi-site field trial. Eur. J. For. Res. 2019, 138, 1015–1032. [Google Scholar] [CrossRef]
- Binder, F. Silberlinde—Baumart mit Chancen im Klimawandel? AFZ-Der Wald 2015, 70, 23–27. [Google Scholar]
- Pigott, C.D. Lime-Trees and Basswoods: A Biological Monograph of the Genus Tilia; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 1–395. [Google Scholar]
- Radoglou, K.; Dobrowolska, D.; Spyroglou, G.; Nicolescu, V.N. A review on the ecology and silviculture of limes (Tilia cordata Mill., Tilia platyphyllos Scop. and Tilia tomentosa Moench.) in Europe. Die Bodenkult. 2009, 60, 7–17. [Google Scholar]
- Sjöman, H.; Oprea, A. Potential of Tilia tomentosa Moench, for use in urban environments in north-west Europe, based on habitat studies in north-east Romania and the Republic of Moldova. Ekológia (Bratislava) 2010, 29, 360–372. [Google Scholar] [CrossRef]
- Purcelean, S.; Pascovschi, S.; Leandru, V.; Drăguț, N.; Stoiculescu, C. Cercetări tipologice în teișuri și arborete amestecate cu participarea teiului. An. ICAS 1970, 27, 135–162. [Google Scholar]
- Zagwijn, W.H. An analysis of Eemian climate in Western and Central Europe. Quat. Sci. Rev. 1996, 15, 451–469. [Google Scholar] [CrossRef]
- Kupryjanowicz, M.; Granoszewski, W.; Fiłoc, M. New finds of Eemian Tilia tomentosa Moench macroremais in NE Poland, and the reconstructed European range of this species during the last interglacial. Quat. Int. 2018, 467, 107–116. [Google Scholar] [CrossRef]
- Horvat, I.; Glavac, H.; Ellenberg, H. Vegetation Südosteuropas; G. Fischer: Jena, Germany, 1974; pp. 1–767. [Google Scholar]
- Marinšek, A.; Šilc, U.; Carni, A. Geographical and ecological differentiation of Fagus forest vegetation in SE Europe. Appl. Veg. Sci. 2012, 16, 131–147. [Google Scholar] [CrossRef]
- Coldea, G.; Indreica, A.; Oprea, A. Les Associations Végétales de Roumanie. Tome 3—Les Associations Forestiéres et Arbustives; Presa Universitară Clujeană: Cluj-Napoca, Romania, 2015; pp. 1–281. [Google Scholar]
- Indreica, A.; Teodosiu, M.; Petrițan, A.M.; Öder, V.; Kasper, J.; Bergmeier, E.; Leuschner, C.; Gailing, O.; Hohnwald, S.; Wildhagen, H.; et al. Nemoral deciduous forests under climatic extremes—Phytosociological studies along climatic gradients in SW Romania. In Proceedings of the Biennial International Symposium “Forest and Sustainable Development”, 8th ed.; Transilvania University Press: Brasov, Romania, 2019; pp. 139–148. [Google Scholar]
- European Commission. Interpretation manual of European Union Habitats. EUR 2013, 28, 1–144. [Google Scholar]
- Doniță, N.; Purcelean, S. Pădurile de șleau din R.S.România și Gospodărirea lor; Ceres Publishing House: Bucharest, Romania, 1975; pp. 1–185. [Google Scholar]
- Dinić, A.L.; Mišić, V.P.; Savić, D.L. Silver Linden (Tilia tomentosa MOENCH) in the community of sessile oak and hornbean (Rusco-Quercetum-Carpinetum, B.Jov. 1979 tilietosum tomentosae subass. nova) on the Fruška Gora Mountain. Matica Srp. Proc. Nat. Sci. 1991, 97, 63–78. [Google Scholar]
- Gafta, D.; Mountford, J.O. (Eds.) Manual de Interpretare a Habitatelor Natura 2000 din România; Risoprint: Cluj-Napoca, Romania, 2008; pp. 1–105. [Google Scholar]
- Jacquemart, A.-L.; Moquet, L.; Ouvrard, P.; Quetin-Leclercq, J.; Hérent, M.-F.; Quinet, M. Tilia trees: Toxic or valuable resources for pollinators? Apidologie 2018, 49, 538–550. [Google Scholar] [CrossRef] [Green Version]
- Hagemeier, M. Funktionale Kronenarchitektur mitteleuropäischer Baumarten am Beispiel von Hängebirke, Waldkiefer, Traubeneiche, Hainbuche, Winterlinde und Rotbuche. Diss. Bot. 2001, 361, 1–154. [Google Scholar]
