Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Coal Samples Preparation
2.3. Acidic Pretreatment of Coal Using Nitric Acid (HNO3)
2.4. Extraction of Humic Acid by KOH
2.5. Extraction of Humic Acid Using NaOH
3. Analysis
3.1. Humic Acid Determination Using Spectrophotometry
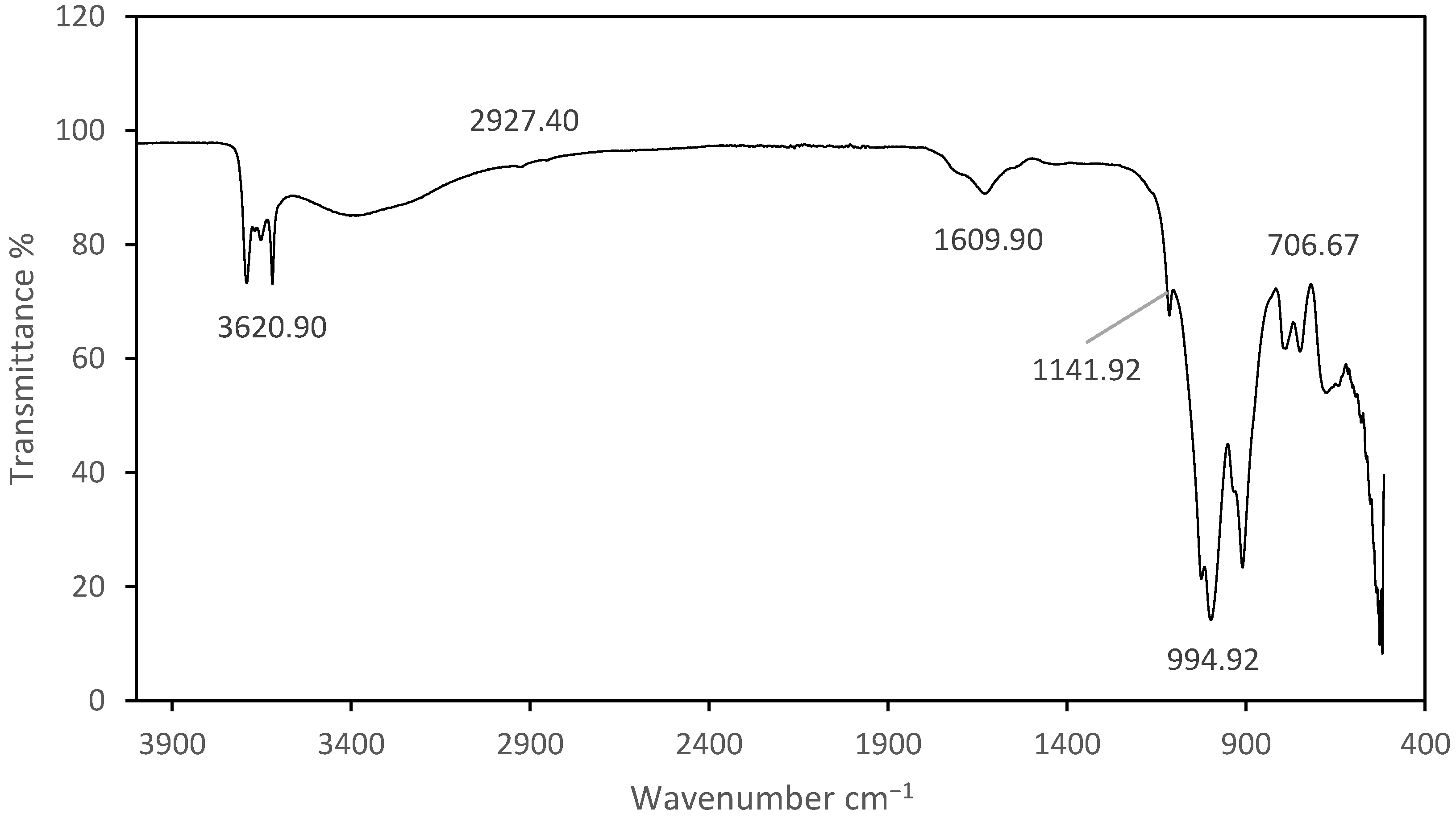
3.2. FTIR Analysis
3.3. CHNSO Elemental Analysis
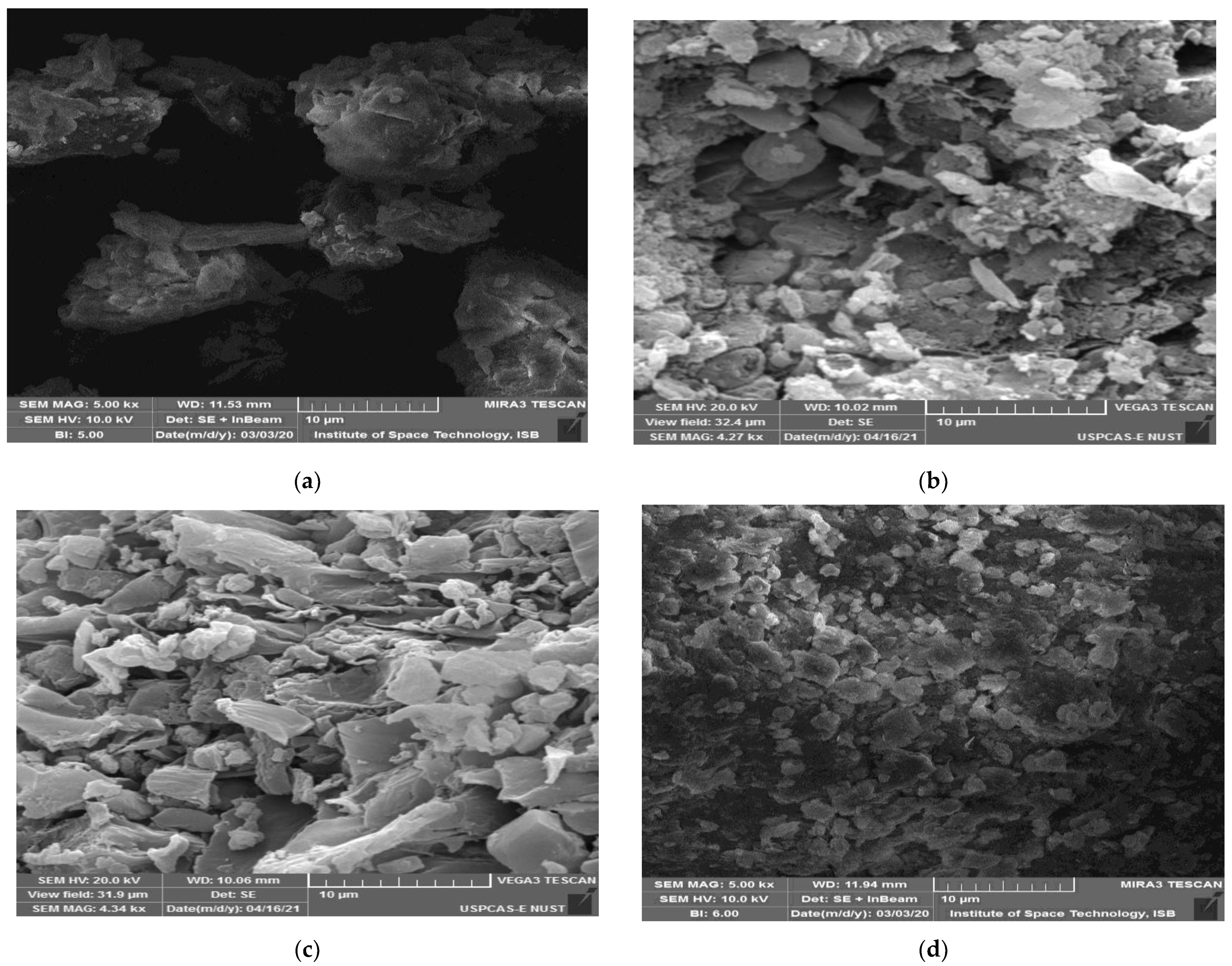
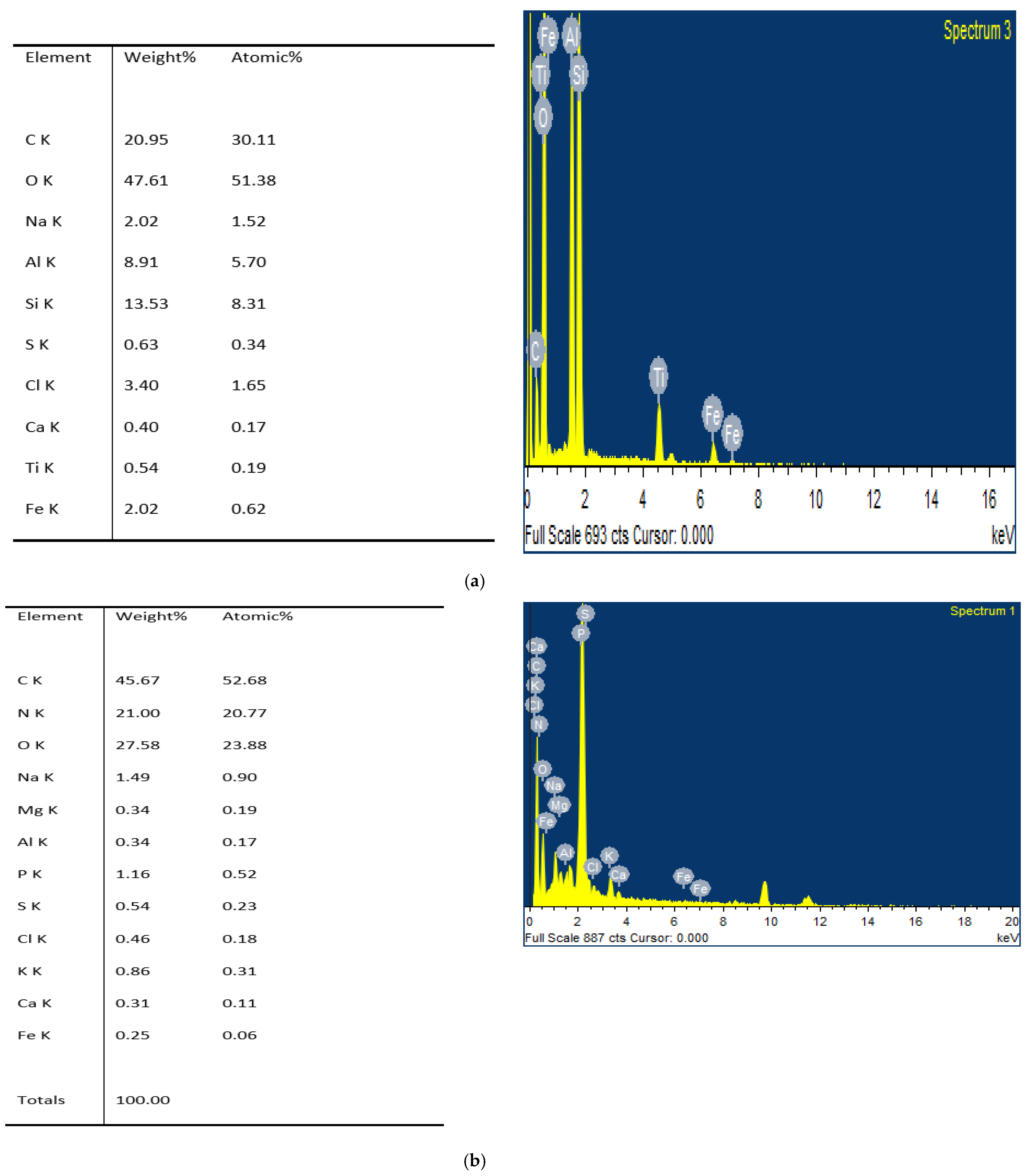
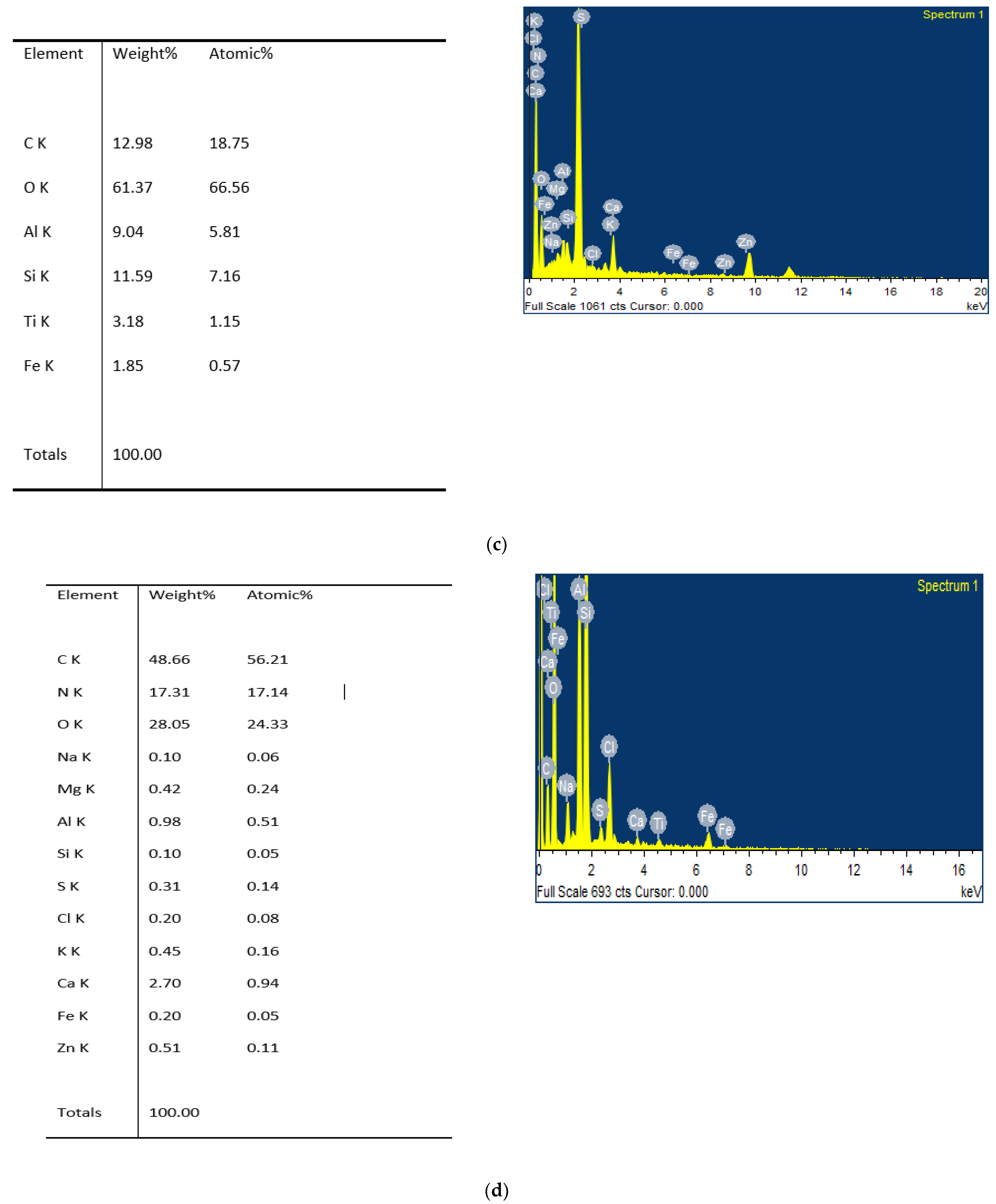
3.4. SEM Analysis
4. Results and Discussion
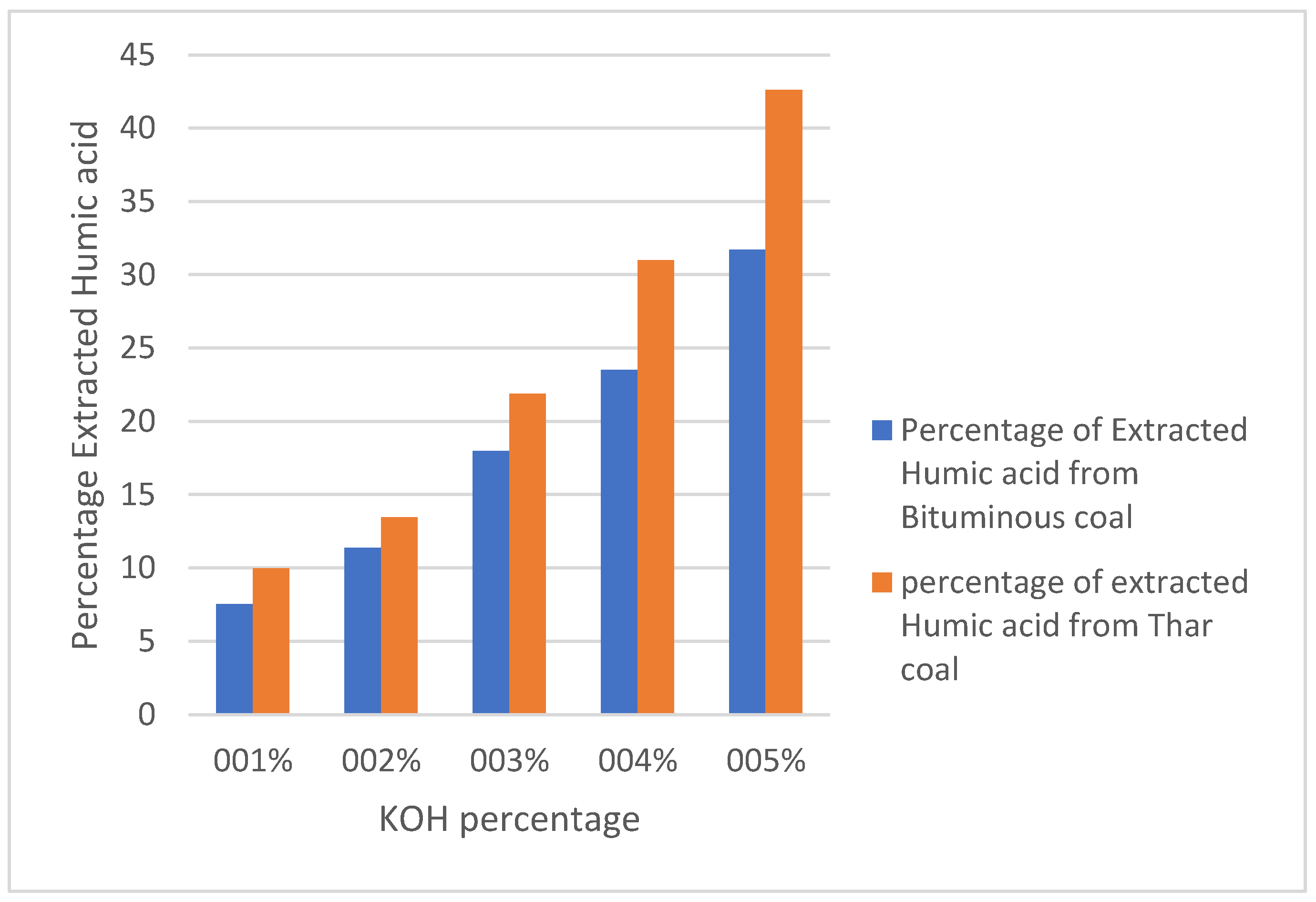
4.1. Gravimetric Determination of Humic Acid Using Different KOH Concentrations
4.2. Humic Acid Yield Using NaOH
4.3. UV-Vis Spectrophotometry of Produced Humic Acid
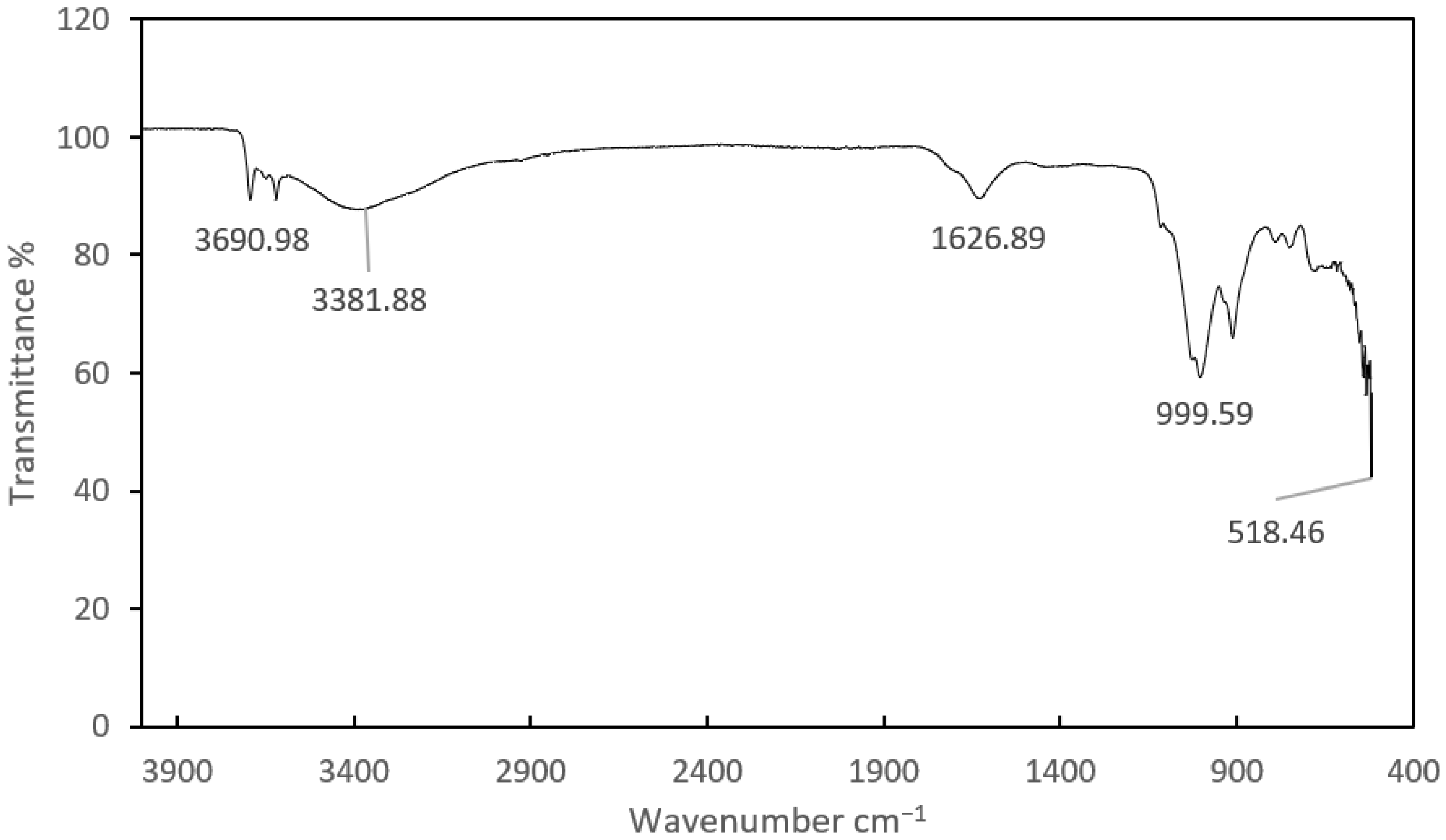
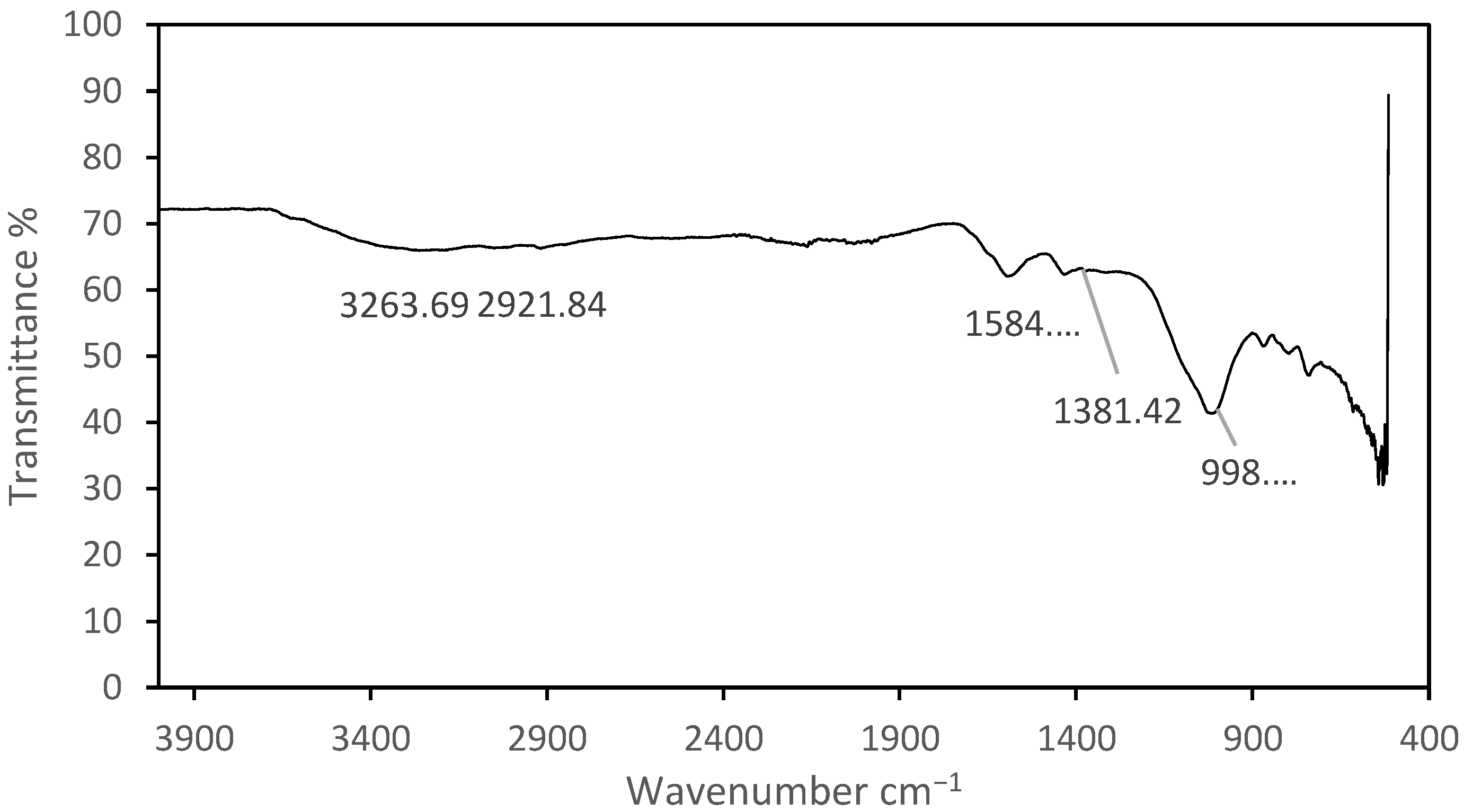
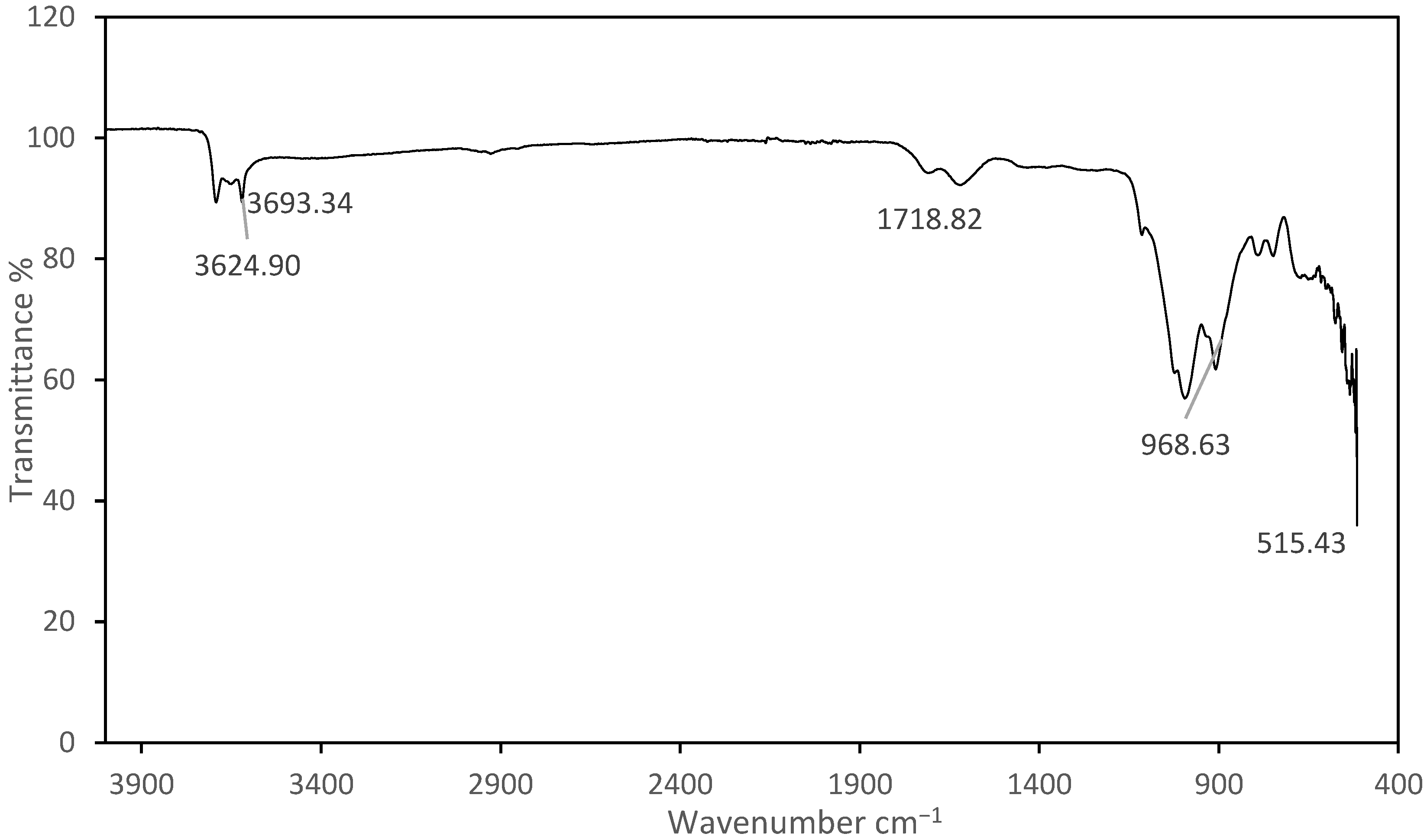
4.4. FTIR Analysis
4.5. Elemental Analysis of Humic Acid
4.6. SEM Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasir, S.; Sarfaraz, T.B.; Verheyen, T.V.; Chaffee, A.L. Structural elucidation of humic acids extracted from Pakistani lignite using spectroscopic and thermal degradative techniques. Fuel Process. Technol. 2011, 92, 983–991. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; SanFilipo, J.R.; Jones, E.J.; Orem, W.H.; Tatu, C.A.; Akhtar, K.; Akhtar, N. Fungal degradation of coal as a pretreatment for methane production. Fuel 2013, 104, 717–725. [Google Scholar] [CrossRef]
