Biodegradation of Keratin-Rich Husbandry Waste as a Path to Sustainable Agriculture
Abstract
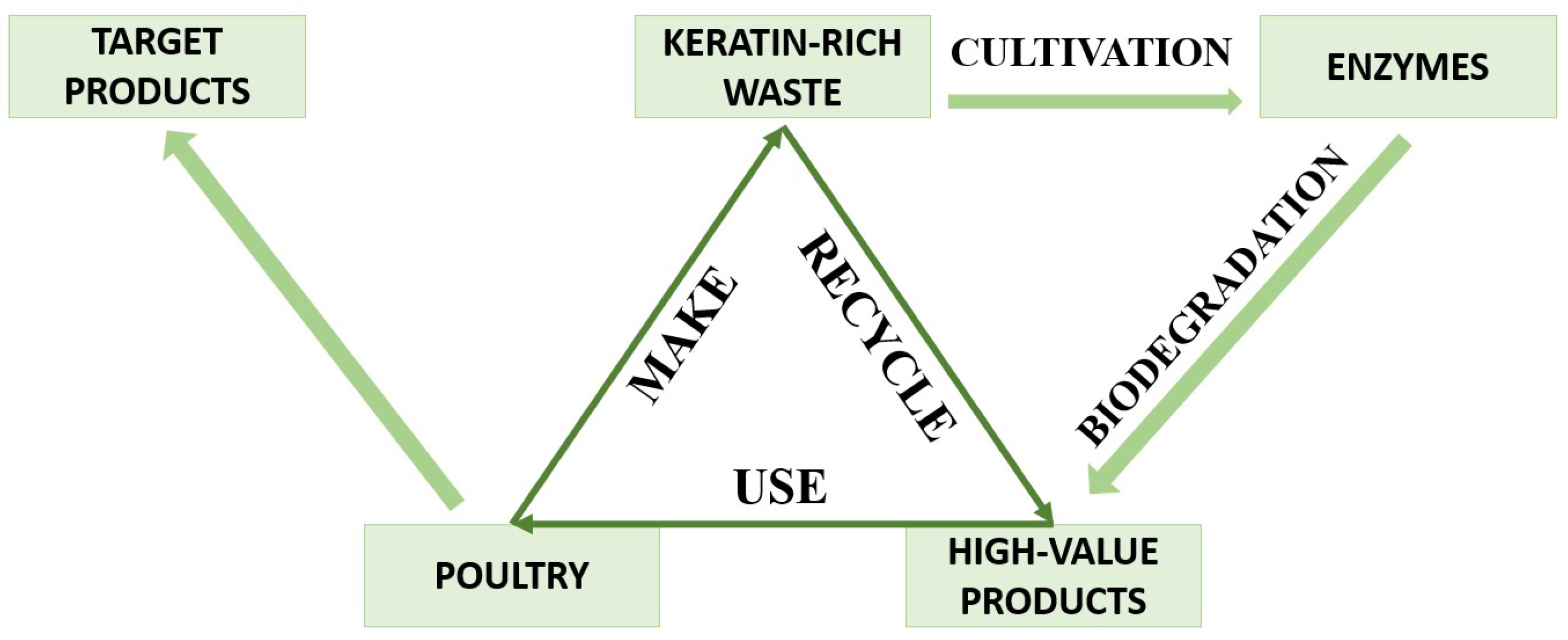
:1. Introduction
2. Poultry Industry Dynamics and Analysis
3. Chicken Feather Waste and Current Waste Management
4. Biodegradation of Keratin Waste
5. Keratinases, Their Sources and Use for Biodegradation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Available online: https://population.un.org/wpp/Download/Probabilistic/Population/ (accessed on 29 June 2021).
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/documents/card/en/c/ca9699en (accessed on 29 June 2021).
- Gulseven, O.; Al Harmoodi, F.; Al Falasi, M.; ALshomali, I. How the COVID-19 Pandemic Will Affect the UN Sustainable Development Goals? SSRN Electron. J. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3592933 (accessed on 29 June 2021). [CrossRef]
- Sustainable Agriculture Research & Education Program. Available online: https://sarep.ucdavis.edu/sustainable-ag (accessed on 29 June 2021).