- Šebesta, J.; Maděra, P.; Řepka, R.; Matula, R. Comparison of vascular plant diversity and species composition of coppice and high beech forest in the Banat region, Romania. Folia Geobot. Phytotaxon. 2017, 52, 33–43. [Google Scholar] [CrossRef]
- Anca, T. Teiul Și Rolul lui în Producția Forestieră din România [Lime and Its Role in Romanian Forestry]. Ph.D. Thesis, University of Brașov, Brasov, Romania, 1969. [Google Scholar]
- Mette, T.; Dolos, K.; Meinardus, C.; Bräuning, A.; Reineking, B.; Blaschke, M.; Pretzsch, H.; Beierkuhnlein, C.; Gohlke, A.; Wellstein, C. Climatic turning point for beech and oak under climate change in Central Europe. Ecosphere 2013, 4, 1–19. [Google Scholar] [CrossRef]
- Öder, V.; Petritan, A.M.; Schellenberg, J.; Bergmeier, E.; Walentowski, H. Patterns and drivers of deadwood quantity and variation in mid-latitude deciduous forests. For. Ecol. Manag. 2021, 487, 118977. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Nicolescu, V.N. The Practice of Silviculture; Aldus: Brasov, Romania, 2018; pp. 1–254. [Google Scholar]
- Doniță, N.; Ivan, D.; Coldea, G.; Sanda, V.; Popescu, A.; Chifu, T.; Paucă-Comănescu, M.; Mititelu, D.; Boscaiu, N. Vegetația României; Tehnică Agricolă: Bucuresti, Romania, 1992. [Google Scholar]
- Karger, D.N.; Conrad, O.; Boehner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [Green Version]
- Van der Maarel, E.; Franklin, J. (Eds.) Vegetation Ecology, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A. Plante Vasculare din România. Determinator Ilustrat de Teren; Victor Publishing House: Bucharest, Romania, 2013. [Google Scholar]
- Hoppmann, D. Die direkte Sonneneinstrahlung. In Die Standortkartierung der hessischen Weinbaugebiete; Löhnertz, O., Hoppmann, D., Emde, K., Friedrich, K., Schmanke, M., Zimmer, T., Eds.; Hessisches Landesamt für Naturschutz, Umwelt und Geologie: Wiesbaden, Germany, 2004; Volume 114, pp. 27–38. [Google Scholar]
- Dormann, C.F.; Bagnara, M.; Boch, S.; Hinderling, J.; Janeiro-Otero, A.; Schäfer, D.; Schall, P.; Hartig, F. Plant species richness increases with light availability, but not variability, in temperate forests understorey. BMC Ecol. 2020, 20, 43. [Google Scholar] [CrossRef]
- Sercu, B.K.; Baeten, L.; Van Coillie, F.; Martel, A.; Lens, L.; Verheyen, K.; Bonte, D. How tree species identity and diversity affect light transmittance to the understory in mature temperate forests. Ecol. Evol. 2017, 7, 10861–10870. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.; Su, Y.-S. Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R package Version 1.11-2. 2020. Available online: https://CRAN.R-project.org/package=arm (accessed on 16 July 2021).
- Grueber, C.E.; Nakagawa, S.; Laws, R.J.; Jamieson, I.G. Multimodel inference in ecology and evolution: Challenges and solutions. J. Evol. Biol. 2011, 24, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Kamil, B. MuMIn: Multi-Model Inference. R Package Version 1.43.17. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 16 July 2021).
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2010, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. lme4: Linear mixed-effects models using Eigen and SR package version 1.1. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Estimating regression models with unknown break-points. Stat. Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen, 5th ed.; Ulmer: Stuttgart, Germany, 1996. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.4-2. Available online: http://CRAN.R-project.org/package=vegan (accessed on 16 July 2021).