- Sarlaki, E.; Paghaleh, A.S.; Kianmehr, M.H.; Vakilian, K.A. Extraction and purification of humic acids from lignite wastes using alkaline treatment and membrane ultrafiltration. J. Clean. Prod. 2019, 235, 712–723. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Al Kahtani, A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Fan, M.; Chen, Y.; Shen, S. The stimulatory effect of humic acid on the co-metabolic biodegradation of tetrabromobisphenol A in bioelectrochemical system. J. Environ. Manag. 2019, 235, 350–356. [Google Scholar] [CrossRef]
- Mustafa, A.; Ahmad, T.; Akhtar, J.; Shahzad, K.; Sheikh, N.; Munir, S. Agglomeration of Makarwal coal using soybean oil as agglomerant. Energy 2016, 38, 733–3739. [Google Scholar] [CrossRef]
- Haider, R.; Ghauri, M.A.; Akhtar, K. Isolation of coal degrading fungus from drilled core coal sample and effect of prior fungal pretreatment on chemical attributes of extracted humic acid. Geomicrobiol. J. 2015, 32, 944–953. [Google Scholar] [CrossRef]
- Asif, M. Comparative Study on Extraction of Humic Acid from Pakistani Coal Samples by Oxidizing the Samples with Hydrogen Peroxide. ASEAN J. Sci. Eng. 2021, 2, 1–8. [Google Scholar]
- Saha, P.; Sarkar, S. Microbial degradation of coal into a value added product. Int J. Coal Prep. Utiliz. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Fong, S.S.; Seng, L.; Majri, N.B.; Mat, H.B. A comparative evaluation on the oxidative approaches for extraction of humic acids from low rank coal of Mukah, Sarawak. J. Braz. Chem. Soc. 2007, 18, 34–40. [Google Scholar] [CrossRef]