- Brown, L. The Emerging Politics of Food Scarcity. In World on the Edge, 1st ed.; W. W. Norton & Company: New York, NY, USA, 2011; pp. 59–71. [Google Scholar]
- Ritchie, H.; Roser, M. Meat and Dairy Production. 2019. Available online: https://ourworldindata.org/meat-production#citation (accessed on 29 June 2021).
- The Business Research Company. Available online: https://www.thebusinessresearchcompany.com/report/poultry-market (accessed on 29 June 2021).
- Singh, P.; Mondal, T.; Sharma, R.; Mahalakshmi, N.; Gupta, M. Poultry Waste Management. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 694–700. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D. Valorisation of chicken feathers: A review on recycling and recovery route—Current status and future prospects. Clean Technol. Environ. Policy 2017, 19, 2363–2378. [Google Scholar] [CrossRef]
- Bouhamed, S.; Kechaou, N. Kinetic study of sulphuric acid hydrolysis of protein feathers. Bioprocess Biosyst. Eng. 2017, 40, 715–721. [Google Scholar] [CrossRef]
- Pahua-Ramos, M.; Hernandez-Melchor, D.; Camacho-Pérez, B.; Quezada-Cruz, M. Degradation of chicken feathers: A review. Biotechnol. Ind. J. 2017, 13, 1–24. [Google Scholar]
- Stingone, J.; Wing, S. Poultry litter incineration as a source of energy reviewing the potential impacts on environmental health and justice. New Solut. 2011, 21, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Chiramba, R.; Charis, G.; Fungura, N.; Danha, G.; Mamvura, T. Production of activated carbon from poultry feathers for waste water treatment. Water Sci. Technol. 2019, 80, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Pajarito, B.; Belarmino, A.; Calimbas, R.; Gonzales, J. Graphite Nanoplatelets from Waste Chicken Feathers. Materials 2020, 13, 2109. [Google Scholar] [CrossRef]
- Williams, K.; Page, M. Biotechnology and Sustainability: A Symbiotic Relationship. Bus. Res. Yearb. 2011, 18, 548–555. [Google Scholar]
- Onifade, A.; Al-Sane, N.; Al-Musallam, A.; Al-Zarban, S. A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 1998, 66, 1–11. [Google Scholar] [CrossRef]
- Nnolim, N.; Udenigwe, C.; Okoh, A.; Nwodo, U. Microbial Keratinase: Next Generation Green Catalyst and Prospective Applications. Front. Microbiol. 2020, 11, 3280. [Google Scholar] [CrossRef]
- Tamreihao, K.; Mukherjee, S.; Khunjamayum, R.; Devi, L.; Asem, R.; Ningthoujam, D. Feather degradation by keratinolytic bacteria and biofertilizing potential for sustainable agricultural production. J. Basic Microbiol. 2019, 59, 4–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Menezes, C.; Santos, R.; Santos, M.; Boscolo, M.; da Silva, R.; Gomes, E.; da Silva, R.R. Industrial sustainability of microbial keratinases: Production and potential applications. World J. Microbiol. Biotechnol. 2021, 37, 86. [Google Scholar] [CrossRef]
- Gupta, V.; Kubicek, C.; Berrin, J.-G.; Wilson, D.; Couturier, M.; Berlin, A.; Filho, E.; Ezeji, T. Fungal Enzymes for Bio-Products from Sustainable and Waste Biomass. Trends Biochem. Sci. 2016, 41, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Abol-Fotouh, D.; Omer, A.; Tamer, T.; Abbas, E. Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review. Int. J. Biol. Macromol. 2020, 154, 567–583. [Google Scholar] [CrossRef]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A. Microbial enzymes catalyzing keratin degradation: Classification, structure, function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/poultry-production-products/production/poultry-species/other-poultry/en/ (accessed on 29 June 2021).