- De Cáceres, M.; Legendre, P. Associations between species and groups of sites: Indices and statistical inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Vilches, B.; De Cáceres, M.; Sánchez-Mata, D.; Gavilán, R.G. Indicator species of broad-leaved oak forests in the eastern Iberian Peninsula. Ecol. Indic. 2013, 26, 44–48. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Heinrichs, S.; Ammer, C.; Mund, M.; Boch, S.; Budde, S.; Fischer, M.; Müller, J.; Schöning, I.; Schulze, E.-D.; Schmidt, W.; et al. Landscape-Scale Mixtures of Tree Species are More Effective than Stand-Scale Mixtures for Biodiversity of Vascular Plants, Bryophytes and Lichens. Forests 2019, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Katona, K.; Kiss, M.; Bleier, N.; Székely, J.; Nyeste, M.; Kovács, V.; Terhes, A.; Fodor, Á.; Olajos, T.; Rasztovits, E.; et al. Ungulate browsing shapes climate change impacts on forest biodiversity in Hungary. Biodivers. Conserv. 2013, 22, 1167–1180. [Google Scholar] [CrossRef] [Green Version]
- Schädler, M.; Brandl, R. Do invertebrate decomposers affect the disappearance rate of litter mixtures? Soil Biol. Biochem. 2005, 37, 329–337. [Google Scholar] [CrossRef]
- Mölder, A.; Bernhardt-Römermann, M.; Leuschner, C.; Schmidt, W. Zur Bedeutung der Winterlinde (Tilia cordata Mill.) in mittel- und nordwestdeutschen Eichen-Hainbuchen-Wäldern. Tuexenia 2009, 29, 9–23. [Google Scholar]
- De Jaegere, T.; Hein, S.; Claessens, H. A Review of the Characteristics of Small-Leaved Lime (Tilia cordata Mill.) and Their Implications for Silviculture in a Changing Climate. Forests 2016, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Mölder, A.; Bernhardt-Römermann, M.; Schmidt, W. Herb-layer diversity in deciduous forests: Raised by tree richness or beaten by beech? For. Ecol. Manag. 2008, 256, 272–281. [Google Scholar] [CrossRef]
- Dimitrova, V.G. Distribution and Assessment of the Nature Conservational Status of the Nature Habitat 91Z0 “Moesian Silver Lime Forests” in SCI “Svishtovska Gora” (BG0000576), Bulgaria. Ecol. Balk. 2015, 7, 13–19. [Google Scholar]
- Vockenhuber, E.A.; Scherber, C.; Langenbruch, C.; Meißner, M.; Seidel, D.; Tscharntke, T. Tree diversity and environmental context predict herb species richness and cover in Germany’s largest connected deciduous forest. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 111–119. [Google Scholar] [CrossRef]
- Le Due, M.; Havill, D. Competition between Quercus petraea and Carpinus betulus in an ancient wood in England: Seedling survivorship. J. Veg. Sci. 1998, 9, 873–880. [Google Scholar] [CrossRef]