- Aravind, M.; Ahmad, A.; Ahmad, I.; Amalanathan, M.; Naseem, K.; Mary, S.M.; Parvathiraja, C.; Hussain, S.; Algarni, T.S.; Pervaiz, M.; et al. Critical green routing synthesis of silver NPs using jasmine flower extract for biological activities and photocatalytical degradation of methylene blue. J. Environ. Chem. Eng. 2021, 9, 104877. [Google Scholar] [CrossRef]
- Ahmad, A.; Jini, D.; Aravind, M.; Parvathiraja, C.; Ali, R.; Kiyani, M.Z.; Alothman, A. A novel study on synthesis of egg shell based activated carbon for degradation of methylene blue via photocatalysis. Arab. J. Chem. 2020, 13, 8717–8722. [Google Scholar] [CrossRef]
- Malik, A.Y.; Ali, M.I.; Jamal, A.; Farooq, U.; Khatoon, N.; Orem, W.H.; Barnhart, E.P.; SanFilipo, J.R.; He, H.; Huang, Z. Coal biomethanation potential of various ranks from Pakistan: A possible alternative energy source. J. Clean. Prod. 2020, 255, 120–177. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P.; Cozzolino, A. Effects of mineral and monocarboxylic acids on the molecular association of dissolved humic substances. Eur. J. Soil Sci. 1999, 50, 687–694. [Google Scholar] [CrossRef]
- Cheng, G.; Niu, Z.; Zhang, C.; Zhang, X.; Li, X. Extraction of humic acid from lignite by KOH-hydrothermal method. Appl. Sci. 2019, 9, 1356. [Google Scholar] [CrossRef] [Green Version]
