Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preliminary Screening
2.3. SCG Extraction Techniques
2.4. Experimental Design
2.5. Chemical Characterization and Antioxidant Activity of the Extracts
2.5.1. Determination of Total Phenolic Content
2.5.2. Determination of Antioxidant Activity
2.5.3. Caffeine and Chlorogenic Acid Content
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Experiments
3.2. FCC Optimization Results
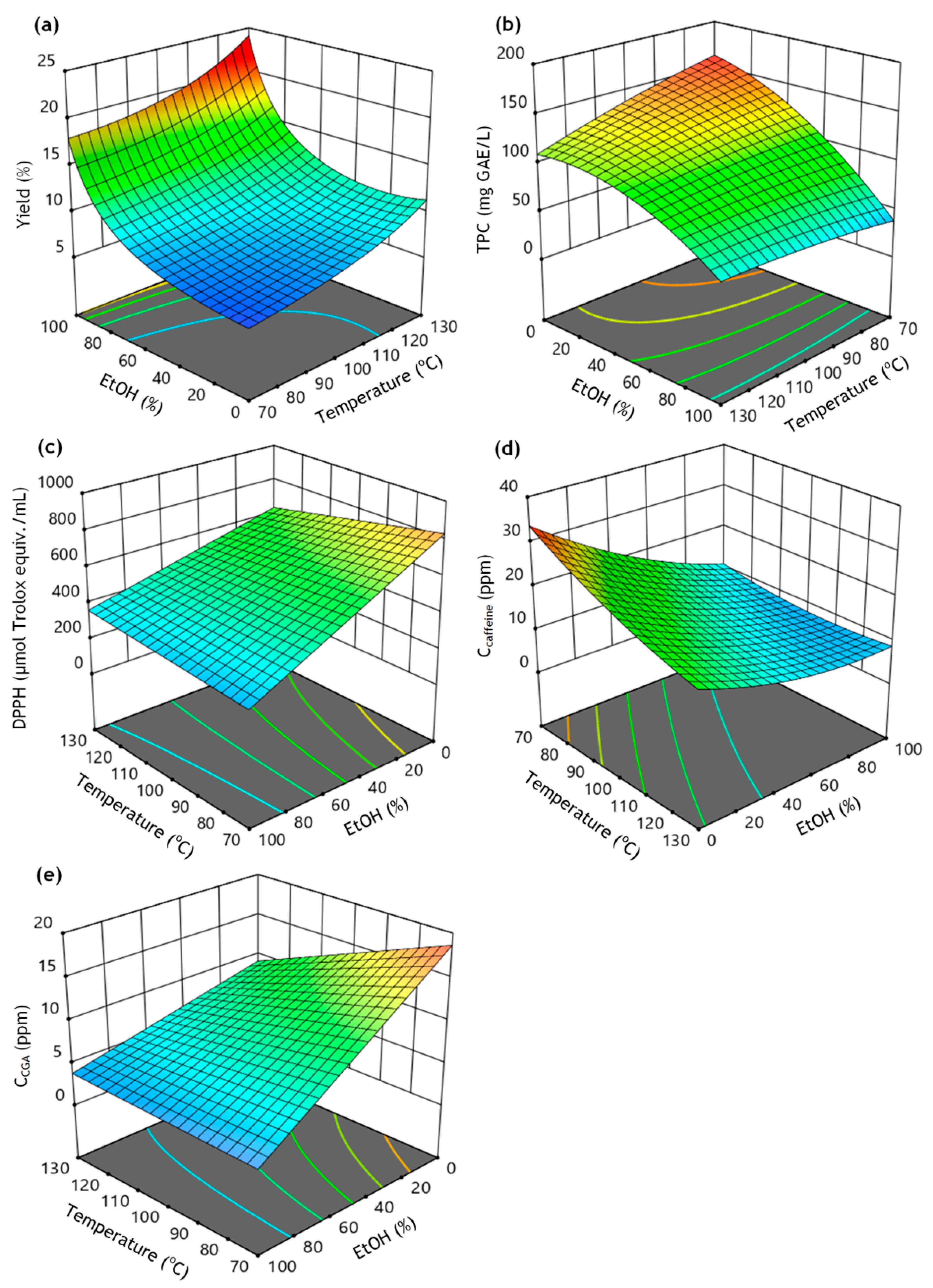
3.3. Response Surface Analysis
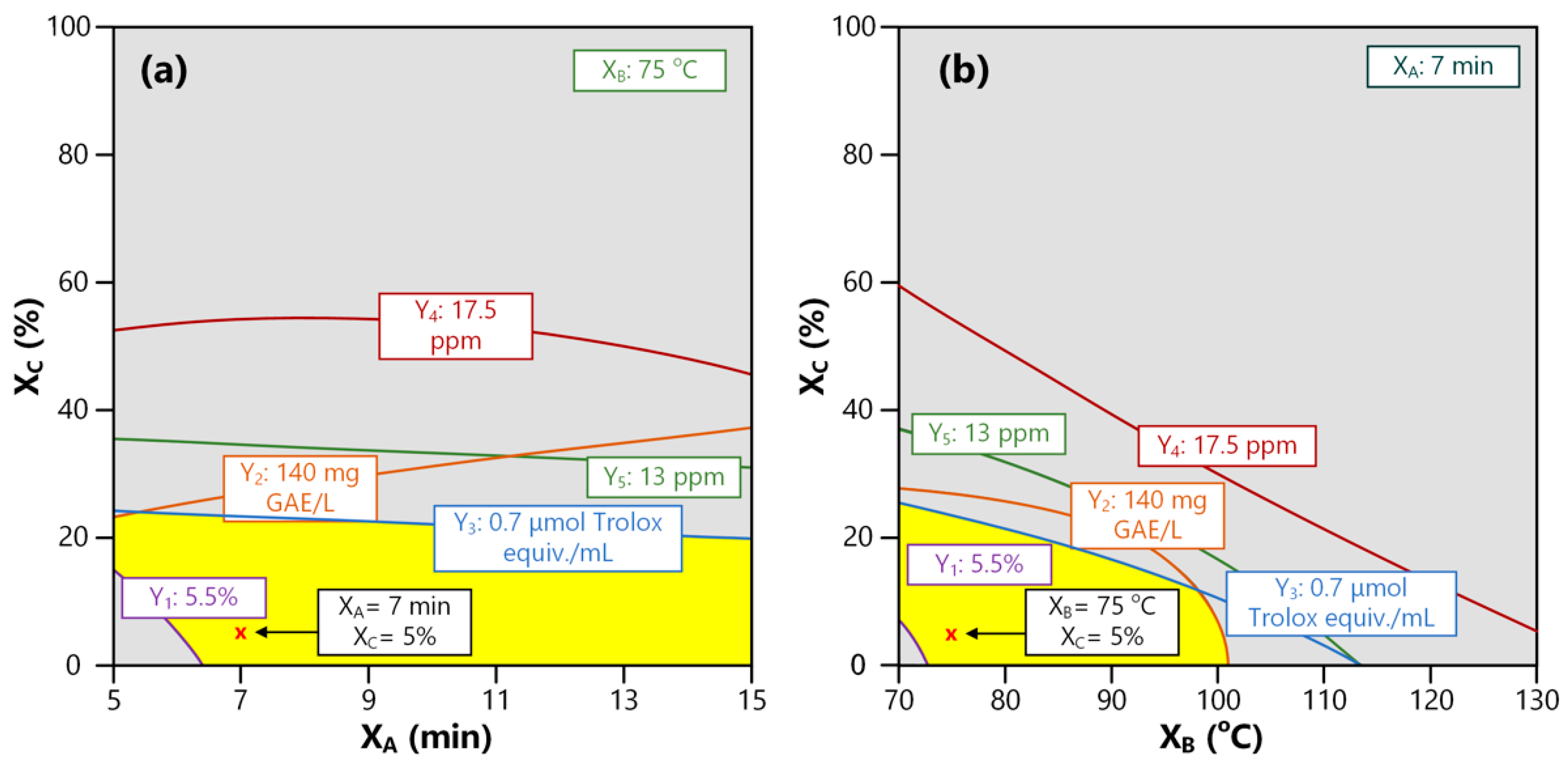
3.4. Optimization of Independent Variables and Validation Experiment