- Chudomelová, M.; Hédl, R.; Zouhar, V.; Szabó, P. Open oakwoods facing modern threats: Will they survive the next fifty years? Biol. Conserv. 2017, 210, 163–173. [Google Scholar] [CrossRef]
- Mölder, A.; Streit, M.; Schmidt, W. When beech strikes back: How strict nature conservation reduces herb-layer diversity and productivity in Central European deciduous forests. For. Ecol. Manag. 2014, 319, 51–61. [Google Scholar] [CrossRef]
- Brändle, M.; Brandl, R. Species richness of insects and mites on trees: Expanding Southwood. J. Anim. Ecol. 2001, 70, 491–504. [Google Scholar] [CrossRef]
- Vogel, S.; Bussler, H.; Finnberg, S.; Müller, J.; Stengel, E.; Thorn, S. Diversity and conservation of saproxylic beetles in 42 European tree species: An experimental approach using early successional stages of branches. Insect Conserv. Divers. 2021, 14, 132–143. [Google Scholar] [CrossRef]
- Machar, I.; Vozenilek, V.; Simon, J.; Pechanec, V.; Brus, J.; Fulnecek, P.; Vitek, T. Joining of the historical research and future prediction as a support tool for the assessment of management strategy for European beech-dominated forests in protected areas. Nat. Conserv. 2017, 22, 51–78. [Google Scholar] [CrossRef] [Green Version]
- Montwé, D.; Isaac-Renton, M.; Hamann, A.; Spiecker, H. Cold adaptation recorded in tree rings highlights risks associated with climate change and assisted migration. Nat. Commun. 2018, 9, 1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walentowski, H.; Falk, W.; Mette, T.; Kunz, J.; Bräuning, A.; Meinardus, C.; Zang, C.; Sutcliffe, L.M.; Leuschner, C. Assessing future suitability of tree species under climate change by multiple methods: A case study in southern Germany. Ann. For. Res. 2014, 60, 101–126. [Google Scholar] [CrossRef] [Green Version]

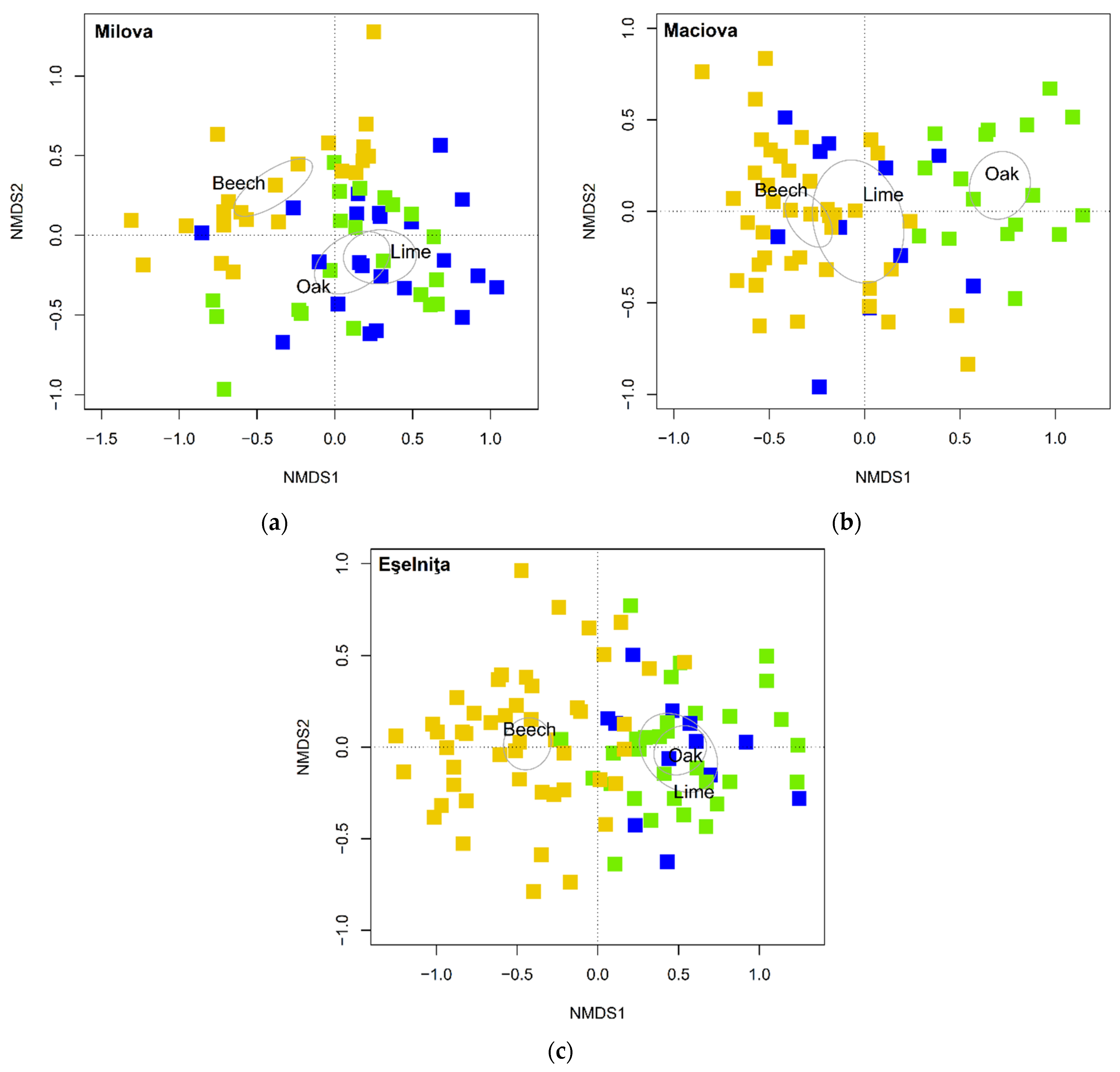
| Milova | Maciova | Eşelniţa | |
|---|---|---|---|
| Location | 46°07.627′ N, 21°47.963′ E | 45°31.488′ N, 22°12.824′ E | 44°44.025′ N, 22°20.710′ E |
| Area | 268 ha | 229 ha | 254 ha |
| Region | Zarand Mountains | Poiana-Ruscă Mountains | Almăj Mountains |
| Bedrock | Slate and granite covered with loess | Sandstone (with some pyroclastic areas) covered with loess | Gneiss and granite covered with loess |
| Soil | Base-rich Luvisols and Cambisols | ||
| Mean stand age (years) | 95 | 70 | 90 |
| Annual temperature (tavg in °C) | 7.9–10.9 | 8.2–11.0 | 7.8–11.8 |
| Annual precipitation (Prec in mm) | 679–892 | 806–951 | 583–844 |
| Investigated elevational range [m a.s.l.] | 253–762 | 290–717 | 170–907 |