- Barhoumi, A.; Ncib, S.; Chibani, A.; Brahmi, K.; Bouguerra, W.; Elaloui, E. High-rate humic acid removal from cellulose and paper industry wastewater by combining electrocoagulation process with adsorption onto granular activated carbon. Ind. Crop. Prod. 2019, 140, 111715. [Google Scholar] [CrossRef]
- Shi, K.; Tao, X.; Hong, F.; He, H.; Ji, Y.; Li, J. Mechanism of oxidation of low rank coal by nitric acid. J. Coal Sci. Eng. 2012, 18, 396–399. [Google Scholar] [CrossRef]
- Ahmad, A.; Mubharak, N.M.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent advancement and development of chitin and chitosan-based nanocomposite for drug delivery: Critical approach to clinical research. Arab. J. Chem. 2020, 13, 8935–8964. [Google Scholar] [CrossRef]
- Skhonde, M.P.; Herod, A.A.; Van der Walt, T.J.; Tsatsi, W.L.; Mokoena, K. The effect of thermal treatment on the compositional structure of humic acids extracted from South African bituminous coal. Int. J. Miner. Process. 2006, 81, 51–57. [Google Scholar] [CrossRef]
- Burange, A.S.; Ahmad, A.; Luque, R. Electrophilicity in heterogeneous catalysis: Role of surface and sub-surface modification. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Xavier, D.; Silva, A.S.; Santos, R.P.; Mesko, M.; Costa, S.; Freire, V.; Cavada, B.; Martins, J. Characterization of the coal humic acids from the Candiota coalfield, Brazil. Int. J. Agric. Sci. 2012, 4, 238–242. [Google Scholar]