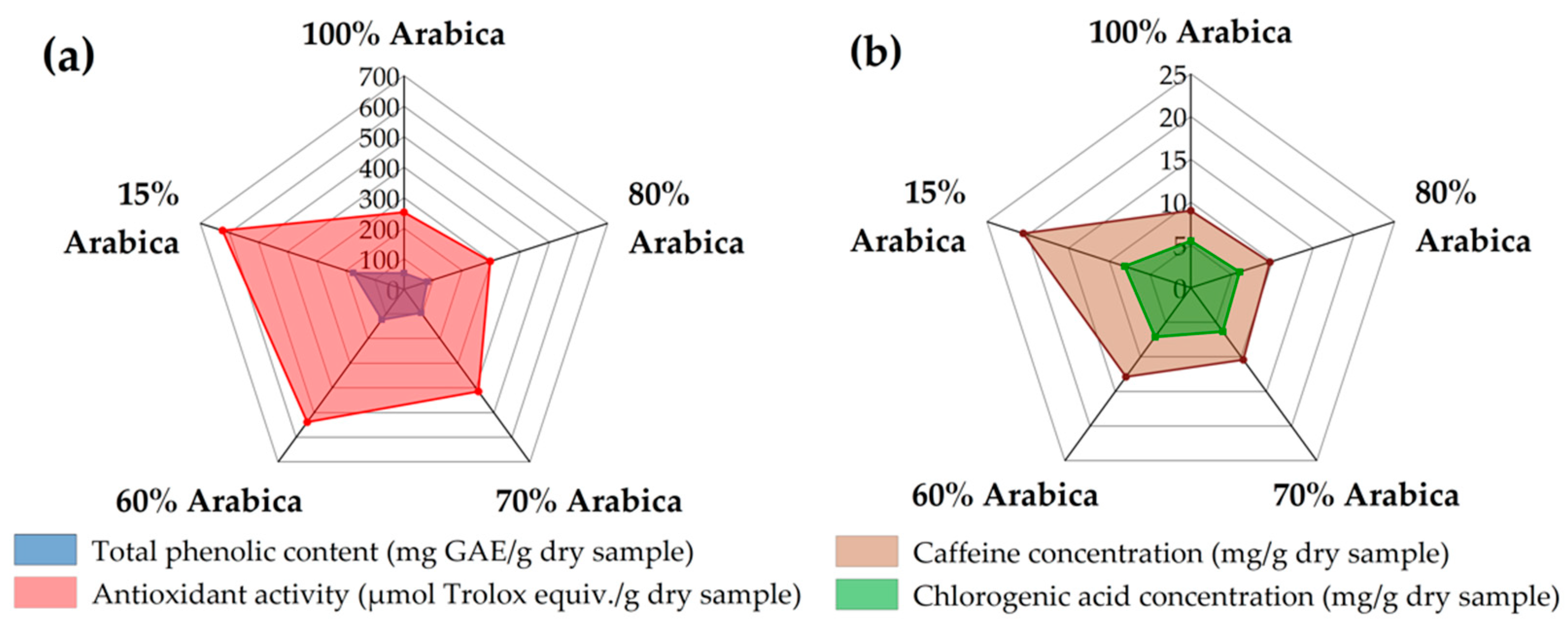
3.5. Extraction of Different Coffee Blends in Optimum Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Coffee Organization. Coffee Market Report-June 2021. Available online: http://www.ico.org/documents/cy2020-21/cmr-0621-e.pdf (accessed on 29 July 2021).