| Elevational range of plots with T. tomentosa [m a.s.l] | 343–729 | 321–650 | 190–795 |
| Number of plots with T. tomentosa | 44 (89) 49.4% | 42 (96) 43.8% | 94 (159) 59.1% |
| Ellenberg Quotient (EQ) of plots with T. tomentosa | 25.8 ± 2.5 a (20.6–30.2) | 23.0 ± 1.2 b (21.2–25.9) | 31.6 ± 3.6 c (23.6–39.3) |
| Tree species shares in plots with T. tomentosa [%] | |||
| T. tomentosa | 49.6 ± 33.4 a (0.5–100) | 26.5 ± 27.4 b (0.1–100) | 26.1 ± 20.8 b (0.4–97.2) |
| F. sylvatica | 11.3 ± 21.4 a (0–85.0) | 30.1 ± 35.6 b (0–97.2) | 27.1 ± 33.5 b (0–96.2) |
| Mesophilous oak (Q. petraea/robur) | 27.3 ± 28.4 ab (0–94.1) | 19.3 ± 27.8 a (0–97.2) | 33.3 ± 28.8 b (0–97.2) |
| Thermophilous oak (Q. frainetto/cerris) | 1.1 ± 5.4 a (0–35.7) | 5.4 ± 15.3 a (0–57.1) | 1.2 ± 9.1 a (0–84.8) |
| Carpinus betulus | 7.1 ± 12.2 ab (0–50.0) | 13.1 ± 17.1 a (0–70.2) | 4.6 ± 12.3 b (0–80.0) |
| Species richness of plots with T. tomentosa | |||
| Tree layer | 3.3 ± 1.3 (1–6) | 3.3 ± 1.3 (1–7) | 3.7 ± 1.1 (2–7) |
| Shrub layer | 1.5 ± 1.3 a (0–6) | 2.1 ± 2.0 ab (0–7) | 2.2 ± 1.6 b (0–8) |
| Herb layer | 15.5 ± 7.2 a (3–37) | 16.4 ± 6.2 a (5–31) | 22.7 ± 8.0 b (6–46) |
| Estimate | SE | z-Value | p-Value | |
|---|---|---|---|---|
| (Intercept) Milova | 39.52 a | 4.57 | 8.602 | <0.001 |
| (Intercept) Maciova | 26.26 ab | 8.26 | 3.161 | <0.001 |
| (Intercept) Eşelniţa | 20.38 b | 3.63 | 5.583 | 0.001 |
| Local abiotic factors | ||||
| Soil reaction x Milova | −37.96 a | 9.56 | 3.948 | <0.001 |
| x Maciova | −7.58 b | 6.29 | 1.198 | 0.231 |
| xEşelniţa | 9.95 b | 7.43 | 1.332 | 0.183 |
| Nitrogen x Milova | −5.01 | 10.41 | 0.480 | 0.631 |
| x Maciova | −3.21 | 8.02 | 0.399 | 0.690 |
| xEşelniţa | −0.12 | 3.93 | 0.031 | 0.975 |
| Moisture x Milova | 2.52 | 10.26 | 0.246 | 0.806 |
| x Maciova | 1.76 | 7.36 | 0.238 | 0.812 |
| xEşelniţa | −1.22 | 4.09 | 0.297 | 0.767 |
| Aspect x Milova | 0.20 | 1.84 | 0.110 | 0.912 |
| x Maciova | −0.76 | 3.53 | 0.214 | 0.830 |
| xEşelniţa | 0.28 | 1.72 | 0.161 | 0.872 |
| Climatic factors | ||||
| Tavg x Milova | −0.86 | 8.95 | 0.095 | 0.924 |
| x Maciova | 1.37 | 6.10 | 0.224 | 0.823 |
| xEşelniţa | 2.89 | 4.65 | 0.620 | 0.535 |
| Prec x Milova | −0.73 | 5.80 | 0.126 | 0.900 |
| x Maciova | −1.81 | 9.93 | 0.181 | 0.856 |
| xEşelniţa | −2.47 | 7.08 | 0.349 | 0.727 |
| Mean R2 | 25.5 ± 1.8 SD (23.6–28.8) | |||
| Estimate | SE | z Value | p-Value | |
|---|---|---|---|---|
| (a) Cover T. tomentosa in the shrub layer (>1 m <9 m height) | ||||
| (Intercept) | 2.28 | 0.36 | 6.31 | <0.001 |
| Local abiotic factors | ||||