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 29 June 2021).
- Poultry Global Market Report 2021: COVID-19 Impact and Recovery to 2030. Available online: https://www.researchandmarkets.com/reports/5240275/poultry-global-market-report-2021-covid-19?utm_source=CI&utm_medium=PressRelease&utm_code=cbjc99&utm_campaign=1502793+-+Global+Poultry+Market+Report+2021&utm_exec=chdo54prd (accessed on 29 June 2021).
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef] [Green Version]
- Seidavi, A.; Zaker-Esteghamati, H.; Scanes, C. Poultry Byproducts. In Byproducts from Agriculture and Fisheries; Simpson, B., Aryee, A., Toldrá, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 123–146. [Google Scholar]
- Kelly, L.; Alworth, L. Techniques for collecting blood from the domestic chicken. Lab. Anim. 2013, 42, 359–361. [Google Scholar] [CrossRef]
- Fleischer, L.-G.; Gerber, G.; Liezenga, R.; Lippert, E.; Scholl, M.; Westphal, G. Blood cells and plasma proteins of chickens fed a diet supplemented with (1->3),(1->6)-β-D-Glucan and enrofloxacin. Arch. Anim. Nutr. 2000, 53, 59–73. [Google Scholar] [CrossRef]
- Hayse, P.; Marion, W. Eviscerated Yield, Component Parts, and Meat, Skin and Bone Ratios in the Chicken Broiler. Poult. Sci. 1973, 52, 718–722. [Google Scholar] [CrossRef]
- Suchý, P.; Straková, E.; Herzig, I.; Steinhauser, L.; Kralik, G.; Zapletal, D. Chemical composition of bone tissue in broiler Chickens intended for slaughter. Czech J. Anim. Sci. 2009, 54, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Grazziotin, A.; Pimentel, F.; Jong, E.; Brandelli, A. Poultry feather hydrolysate as a protein source for growing rats. Braz. J. Vet. Res. Anim. Sci. 2008, 45, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, F.; Buchtová, H. Comparison of qualitative and quantitative properties of the wings, necks and offal of chicken broilers from organic and conventional production systems. Veterinární Med. 2016, 61, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of keratin protein extraction from waste chicken feathers using hybrid pre-treatment techniques. Sustain. Chem. Pharm. 2020, 17, 100267. [Google Scholar] [CrossRef]
- Thyagarajan, D.; Barathi, M.; Sakthivadivu, R. Scope of Poultry Waste Utilization. J. Agric. Vet. Sci. 2013, 6, 29–35. [Google Scholar]
- Cornejo, J.; Pokrant, E.; Carvallo, C.; Maddaleno, A.; San Martín, B. Depletion of tylosin residues in feathers, muscle and liver from broiler chickens after completion of antimicrobial therapy. Food Addit. Contam. Part A 2018, 35, 448–457. [Google Scholar] [CrossRef]
- Kadir, M.; Wang, X.; Zhu, B.; Liu, J.; Harland, D.; Popescu, C. The structure of the “amorphous” matrix of keratins. J. Struct. Biol. 2017, 198, 116–123. [Google Scholar] [CrossRef]
- Strasser, B.; Mlitz, V.; Hermann, M.; Tschachler, E.; Eckhart, L. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evol. Biol. 2015, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Alibardi, L. Cornification in reptilian epidermis occurs through the deposition of keratin-associated beta-proteins (beta-keratins) onto a scaffold of intermediate filament keratins. J. Morphol. 2013, 274, 175–193. [Google Scholar] [CrossRef]
- Sakudo, A. Inactivation Methods for Prions. Curr. Issues Mol. Biol. 2020, 36, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Staroń, P.; Kowalski, Z.; Staroń, A.; Banach, M. Thermal treatment of waste from the meat industry in high scale rotary kiln. Int. J. Environ. Sci. Technol. 2017, 14, 1157–1168. [Google Scholar] [CrossRef] [Green Version]
- Marculescu, C.; Stan, C. Poultry processing industry waste to energy conversion. Energy Procedia 2011, 6, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Glanville, T.; Ahn, H.; Richard, T.; Shiers, L.; Harmon, J. Soil Contamination Caused by Emergency Bio-Reduction of Catastrophic Livestock Mortalities. Water Air Soil Pollut. 2009, 198, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Carcass Disposal: A Comprehensive Review. Available online: https://amarillo.tamu.edu/files/2011/01/draftreport.pdf (accessed on 29 June 2021).