- Galanakis, C. Handbook of Coffee Processing By-Products: Sustainable Applications; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [Green Version]
- Kamil, M.; Ramadan, K.M.; Olabi, A.G.; Shanableh, A.; Ghenai, C.; Al Naqbi, A.K.; Awad, O.I.; Ma, X. Comprehensive evaluation of the life cycle of liquid and solid fuels derived from recycled coffee waste. Resour. Conserv. Recycl. 2019, 150, 104446. [Google Scholar] [CrossRef]
- Mata, T.; Martins, A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating Circular Bioeconomy—A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- Vítěz, T.; Koutný, T.; Šotnar, M.; Chovanec, J. On the spent coffee grounds biogas production. Acta Univ. Agric. et Silvic. Mendel. Brun. 2016, 64, 1279–1282. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesias, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-S.; Park, E.D.; Park, Y.; Han, S.H.; Hong, K.B.; Suh, H.J. Topical application of spent coffee ground extracts protects skin from ultraviolet B-induced photoaging in hairless mice. Photochem. Photobiol. Sci. 2016, 15, 779–790. [Google Scholar] [CrossRef]
- Marto, J.; Gouveia, L.; Chiari-Andréo, B.G.; Paiva, A.; Isaac, V.; Pinto, P.; Simões, P.; Almeida, A.; Ribeiro, H. The green generation of sunscreens: Using coffee industrial sub-products. Ind. Crop. Prod. 2016, 80, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Nasution, S.L.; Suhartina, S.; Munshy, U.Z.; Rizal, R.; Widowati, W. Antioxidant and anti-inflammatory activity of chlorogenic acid on lead-induced fibroblast cells. J. Phys. Conf. Ser. 2019, 1374. [Google Scholar] [CrossRef]
- Massaya, J.; Pereira, A.P.; Mills-Lamptey, B.; Benjamin, J.; Chuck, C.J. Conceptualization of a spent coffee grounds biorefinery: A review of existing valorisation approaches. Food Bioprod. Process. 2019, 118, 149–166. [Google Scholar] [CrossRef]
- Matrapazi, V.; Zabaniotou, A. Experimental and feasibility study of spent coffee grounds upscaling via pyrolysis towards proposing an eco-social innovation circular economy solution. Sci. Total Environ. 2020, 718, 137316. [Google Scholar] [CrossRef]
- Rivera, X.C.S.; Gallego-Schmid, A.; Najdanovic-Visak, V.; Azapagic, A. Life cycle environmental sustainability of valorisation routes for spent coffee grounds: From waste to resources. Resour. Conserv. Recycl. 2020, 157, 104751. [Google Scholar] [CrossRef]
- Stylianou, M.; Agapiou, A.; Omirou, M.; Vyrides, I.; Ioannides, I.M.; Maratheftis, G.; Fasoula, D. Converting environmental risks to benefits by using spent coffee grounds (SCG) as a valuable resource. Environ. Sci. Pollut. Res. 2018, 25, 35776–35790. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Spent coffee grounds as a valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 2012, 34, 49–56. [Google Scholar] [CrossRef]
- Bravo, J.; Juaniz, I.; Monente, C.; Caemmerer, B.; Kroh, L.; Lothar, W.; de Peña, M.-P.; Cid, C. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. J. Agric. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef] [PubMed]
- Battista, F.; Barampouti, E.M.; Mai, S.; Bolzonella, D.; Malamis, D.; Moustakas, K.; Loizidou, M. Added-value molecules recovery and biofuels production from spent coffee grounds. Renew. Sustain. Energy Rev. 2020, 131, 110007. [Google Scholar] [CrossRef]
- Martin, D.S.; Orive, M.; Iñarra, B.; Garcia, A.; Goiri, I.; Atxaerandio, R.; Urkiza, J.; Zufía, J. Spent coffee ground as second-generation feedstuff for dairy cattle. Biomass Convers. Biorefinery 2021, 11, 589–599. [Google Scholar] [CrossRef]