- Shakiba, N. Investigation of the Effective Parameters on Separation and Purification of Humic Acid from the Leonardite Humate Using a Proper Filter. Master’s Thesis, University of Tehran, Tehran, Iran, 2016. [Google Scholar]
- Zara, M.; Ahmad, Z.; Akhtar, J.; Shahzad, K.; Sheikh, N.; Munir, S. Extraction and characterization of humic acid from Pakistani lignite coals. Energy Sources Part A Recovery Util. Environ. Effects 2017, 39, 1159–1166. [Google Scholar] [CrossRef]
- Saleem, M.; Irfan, M.; Tabassum, S.; Albaqami, M.D.; Javed, M.S.; Hussain, S.; Pervaiz, M.; Ahmad, I.; Ahmad, A.; Zuber, M. Experimental and theoretical study of highly porous lignocellulose assisted metal oxide photoelectrodes for dye-sensitized solar cells. Arab. J. Chem. 2021, 14, 102937. [Google Scholar] [CrossRef]
- Hofrichter, M.; Fritsche, W. Depolymerization of low-rank coal by extracellular fungal enzyme systems. II. The ligninolytic enzymes of the coal-humic-acid-depolymerizing fungus Nematoloma frowardii b19. Appl. Microbiol. Biotechnol. 1997, 47, 419–424. [Google Scholar] [CrossRef]
- Sabar, M.A.; Ali, M.I.; Fatima, N.; Malik, A.Y.; Jamal, A.; Liaquat, R.; He, H.; Liu, F.-J.; Guo, H.; Urynowicz, M.; et al. Evaluation of humic acids produced from Pakistani subbituminous coal by chemical and fungal treatments. Fuel 2020, 278, 118301. [Google Scholar] [CrossRef]