- Ghaffar, I.; Imtiaz, A.; Hussain, A.; Javid, A.; Jabeen, F.; Akmal, M.; Qazi, J. Microbial production and industrial applications of keratinases: An overview. Int. Microbiol. 2018, 21, 163–174. [Google Scholar] [CrossRef]
- Kodak, S.; Gharge, T.; Chavan, V.; Sibi, G. Microbial Degradation of Poultry Feather Wastes under the Influence of Temperature and Ph—A Review. Int. J. Environ. Sci. Nat. Res. 2019, 21, 556063. [Google Scholar]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [Green Version]
- Watts, C.; Wagner, D.; Sohnle, P. Fungal infections, cutaneous. In Encyclopedia of Microbiology, 2nd ed.; Academic: San Diego, CA, USA, 2000; pp. 382–388. [Google Scholar]
- Gupta, R.; Ramnani, P. Microbial keratinases and their prospective applications: An overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [CrossRef]
- Mazotto, A.; Courib, S.; Damasod, M.; Vermelho, A. Degradation of feather waste by Aspergillus niger keratinases: Comparison of submerged and solid-state fermentation. Int. Biodeterior. Biodegrad. 2013, 85, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Casarin, F.; Cladera-Olivera, F.; Brandelli, A. Use of Poultry Byproduct for Production of Keratinolytic Enzymes. Food Bioprocess. Technol. 2008, 1, 301–305. [Google Scholar] [CrossRef]
- Kang, E.; Jin, H.; La, J.; Sung, J.; Park, S.; Kim, W.; Lee, D. Identification of keratinases from Fervidobacterium islandicum AW-1 using dynamic gene expression profiling. Microb. Biotechnol. 2020, 13, 442–457. [Google Scholar] [CrossRef] [Green Version]
- Duffeck, C.; de Menezes, C.; Boscolo, M.; da Silva, R.; Gomes, E.; da Silva, R.R. Keratinases from Coriolopsis byrsina as an alternative for feather degradation: Applications for cloth cleaning based on commercial detergent compatibility and for the production of collagen hydrolysate. Biotechnol. Lett. 2020, 42, 2403–2412. [Google Scholar] [CrossRef]
- González, V.; Vargas-Straube, M.; Beys-da-Silva, W.; Santi, L.; Valencia, P.; Beltrametti, F.; Cámara, B. Enzyme Bioprospection of Marine-Derived Actinobacteria from the Chilean Coast and New Insight in the Mechanism of Keratin Degradation in Streptomyces sp. G11C. Mar. Drugs 2020, 18, 537. [Google Scholar] [CrossRef]
- Nnolim, N.; Okoh, A.; Nwodo, U. Proteolytic bacteria isolated from agro-waste dumpsites produced keratinolytic enzymes. Biotechnol. Rep. 2020, 27, e00483. [Google Scholar] [CrossRef]
- Li, Z.; Liang, S.; Ke, Y.; Deng, J.; Zhang, M.; Lu, D.; Li, J.; Luo, X. The feather degradation mechanisms of a new Streptomyces sp. isolate SCUT-3. Commun. Biol. 2020, 3, 191. [Google Scholar] [CrossRef] [Green Version]
- Nnolim, N.; Ntozonke, N.; Okoh, A.; Nwodo, U. Exoproduction and characterization of a detergent-stable alkaline keratinase from Arthrobacter sp. KFS-1. Biochimie 2020, 177, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.; Beys-da-Silva, W.; Tirloni, L.; Santi, L.; Seixas, A.; Termignoni, C.; Silva, M.; Macedo, A. The extremophile Anoxybacillus sp. PC2 isolated from Brazilian semiarid region (Caatinga) produces a thermostable keratinase. J. Basic Microbiol. 2020, 60, 809–815. [Google Scholar] [CrossRef]