| Share Beech | −2.08 | 0.76 | 2.74 | 0.006 |
| Species richness tree layer | −0.14 | 0.45 | 0.32 | 0.748 |
| Nitrogen | −0.43 | 0.70 | 0.61 | 0.541 |
| Soil reaction | −0.02 | 0.21 | 0.12 | 0.905 |
| Moisture | −0.02 | 0.21 | 0.08 | 0.934 |
| Aspect | 0.20 | 0.51 | 0.39 | 0.700 |
| Climatic Factors | ||||
| Tavg | −0.09 | 0.37 | 0.25 | 0.801 |
| Mean R2 | 5.8 ± 0.6 (5.0–6.6) | |||
| (b) Cover T. tomentosa in the herb layer (≤1 m height) | ||||
| (Intercept) Milova | 0.75 | 0.71 | 1.06 | 0.290 |
| (Intercept) Maciova | 2.34 | 1.04 | 2.23 | 0.025 |
| (Intercept) Eşelniţa | 1.34 | 0.58 | 2.28 | 0.022 |
| Local abiotic factors | ||||
| Species richness tree layer x Milova | 0.36 a | 0.98 | 0.37 | 0.711 |
| x Maciova | 4.76 b | 0.91 | 5.21 | <0.001 |
| x Eşelniţa | −0.67 a | 0.81 | 0.83 | 0.409 |
| Total tree layer cover | −0.27 | 0.47 | 0.56 | 0.575 |
| Share beech | −0.32 | 0.58 | 0.55 | 0.581 |
| Slope | −0.03 | 0.19 | 0.16 | 0.877 |
| Moisture x Milova | 1.33 | 1.65 | 0.80 | 0.422 |
| x Maciova | 2.39 | 1.23 | 1.94 | 0.053 |
| xEşelniţa | 1.96 | 0.94 | 2.07 | 0.039 |
| Soil reaction | −0.03 | 0.18 | 0.15 | 0.880 |
| Climatic factors | ||||
| Tavg x Milova | 1.30 a | 1.93 | 0.67 | 0.504 |
| x Maciova | 6.87 b | 1.86 | 3.66 | <0.001 |
| xEşelniţa | −0.02 a | 0.84 | 0.02 | 0.982 |
| Prec | −0.19 | 1.17 | 0.16 | 0.870 |
| Mean R2 | 26.1 ± 1.0 (25.0–28.1) | |||
| Milova | Maciova | Eşelniţa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beech | Oak | Lime | Beech | Oak | Lime | Beech | Oak | Lime | |
| n | 22 | 21 | 22 | 40 | 17 | 11 | 50 | 31 | 12 |
| Local abiotic factors | |||||||||
| Moisture | 5.1 ± 0.3 a | 4.8 ± 0.2 b | 4.8 ± 0.2 b | 5.2 ± 0.3 a | 4.5 ± 0.2 b | 4.9 ± 0.2 c | 4.7 ± 0.3 a | 4.3 ± 0.2 b | 4.1 ± 0.2 b |
| Nitrogen | 5.6 ± 0.8 a | 5.2 ± 0.5 ab | 5.0 ± 0.5 b | 5.4 ± 0.4 a | 4.5 ± 0.5 b | 4.9 ± 0.4 c | 4.7 ± 0.7 | 4.9 ± 0.5 | 4.9 ± 0.5 |
| Soil reaction | 6.4 ± 0.4 | 6.4 ± 0.3 | 6.3 ± 0.4 | 6.2 ± 0.4 | 6.2 ± 0.4 | 6.1 ± 0.7 | 6.3 ± 0.5 a | 6.6 ± 0.3 b | 6.7 ± 0.2 b |
| Slope [°] | 12.6 ± 8.6 | 13.7 ± 9.8 | 15.2 ± 9.0 | 17.6 ± 0.8 | 20.0 ± 9.5 | 22.2 ± 9.1 | 16.0 ± 9.8 | 21.1 ± 11.8 | 21.7 ± 8.6 |
| Aspect index | 6.5 ± 2.2 | 6.2 ± 1.9 | 5.5 ± 2.6 | 6.2 ± 2.5 | 7.5 ± 1.5 | 5.9 ± 2.7 | 5.1 ± 2.3 a | 7.4 ± 1.4 b | 7.2 ± 2.1 b |
| Elevation [m a.s.l] | 655.7 ± 91.2 a | 485.5 ± 89.1 b | 506.8 ± 76.5 b | 567.8 ± 107.3 a | 448.8 ± 61.7 b | 481.9 ± 69.0 b | 540. 8 ± 94.7 a | 477.7 ± 154.7 b | 377.9 ± 129.4 c |
| Climatic factors | |||||||||
| Tavg [°C] | 8.3 ± 0.6 a | 9.4 ± 0.7 b | 9.4 ± 0.6 b | 9.2 ± 0.6 a | 10.1 ± 0.4 b | 9.7 ± 0.4 b | 9.7 ± 0.7 a | 10.0 ± 1.1 a | 10.7 ± 0.8 b |