- Boral, P.; Varma, A.K.; Maity, S. Nitration of jharia basin coals, india: A study of structural modifications by XRD and FTIR techniques. Int. J. Coal Sci. Technol. 2021, 1–12. [Google Scholar]
- Das, T.; Bora, M.; Tamuly, J.; Benoy, S.M.; Baruah, B.P.; Saikia, P.; Saikia, B.K. Coal-derived humic acid for application in acid mine drainage (AMD) water treatment and electrochemical devices. Int. J. Coal Sci. Technol. 2021, 1–12. [Google Scholar]
- Syahren, A.M.; Wong, N.C. Extraction and chemical characteristics of nitro-humic acids from coals and composts. J. Trop Agric. Fd. Sc. 2008, 36, 269–279. [Google Scholar]
- Manoj, B.; Narayanan, P. Study of changes to the organic functional groups of a high volatile bituminous coal during organic acid treatment process by FTIR spectroscopy. J. Miner. Mater. Characterisation Eng. 2013, 1, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Patti, A.F.; Verheyen, T.V.; Douglas, L.; Wang, X. Nitrohumic acids from Victorian brown coal. Sci. Tot. Environ. 1992, 113, 49–65. [Google Scholar] [CrossRef]
- Macphee, J.M.; Giroux, T.P.; Charland, J.F.; Price, J.T. Detection of natural oxidation of coking coal by TG- FTIR mechanistic implications. Fuel 2004, 83, 1855–1860. [Google Scholar] [CrossRef]
- Dong, L.H.; Yuan, Q.; Yuan, H. Changes of chemical properties of humic acids from crude and fungal transformed lignite. Fuel 2006, 85, 2402–2407. [Google Scholar] [CrossRef]