- Preczeski, K.; Dalastra, C.; Czapela, F.; Kubeneck, S.; Scapini, T.; Camargo, A.; Zanivan, J.; Bonatto, C.; Stefanski, F.; Venturin, B.; et al. Fusarium oxysporum and Aspergillus sp. as Keratinase Producers Using Swine Hair From Agroindustrial Residues. Front. Bioeng. Biotechnol. 2020, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavello, I.; Urbieta, M.; Cavalitto, S.; Donati, E. Bacillus cytotoxicus Isolated from a Pristine Natural Geothermal Area Reveals High Keratinolytic Activity. Microorganisms 2020, 8, 796. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Kango, N. Production and characterization of keratinase by Ochrobactrum intermedium for feather keratin utilization. Int. J. Biol. Macromol. 2021, 166, 1046–1056. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 61. [Google Scholar] [CrossRef]
- Forgács, G.; Alinezhad, S.; Mirabdollah, A.; Feuk-Lagerstedt, E.; Horváth, I. Biological treatment of chicken feather waste for improved biogas production. J. Environ. Sci. 2011, 23, 1747–1753. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Verma, P. Metabolism of Chicken Feathers and Concomitant Electricity Generation by Pseudomonas aeruginosa by Employing Microbial Fuel Cell (MFC). J. Waste Manag. 2014, 2014, 928618. [Google Scholar] [CrossRef]
- Patinvoh, R.; Feuk-Lagerstedt, E.; Lundin, M.; Sárvári Horváth, I.; Taherzadeh, M. Biological Pretreatment of Chicken Feather and Biogas Production from Total Broth. Appl. Biochem. Biotechnol. 2016, 180, 1401–1415. [Google Scholar] [CrossRef]
- Wawrzkiewicz, K.; Lobarzewski, J.; Wolski, T. Intracellular keratinase of Trichophyton gallinae. J. Med. Vet. Mycol. 1987, 25, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Riessen, S.; Antranikian, G. Isolation of Thermoanaerobacter keratinophilus sp. nov., a novel thermophilic, anaerobic bacterium with keratinolytic activity. Extremophiles 2001, 5, 399–408. [Google Scholar] [CrossRef]
- Nam, G.; Lee, D.; Lee, H.; Lee, N.; Kim, B.; Choe, E.; Hwang, J.; Suhartono, M.; Pyun, Y. Native-feather degradation by Fervidobacterium islandicum AW-1, a newly isolated keratinase-producing thermophilic anaerobe. Arch. Microbiol. 2002, 178, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Bidzhieva, S.; Derbikova, K.; Kublanov, I.; Bonch-Osmolovskaya, E. Capacity of Hyperthermophilic Crenarchaeota for Decomposition of Refractory Proteins (α- and β-keratins). Microbiology 2014, 83, 880–887. [Google Scholar] [CrossRef]
- Dalmaso, G.; Lage, C.; Mazotto, A.; Dias, E.; Caldas, L.; Ferreira, D.; Vermelho, A. Extracellular peptidases from Deinococcus radiodurans. Extremophiles 2015, 19, 989–999. [Google Scholar] [CrossRef]
- Manczinger, L.; Rozs, M.; Vágvölgyi, C.; Kevei, F. Isolation and characterization of a new keratinolytic Bacillus licheniformis strain. W. J. Microbiol. Biotechnol. 2003, 19, 35–39. [Google Scholar] [CrossRef]
- Li, Q. Structure, Application, and Biochemistry of Microbial Keratinases. Front. Microbiol. 2021, 12, 1510. [Google Scholar] [CrossRef]
- Kumar, R.; Balaji, S.; Uma, T.; Mandal, A.; Sehgal, P. Optimization of influential parameters for extracellular keratinase production by Bacillus subtilis (MTCC9102) in solid state fermentation using Horn meal--a biowaste management. Appl. Biochem. Biotechnol. 2010, 160, 30–39. [Google Scholar] [CrossRef]
- Prakash, P.; Jayalakshmi, S.; Sreeramulu, K. Production of keratinase by free and immobilized cells of Bacillus halodurans strain PPKS-2: Partial characterization and its application in feather degradation and dehairing of the goat skin. Appl. Biochem. Biotechnol. 2010, 160, 1909–1920. [Google Scholar] [CrossRef]