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.F.; Oliveira, M.B.P.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–26. [Google Scholar]
- Severini, C.; Derossi, A.; Fiore, A. Ultrasound-assisted extraction to improve the recovery of phenols and antioxidants from spent espresso coffee ground: A study by response surface methodology and desirability approach. Eur. Food Res. Technol. 2017, 243, 835–847. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the recovery of phenolic compounds from Spent Coffee Grounds (SCG) by environmentally friendly extraction techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef] [PubMed]
- Andrade, K.; Gonçalvez, R.T.; Maraschin, M.; Ribeiro-Do-Valle, R.M.; Martínez, J.; Ferreira, S.R.S. Supercritical fluid extraction from spent coffee grounds and coffee husks: Antioxidant activity and effect of operational variables on extract composition. Talanta 2012, 88, 544–552. [Google Scholar] [CrossRef]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso coffee residues: A valuable source of unextracted compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R. Polyphenols and energy recovery from spent coffee grounds. Chem. Eng. Trans. 2011, 25, 285–290. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Prado, J.; Vardanega, R.; Debien, I.C.; Meireles, M.A.D.A.; Gerschenson, L.N.; Sowbhagya, H.B.; Chemat, S. Conventional extraction. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2015; pp. 127–148. [Google Scholar]
- Pavlović, M.D.; Buntić, A.; Šiler-Marinković, S.S.; Dimitrijevic-Brankovic, S. Ethanol influenced fast microwave-assisted extraction for natural antioxidants obtaining from spent filter coffee. Sep. Purif. Technol. 2013, 118, 503–510. [Google Scholar] [CrossRef]
- De Melo, M.M.; Silvestre, A.J.; Portugal, I.; Silva, C.M. Emerging technologies for the recovery of valuable compounds from coffee processing by-products. In Handbook of Coffee Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 141–169. [Google Scholar]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of Agrifood By-Products by Extracting Valuable Bioactive Compounds Using Green Processes. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–228. [Google Scholar]
- Shang, Y.-F.; Xu, J.-L.; Lee, W.-J.; Um, B.-H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Vargas-González, M.; Pedreschi, F.; Pérez-Correa, J.R. The antioxidant and safety properties of spent coffee ground extracts impacted by the combined hot pressurized liquid extraction-resin purification process. Molecules 2017, 23, 21. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. The universal recovery strategy. In Food Waste Recovery; Elsevier: Amsterdam, The Netherlands, 2015; pp. 59–81. [Google Scholar]
- Osorio-Tobón, J.F.; Meireles, M.A.A. Recent applications of pressurized fluid extraction: Curcuminoids extraction with pressurized liquids. Food Public Health 2013, 3, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Eveleigh, A.; Mills-Lamptey, B. Factors affecting the efficiency of pres-surized solvent extraction of oil from spent coffee grounds. Detritus 2019, in press. [Google Scholar] [CrossRef] [Green Version]
- Mottaleb, M.A.; Sarker, S.D. Accelerated solvent extraction for natural products isolation. In Methods in Molecular Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; Volume 864, pp. 75–87. [Google Scholar]
- NIST/SEMATECH e-Handbook of Statistical Methods. Available online: https://www.itl.nist.gov/div898/handbook/ (accessed on 14 March 2020).