- Wang, C.F.; Fan, X.; Zhang, F.; Wang, S.Z.; Zhao, Y.P.; Zhao, X.Y.; Zhao, W.; Zhu, T.G.; Lu, J.L.; Wei, X.Y. Characterization of humic acids extracted from a lignite and interpretation for the mass spectra. RSC Adv. 2017, 7, 20677–20684. [Google Scholar] [CrossRef] [Green Version]
- Kashif, M.; Jaafar, E.; Bhadja, P.; Low, F.W.; Sahari, S.K.; Hussain, S.; Loong, F.K.; Ahmad, A.; AlGarni, T.S.; Shafa, M.; et al. Effect of potassium permanganate on morphological, structural and electro-optical properties of graphene oxide thin films. Arab. J. Chem. 2021, 14, 102953. [Google Scholar] [CrossRef]
| S.No | Types of Treated Coal | % Yield of Humic Acid Using NaOH |
|---|---|---|
| 1. | Native lignite coal | 21.15 |
| 2. | Native bituminous coal | 11.6 |
| 3. | HNO3 treated lignite coal | 57.8 |
| 4. | HNO3 treated bituminous coal | 49.6 |
| S.No | Types of Treated Coal | E4/E6 Ratio |
|---|---|---|
| 1. | Native Thar lignite coal | 1.503 |
| 2. | Native bituminous coal | 1.405 |
| 3. | HNO3 treated Thar lignite coal | 1.879 |
| 4. | HNO3 treated bituminous coal | 1.660 |
| HA From | Elemental Analysis | Atomic Ratio | Yield (%) | |||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | H/C | O/C | ||
| Raw Lignite coal | 72.2 | 4.44 | 1.97 | 18.07 | 3.31 | 0.06 | 0.25 | 44.2 |
| HNO3 treated lignite coal | 69.21 | 4.35 | 2.77 | 20.37 | 3.30 | 0.06 | 0.29 | 64.1 |