- El-Gendy, M. Keratinase production by endophytic Penicillium spp. Morsy1 under solid-state fermentation using rice straw. Appl. Biochem. Biotechnol. 2010, 162, 780–794. [Google Scholar] [CrossRef]
- da Gioppo, N.; Moreira-Gasparin, F.; Costa, A.; Alexandrino, A.; de Souza, C.; Peralta, R. Influence of the carbon and nitrogen sources on keratinase production by Myrothecium verrucaria in submerged and solid-state cultures. J. Ind. Microbiol. Biotechnol. 2009, 36, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Ferrante, V.; Guarino, M.; Bacenetti, J. Environmental sustainability assessment of poultry productions through life cycle approaches: A critical review. Trends Food Sci. Technol. 2021, 110, 201–212. [Google Scholar] [CrossRef]
- Bagewadi, Z.; Mulla, S.; Ninnekar, H. Response surface methodology based optimization of keratinase production from Trichoderma harzianum isolate HZN12 using chicken feather waste and its application in dehairing of hide. J. Environ. Chem. Eng. 2018, 6, 4828–4839. [Google Scholar] [CrossRef]
- de Oliveira, C.; de Souza, A.; de Castro, R. Bioconversion of chicken feather meal by Aspergillus niger: Simultaneous enzymes production using a cost-effective feedstock under solid state fermentation. Indian J. Microbiol. 2019, 59, 209–216. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.; Bittencourt, M.; Caprara, C.; de Freitas, M.; de Almeida, R.; Silveira, D.; Fonseca, Y.; Ferreira Filho, E.; Pessoa Junior, A.; Magalhães, P. A biotechnology perspective of fungal proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Ye, J.; Tao, L.; Su, C.; Qin, J.; Zhang, Y.; Li, H.; Li, H.; Xu, Z.; Shi, J. Efficient keratinase expression via promoter engineering strategies for degradation of feather wastes. Enzyme Microb. Technol. 2020, 137, 109550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gong, J.; Su, C.; Qin, J.; Li, H.; Li, H.; Shi, J.; Xu, Z. Recombinant expression and molecular engineering of the keratinase from Brevibacillus parabrevis for dehairing performance. J. Biotechnol. 2020, 320, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Peng, B.; Su, Z.; Liu, A.; Hu, Y.; Nomura, C.; Chen, S.; Wang, Q. Facilitating Protein Expression with Portable 5′-UTR Secondary Structures in Bacillus licheniformis. ACS Synth. Biol. 2020, 9, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Ben Elhoul, M.; Zaraî Jaouadi, N.; Bouacem, K.; Allala, F.; Rekik, H.; Mechri, S.; Khemir Ezzine, H.; Miled, N.; Jaouadi, B. Heterologous expression and purification of keratinase from Actinomadura viridilutea DZ50: Feather biodegradation and animal hide dehairing bioprocesses. Environ. Sci. Pollut. Res. Int. 2021, 28, 9921–9934. [Google Scholar] [CrossRef]
- Cavello, I.; Chesini, M.; Hours, R.; Cavalitto, S. Study of the production of alkaline keratinases in submerged cultures as an alternative for solid waste treatment generated in leather technology. J. Microbiol. Biotechnol. 2013, 23, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Aspergillus nidulans: A Potential Resource of the Production of the Native and Heterologous Enzymes for Industrial Applications. Int. J. Microbiol. 2020, 2020, 8894215. [Google Scholar] [CrossRef]
| By-Product | Percentage of Body Mass per Component, % | Protein Content, % | References |
|---|---|---|---|
| Blood | 6–7.5 | 28–31 | [29,30] |
| Bones and cartilage | 22–24 | 20–24 | [31,32] |
| Feathers | 7–9 | up to 90 | [33] |
| Offal | 6 | 32 | [8,34] |