- Montgomery, D.C. Design and Analysis of Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1991; pp. 521–568. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin-ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Somnuk, K.; Eawlex, P.; Prateepchaikul, G. Optimization of coffee oil extraction from spent coffee grounds using four solvents and prototype-scale extraction using circulation process. Agric. Nat. Resour. 2017, 51, 181–189. [Google Scholar] [CrossRef]
- Ahangari, B.; Sargolzaei, J. Extraction of lipids from spent coffee grounds using organic solvents and supercritical carbon dioxide. J. Food Process. Preserv. 2012, 37, 1014–1021. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a re-newable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Sun, H.; Ge, X.; Lv, Y.-K.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef]
- Le Man, H.; Behera, S.K.; Park, H.S. Optimization of operational parameters for ethanol production from Korean food waste leachate. Int. J. Environ. Sci. Technol. 2010, 7, 157–164. [Google Scholar] [CrossRef] [Green Version]
- López-Barrera, D.M.; Vázquez-Sánchez, K.; Loarca-Piña, G.; Campos-Vega, R. Spent coffee grounds, an innovative source of colonic fermentable compounds, inhibit inflammatory mediators in vitro. Food Chem. 2016, 212, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nuñez, O.; Fiorini, D.; Giardinieri, A.; Frega, N.G.; Pacetti, D. Spent espresso coffee grounds as a source of anti-proliferative and antioxidant compounds. Innov. Food Sci. Emerg. Technol. 2020, 59, 102254. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.; Roberto, I.; Teixeira, J. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]


| Accelerated Solvent Extraction | |||
|---|---|---|---|
| Solvent | n-Hexane | EtOH | EtOH: H2O (50:50 v/v) |
| Solvent vol. (mL) | 199 | 187 | 163 |
| Dry sample (g) | 11.67 | 11.32 | 11.51 |
| Static time (min) | 10 | 10 | 10 |
| Cycles | 3 | 3 | 3 |
| Rinse volume (%) | 50 | 50 | 50 |
| Temperature (°C) | 90 | 90 | 90 |
| Run | Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| XA (min) | XB (°C) | XC (%) | Y1 (% w/w) | Y2 (mg GAE/L) | Y3 (μmol Trolox equiv./mL) | Y4 (ppm) | Y5 (ppm) | |
| 1 | 15 | 100 | 50 | 8.69 | 117.47 | 0.48 | 11.76 | 7.52 |
| 2 | 10 | 130 | 50 | 11.82 | 88.80 | 0.41 | 10.00 | 5.73 |
| 3 | 15 | 70 | 100 | 15.21 | 66.97 | 0.30 | 10.86 | 4.17 |
| 4 | 10 | 100 | 0 | 7.32 | 152.24 | 0.92 | 24.79 | 13.68 |
| 5 | 15 | 130 | 100 | 19.75 | 34.80 | 0.42 | 5.91 | 2.70 |
| 6 | 5 | 70 | 0 | 5.20 | 146.80 | 0.78 | 32.00 | 18.74 |
| 7 | 15 | 100 | 50 | 8.97 | 75.16 | 0.37 | 2.77 | 5.29 |
| 8 | 5 | 70 | 100 | 15.65 | 28.69 | 0.27 | 10.68 | 2.70 |
| 9 | 5 | 70 | 0 | 5.18 | 150.29 | 0.76 | 35.04 | 19.31 |
| 10 | 10 | 70 | 50 | 7.02 | 148.92 | 0.68 | 22.19 | 14.56 |
| 11 | 15 | 130 | 100 | 17.75 | 25.99 | 0.36 | 4.04 | 2.39 |
| 12 | 10 | 100 | 100 | 17.88 | 62.87 | 0.29 | 12.84 | 3.55 |
| 13 | 10 | 100 | 100 | 19.49 | 49.24 | 0.29 | 9.99 | 3.29 |
| 14 | 5 | 100 | 50 | 7.46 | 125.76 | 0.52 | 17.99 | 9.30 |
| 15 | 5 | 130 | 0 | 8.08 | 121.60 | 0.69 | 19.76 | 13.73 |
| 16 | 15 | 100 | 50 | 8.56 | 159.85 | 0.60 | 19.74 | 9.74 |
| 17 | 5 | 130 | 100 | 18.64 | 78.54 | 0.37 | 6.85 | 3.86 |
| 18 | 10 | 100 | 50 | 8.62 | 95.68 | 0.48 | 8.97 | 6.60 |
| 19 | 5 | 70 | 0 | 5.24 | 143.46 | 0.80 | 30.69 | 18.16 |
| 20 | 15 | 70 | 0 | 6.00 | 162.58 | 0.81 | 31.50 | 18.59 |
| 21 | 10 | 70 | 50 | 7.08 | 146.86 | 0.70 | 20.89 | 14.01 |