| HA from native bituminous Coal | 56.20 | 10.99 | 3.07 | 18.59 | 11.15 | 0.19 | 0.18 | 38.8 |
| HA from HNO3 treated bituminous coal | 53.2 | 9.89 | 5.37 | 21.38 | 10.07 | 0.185 | 0.40 | 58.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, N.; Jamal, A.; Huang, Z.; Liaquat, R.; Ahmad, B.; Haider, R.; Ali, M.I.; Shoukat, T.; ALOthman, Z.A.; Ouladsmane, M.; et al. Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples. Sustainability 2021, 13, 8969. https://doi.org/10.3390/su13168969
Fatima N, Jamal A, Huang Z, Liaquat R, Ahmad B, Haider R, Ali MI, Shoukat T, ALOthman ZA, Ouladsmane M, et al. Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples. Sustainability. 2021; 13(16):8969. https://doi.org/10.3390/su13168969
Chicago/Turabian StyleFatima, Noureen, Asif Jamal, Zaixing Huang, Rabia Liaquat, Bashir Ahmad, Rizwan Haider, Muhammad Ishtiaq Ali, Tayyba Shoukat, Zeid A. ALOthman, Mohamed Ouladsmane, and et al. 2021. "Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples" Sustainability 13, no. 16: 8969. https://doi.org/10.3390/su13168969
APA StyleFatima, N., Jamal, A., Huang, Z., Liaquat, R., Ahmad, B., Haider, R., Ali, M. I., Shoukat, T., ALOthman, Z. A., Ouladsmane, M., Ali, T., Ali, S., Akhtar, N., & Sillanpää, M. (2021). Extraction and Chemical Characterization of Humic Acid from Nitric Acid Treated Lignite and Bituminous Coal Samples. Sustainability, 13(16), 8969. https://doi.org/10.3390/su13168969