| Method of Treatment | Advantages | Disadvantages | References |
|---|---|---|---|
| Incineration | Ash produced is safe and decontaminated | Air pollution | [12,41,42,43] |
| Ash may be used as a fertilizer | Causes bad smell, fumes, and smog | ||
| Allows the disposal of large volumes of waste | Special equipment required | ||
| Burial | Avoids unpleasant odors | Groundwater contamination risk | [9,37,44,45] |
| A relatively economical option | Soil and water pollution risk | ||
| Landfill area required | |||
| Burial is difficult when the ground is wet or frozen | |||
| Chemical hydrolysis | Results in the production of low molecular weight components | Requires dangerous, harsh chemicals | [10,11] |
| Requires disposal or recycling of residues and undesirable salts needed | |||
| Large amounts of wastewater produced | |||
| Biodegradation | Allows the obtainment of hydrolysate containing single compounds such as oligopeptides and amino acids | Potentially difficult to scale up | [18,46] |
| A relatively energy-efficient process | |||
| No emissions and environmental pollution | |||
| Safe for people and animals |
| Product Name | Source | Area of Application | Manufacturer |
|---|---|---|---|
| FEED-0001 | Bacillus licheniformis | Production of feed additives for animals | Creative Enzymes |
| Valkerase | Production of feed additives for animals | BioResource International, Inc. | |
| Versazyme | Production of feed additives for animals | BioResource International, Inc. | |
| Prionzyme TM | Prion degradation | Genencor International, Inc. | |
| Keratoclean PB | Cosmetology | Proteos Biotech | |
| Keratoclean HYDRA PB | Cosmetology | Proteos Biotech | |
| Keratoclean sensitive PB | Cosmetology | Proteos Biotech | |
| Keratopeel PB | Cosmetology | Proteos Biotech | |
| PURE100 KERATINASE | Cosmetology, prion degradation | Proteos Biotech | |
| Alcalase (protease P4860) | Scientific research | Novozymes Crop. | |
| Esperase (protease P5860) | Bacillus sp. | Scientific research | Novozymes Crop. |
| Savinase (protease P3111) | Scientific research | Novozymes Crop. | |
| NATE-0853 | recombinant strain Escherichia coli BL21 | Scientific research | Creative Enzymes |
| Proteinase k | Engyodontium album (earlier Tritirachium album) and recombinant strains | Scientific research | Various |
| FixaFungus | - | Treatments of toe nail | FixaFungus |
| Bioguard Plus | Proprietary blend of multiple microorganisms cultures including keratinolytic | Cleaning agents | RuShay, Inc. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shestakova, A.; Timorshina, S.; Osmolovskiy, A. Biodegradation of Keratin-Rich Husbandry Waste as a Path to Sustainable Agriculture. Sustainability 2021, 13, 8691. https://doi.org/10.3390/su13168691
Shestakova A, Timorshina S, Osmolovskiy A. Biodegradation of Keratin-Rich Husbandry Waste as a Path to Sustainable Agriculture. Sustainability. 2021; 13(16):8691. https://doi.org/10.3390/su13168691
Chicago/Turabian StyleShestakova, Anna, Svetlana Timorshina, and Alexander Osmolovskiy. 2021. "Biodegradation of Keratin-Rich Husbandry Waste as a Path to Sustainable Agriculture" Sustainability 13, no. 16: 8691. https://doi.org/10.3390/su13168691
APA StyleShestakova, A., Timorshina, S., & Osmolovskiy, A. (2021). Biodegradation of Keratin-Rich Husbandry Waste as a Path to Sustainable Agriculture. Sustainability, 13(16), 8691. https://doi.org/10.3390/su13168691