| Prec [mm] | 869.0 ± 48.1 a | 787.8 ± 55.7 b | 786.9 ± 48.5 b | 897.8 ± 20.0 a | 877.9 ± 29.7 b | 888.4 1 ± 3.0 ab | 696.3 ± 30.5 a | 679.9 ± 53.5 ab | 652.2 ± 38.7 b |
| EQ | 21.8 ± 2.2 | 25.6 ± 2.8 | 25.5 ± 2.3 | 22.2 ± 1.2 a | 23.7 ± 1.3 b | 23.0 ± 0.8 ab | 29.7 ± 2.3 a | 31.1 ± 3.9 b | 33.4 ± 3.3 b |
| Canopy characteristics | |||||||||
| Total tree layer cover [%] | 93.1 ± 23.6 | 93.5 ± 30.7 | 94.7 ± 20.1 | 96.8 ± 19.1 | 90.2 ± 20.5 | 105.5 ± 15.8 | 89.5 ± 18.0 a | 86.2 ± 21.7 a | 104.9 ± 22.2 b |
| Tree species share of total tree layer cover [%] | |||||||||
| Beech | 81.0 ± 17.0 a | 3.0 ± 9.5 b | 3.6 ± 10.4 b | 81.9 ± 17.2 a | 2.3 ± 8.9 b | 11.3 ± 13.0 b | 84.8 ± 15.6 a | 5.1 ± 12.5 b | 7.0 ± 14.8 b |
| Mesophil. oak | 8.3 ± 12.4 a | 68.3 ± 15.3b | 11.5 ± 14.4 a | 2.4 ± 7.5 a | 75.6 ± 15.1 b | 7.1 ± 13.9 a | 4.8 ± 8.7 a | 73.6 ± 16.2 b | 22.3 ± 15.2 c |
| Thermophil. oak | 0 | 2.3 ± 8.0 | 0 | 0.5 ± 2.5 a | 6.7 ± 13.4 b | 0 a | 0 | 0.8 ± 4.2 | 0.1 ± 0.2 |
| Silver lime | 2.9 ± 8.1 a | 7.5 ± 11.9 a | 78.0 ± 17.9 b | 3.1 ± 7.8 a | 2.1 ± 4.6 a | 66.7 ± 14.7 b | 7.5 ± 9.9 a | 12.6 ± 12.5 a | 62.9 ± 17.2 b |
| Hornbeam | 4.0 ± 10.9 a | 16.6 ± 18.7 b | 3.8 ± 7.8 a | 3.8 ± 8.8 a | 8.3 ± 12.4 ab | 14.3 ± 15.8 b | 0.3 ± 1.0 a | 1.1 ± 3.5 ab | 2.4 ± 4.4 b |
| Mean species richness per plot | |||||||||
| Tree layer | 2.6 ± 1.0 | 3.2 ± 1.3 | 2.6 ± 1.1 | 2.9 ± 1.4 | 2.8 ± 1.0 | 2.9 ± 0.9 | 2.7 ± 1.5 | 2.9 ± 1.1 | 3.6 ± 0.9 |
| Shrub layer | 1.6 ± 1.7 | 1.2 ± 1.3 | 1.7 ± 1.5 | 0.9 ± 0.8 a | 3.1 ± 1.9 b | 1.3 ± 0.9 a | 1.0 ± 0.8 a | 2.8 ± 1.4 b | 2.9 ± 1.5 b |
| Herb layer | 18.6 ± 5.1 a | 17.4 ± 6.4 a | 12.8 ± 7.3 b | 12.5 ± 4.8 a | 22.0 ± 8.1 b | 13.5 ± 3.8 a | 13.8 ± 7.8 a | 25.6 ± 6.5 b | 22.2 ± 5.4 b |
| Milova | Maciova | Eşelniţa | EIV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | T | C | M | R | N | ||||
| Beech and/or oak | |||||||||
| Dentaria bulbifera | Beech&Oak | Beech | 3 | 5 | 4 | 5 | 7 | 6 | |
| Galium schultesii | Oak | Oak | 5 | 5 | 5 | 4 | 7 | 4 | |
| Beech & Lime | |||||||||
| Carex digitata | Lime | Beech&Oak | 3 | x | 4 | 5 | x | 4 | |
| Fagus sylvatica | Beech | Beech&Lime | Beech | 2 | 5 | 3 | 6 | x | x |
| Fagus sylvatica_sl | Beech | Beech&Lime | |||||||
| Galium odoratum | Beech | Beech&Lime | Beech | 2 | 5 | 2 | 5 | 6 | 5 |
| Mercurialis perennis | Beech | Beech&Lime | 3 | x | 3 | 5 | 8 | 7 | |
| ∅ EIV | 2.5 | 5.0 | 3.0 | 5.3 | 7.0 | 5.3 | |||
| Oak & Lime | |||||||||
| Cornus mas_sl | Oak&Lime | Oak&Lime | Oak&Lime | 6 | 7 | 4 | 4 | 8 | 4 |