| 22 | 5 | 100 | 50 | 6.84 | 163.95 | 0.64 | 18.13 | 13.13 |
| 23 | 5 | 100 | 50 | 8.22 | 87.46 | 0.41 | 10.65 | 5.47 |
| 24 | 5 | 130 | 100 | 16.29 | 76.73 | 0.34 | 8.65 | 7.90 |
| 25 | 10 | 100 | 0 | 8.55 | 146.19 | 0.92 | 21.63 | 12.52 |
| 26 | 15 | 70 | 0 | 5.62 | 162.59 | 0.76 | 33.83 | 17.52 |
| 27 | 15 | 130 | 0 | 12.50 | 86.09 | 0.52 | 12.79 | 6.72 |
| 28 | 5 | 70 | 100 | 18.38 | 43.75 | 0.34 | 8.75 | 3.81 |
| 29 | 10 | 100 | 50 | 8.44 | 148.92 | 0.66 | 17.46 | 9.93 |
| 30 | 10 | 70 | 50 | 7.30 | 144.82 | 0.72 | 19.15 | 13.47 |
| 31 | 5 | 130 | 0 | 8.52 | 122.45 | 0.67 | 19.88 | 12.54 |
| 32 | 15 | 130 | 0 | 12.79 | 99.73 | 0.55 | 12.32 | 7.31 |
| 33 | 5 | 130 | 100 | 21.10 | 80.63 | 0.40 | 10.39 | 6.46 |
| 34 | 15 | 130 | 0 | 13.11 | 113.41 | 0.77 | 12.16 | 7.90 |
| 35 | 5 | 70 | 100 | 13.34 | 13.70 | 0.20 | 11.41 | 1.58 |
| 36 | 5 | 130 | 0 | 8.83 | 122.97 | 0.66 | 18.77 | 11.34 |
| 37 | 10 | 100 | 0 | 6.59 | 158.48 | 0.69 | 27.19 | 14.83 |
| 38 | 15 | 70 | 0 | 5.75 | 161.99 | 0.79 | 32.21 | 18.05 |
| 39 | 10 | 130 | 50 | 11.11 | 132.53 | 0.57 | 16.36 | 8.88 |
| 40 | 10 | 130 | 50 | 12.66 | 45.11 | 0.26 | 8.34 | 2.59 |
| 41 | 15 | 70 | 100 | 15.34 | 38.33 | 0.27 | 7.99 | 3.17 |
| 42 | 15 | 70 | 100 | 15.24 | 9.60 | 0.24 | 6.74 | 2.17 |
| 43 | 10 | 100 | 50 | 8.98 | 58.77 | 0.35 | 5.89 | 5.05 |
| 44 | 15 | 130 | 100 | 21.93 | 43.75 | 0.48 | 8.90 | 4.00 |
| 45 | 10 | 100 | 50 | 8.29 | 79.26 | 0.41 | 6.65 | 4.82 |
| 46 | 10 | 100 | 100 | 21.35 | 35.55 | 0.48 | 6.18 | 3.02 |
| Factors | Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y5 | ||||||
| F | p-Value | F | p-Value | F | p-Value | F | p-Value | F | p-Value | |
| XA | 39.68 | <0.0001 | - | - | - | - | 5.27 | 0.0277 | 7.95 | 0.0075 |
| XB | 376.12 | <0.0001 | 4.51 | 0.0407 | - | - | 47.71 | <0.0001 | 36.39 | <0.0001 |
| XC | 1017.57 | <0.0001 | 95.21 | <0.0001 | 107.24 | <0.0001 | 136.05 | <0.0001 | 204.1 | <0.0001 |
| XAXB | 4.74 | 0.0361 | 4.84 | 0.0344 | - | - | - | - | 6.12 | 0.0178 |
| XAXC | 24.64 | <0.0001 | - | - | - | - | - | - | - | - |
| XCXB | 156.25 | <0.0001 | 10.31 | 0.0028 | 9.35 | 0,0040 | 23.94 | <0.0001 | 38.32 | <0.0001 |
| XA2 | 12.34 | 0.0012 | - | - | - | - | ||||
| XB2 | - | - | - | - | - | - | ||||
| XC2 | 76.90 | <0.0001 | 5.19 | 0.0287 | 4.00 | 0.0530 | ||||
| R2 | 0.9794 | 0.7759 | 0.7543 | 0.8611 | 0.8829 | |||||
| Response | Predicted Values | Experimental Values (Mean ± SD) | Confirmation Factor (%) |
|---|---|---|---|
| Y1: Yield (% w/w) | 5.65 | 6.09 ± 0.22 | 93 |
| Y2: ΤPC (mg GAE/L) | 152.67 | 166.77 ± 4.26 | 92 |
| Y3: DPPH (μmol Trolox equiv./mL) | 0.79 | 0.80 ± 0.04 | 99 |
| Y4: Ccaffeine (ppm) | 30.50 | 28.72 ± 1.20 | 94 |
| Y5: CCGA (ppm) | 17.40 | 16.72 ± 0.73 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitraka, G.-C.; Kontogiannopoulos, K.N.; Batsioula, M.; Banias, G.F.; Assimopoulou, A.N. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability 2021, 13, 8818. https://doi.org/10.3390/su13168818
Mitraka G-C, Kontogiannopoulos KN, Batsioula M, Banias GF, Assimopoulou AN. Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach. Sustainability. 2021; 13(16):8818. https://doi.org/10.3390/su13168818
Chicago/Turabian StyleMitraka, Georgia-Christina, Konstantinos N. Kontogiannopoulos, Maria Batsioula, George F. Banias, and Andreana N. Assimopoulou. 2021. "Spent Coffee Grounds’ Valorization towards the Recovery of Caffeine and Chlorogenic Acid: A Response Surface Methodology Approach" Sustainability 13, no. 16: 8818. https://doi.org/10.3390/su13168818