| Quercus petraea | Oak&Lime | Oak&Lime | Oak&Lime | 6 | 5 | 2 | 4 | x | x |
| Tilia tomentosa_sl | Oak&Lime | Oak&Lime | Oak&Lime | 5 | 7 | 6 | 5 | 7 | 5 |
| Poa nemoralis | Oak&Lime | Oak&Lime | 5 | x | 5 | 5 | 5 | 4 | |
| Prunus avium | Oak&Lime | Oak&Lime | 5 | 5 | 4 | 5 | 7 | 5 | |
| Brachypodium sylvaticum | Oak | Oak&Lime | 3 | 5 | 3 | 5 | 6 | 6 | |
| Clinopodium vulgare | Oak | Oak&Lime | 7 | x | 3 | 4 | 7 | 3 | |
| Dactylis glomerata | Oak | Oak&Lime | 6 | x | 3 | 4 | x | 6 | |
| Festuca heterophylla | Oak | Oak&Lime | 5 | 6 | 4 | 4 | 5 | 5 | |
| Fraxinus ornus_sl | Oak | Oak&Lime | 6 | 8 | 4 | 3 | 8 | 3 | |
| Lathyrus niger | Oak | Oak&Lime | 5 | 6 | 4 | 3 | 7 | 3 | |
| Lathyrus venetus | Lime | Oak&Lime | 3 | 7 | 6 | 4 | 8 | 4 | |
| Potentilla micrantha | Oak&Lime | Oak | Oak&Lime | 5 | 7 | 4 | 3 | 7 | 4 |
| Rubus canescens | Oak | Oak&Lime | 7 | 7 | 5 | 3 | x | 5 | |
| Sorbus torminalis | Oak | Oak&Lime | 5 | 7 | 4 | 3 | 7 | 4 | |
| Verbascum glabratum | Lime | Oak&Lime | 7 | 7 | 7 | 3 | 7 | x | |
| ∅ EIV | 5.4 | 6.5 | 4.3 | 3.9 | 6.8 | 4.4 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinrichs, S.; Öder, V.; Indreica, A.; Bergmeier, E.; Leuschner, C.; Walentowski, H. The Influence of Tilia tomentosa Moench on Plant Species Diversity and Composition in Mesophilic Forests of Western Romania–A Potential Tree Species for Warming Forests in Central Europe? Sustainability 2021, 13, 7996. https://doi.org/10.3390/su13147996
Heinrichs S, Öder V, Indreica A, Bergmeier E, Leuschner C, Walentowski H. The Influence of Tilia tomentosa Moench on Plant Species Diversity and Composition in Mesophilic Forests of Western Romania–A Potential Tree Species for Warming Forests in Central Europe? Sustainability. 2021; 13(14):7996. https://doi.org/10.3390/su13147996
Chicago/Turabian StyleHeinrichs, Steffi, Veronika Öder, Adrian Indreica, Erwin Bergmeier, Christoph Leuschner, and Helge Walentowski. 2021. "The Influence of Tilia tomentosa Moench on Plant Species Diversity and Composition in Mesophilic Forests of Western Romania–A Potential Tree Species for Warming Forests in Central Europe?" Sustainability 13, no. 14: 7996. https://doi.org/10.3390/su13147996
APA StyleHeinrichs, S., Öder, V., Indreica, A., Bergmeier, E., Leuschner, C., & Walentowski, H. (2021). The Influence of Tilia tomentosa Moench on Plant Species Diversity and Composition in Mesophilic Forests of Western Romania–A Potential Tree Species for Warming Forests in Central Europe? Sustainability, 13(14), 7996. https://doi.org/10.3390/su13147996







