Sanky (Corryocactus brevistylus) Peel as Low-Cost Adsorbent for Removal of Phosphate from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbents
2.2. Adsorbate
2.3. Selection of Adsorbent
2.4. Physicochemical Characterization of the Selected Adsorbent
2.5. Batch Adsorption Studies
2.6. Adsorption Kinetics
2.7. Adsorption Equilibrium Studies
2.8. Statistical Analysis
2.8.1. Selection of the Sanky Peel Adsorbent
2.8.2. Batch Adsorption
3. Results and Discussion
3.1. Adsorbent Material
3.2. Adsorption Kinetics
3.3. Kinetic Modeling
3.4. Adsorption Isotherms
3.5. Comparison of the Adsorption Capacity with the Adsorbents Reported in the Literature
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Damania, R.; Desbureaux, S.; Rodella, A.-S.; Russ, J.; Zaveri, E. Quality Unknown: The Invisible Water Crisis; World Bank: Washington, DC, USA, 2019. [Google Scholar]
- Marshall, J.A.; Morton, B.J.; Muhlack, R.; Chittleborough, D.; Waikwong, C. Recovery of phosphate from calcium-containing aqueous solution resulting from biochar-induced calcium phosphate precipitation. J. Clean. Prod. 2017, 165, 27–35. [Google Scholar] [CrossRef]
- Bolaños-Alfaro, J.D.; Cordero-Castro, G.; Segura-Araya, G. Determinación de nitritos, nitratos, sulfatos y fosfatos en agua potable como indicadores de contaminación ocasionada por el hombre, en dos cantones de Alajuela (Costa Rica). Tecnol. Marcha 2017, 30, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Roy-Poirier, A. Bioretention for Phosphorus Removal: Modelling Stormwater Quality Improvements; Universidad de la reina en Kingston: Kingston, ON, Canada, 2009. [Google Scholar]
- Xie, Q.; Li, Y.; Lv, Z.; Zhou, H.; Yang, X.; Chen, J.; Guo, H. Effective adsorption and removal of phosphate from aqueous solutions and eutrophic water by Fe-based MOFs of MIL-101. Sci. Rep. 2017, 7, 3316. [Google Scholar] [CrossRef] [PubMed]
- Tareq, R.; Akter, N.; Azam, M.S. Biochars and biochar composites: Low-cost adsorbents for environmental remediation. In Biochar from Biomass and Waste; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–209. [Google Scholar]
- Wang, W.; Huang, G.; An, C.; Zhao, S.; Chen, X.; Zhang, P. Adsorption of anionic azo dyes from aqueous solution on cationic gemini surfactant-modified flax shives: Synchrotron infrared, optimization and modeling studies. J. Clean. Prod. 2018, 172, 1986–1997. [Google Scholar] [CrossRef]
- Pujante, A.M. Valorización de Residuos Sólidos de Lodos de Purín Mediante la Obtención de Carbón Activo; Universitat Politècnica de València: Valencia, Spain, 2016; Available online: https://riunet.upv.es/handle/10251/68389 (accessed on 4 June 2021).
- Camero, C.R.L. Efecto Hepatoprotector del Zumo del Fruto de Corryocactus Brevistylus (Sanky) en Ratones con Daño Hepático Inducido por Etanol; Universidad Nacional Mayor de San Marcos: Lima, Peru, 2016. [Google Scholar]
- Cáceres, F.; Roque, J.; Ostalaza, C.; Walter, H.E. Corryocactus brevistylus (Amended Version of 2013 Assessment). In The IUCN Red List of Threatened Species; Santiago de Chile, Chile. 2017. Available online: https://www.iucnredlist.org/species/152049/121456167 (accessed on 27 May 2021).
- Ostolaza, C.N. Todos los Cactus del Perú; Ministerio del Ambiente: Lima, Perú, 2014. [Google Scholar]
- Pauca, A.; Quipuscoa, V. Catálogo de las cactáceas del departamento de Arequipa, Perú. Arnaldoa 2017, 24, 447–496. [Google Scholar] [CrossRef] [Green Version]
- Arakaki, M.; Ostolaza, C.; Caceres, F.; Roque, J. Cactaceae endémicas del Perú. Rev. Peru. Biol. 2006, 13, 193–219. [Google Scholar] [CrossRef] [Green Version]
- Municipalidad-Distrital-de-Saisa. Plan de Desarrollo Concertado local del Distrito de Saisa 2011–2121; Ayacucho, Perú, 2010. Available online: https://leyes.congreso.gob.pe/Documentos/2016_2021/Consejo_Directivo/Documentos_Otras_Instituciones/OFICIO-0201-2019-MDS-A.pdf (accessed on 10 May 2021).
- SERFOR. SERFOR Promueve Plantaciones de Especie Nativa Medicinal “Sanky” en Comunidades Campesinas de Ica y Huancavelica. 2016. Available online: https://www.serfor.gob.pe/portal/noticias/forestal/serfor-promueve-plantaciones-de-especie-nativa-medicinal-sanky-en-comunidades-campesinas-de-ica-y-huancavelica (accessed on 3 May 2021).
- GOB.REG.-HVCA. Aprobación y Ampliación del Plazo del Proyecto Recuperación del Servicio Ambiental a Través de la Especie Corryocactus Brevistylus (Sanky) en las Provincias de Castrovirreyna y Huaytará Departamento de Huancavelica; Resolución Gerencial General Regional 131-2028/GOB.REG.-HVCA/GGR; Gobierno Regional de Huancavelica: Huancavelica, Peru, 2018. [Google Scholar]
- Lima, D.O.; Crouzeilles, R.; Vieira, M.V. Integrating strict protection and sustainable use areas to preserve the Brazilian Pampa biome through conservation planning. Land Use Policy 2020, 99, 104836. [Google Scholar] [CrossRef]
- Málaga Villanueva, C.N.; Rodríguez Coaguila, M.D.P. Proceso para la obtención de un Néctar Funcional a Partir de Sanky (Corryocactus Brevistylus) Maracuyá (Passiflora edulis), y agua Mineral Obtenida de Yura; Universidad Nacional de San Agustín: Arequipa, Perú, 2014; Available online: http://repositorio.unsa.edu.pe/handle/UNSA/4189 (accessed on 2 July 2021).
- Contreras-López, E.; Salvá Ruiz, B.K. Caracterización sensorial de hamburguesa de llama con cáscara de sanky. Rev. Investig. Altoandin 2018, 20, 155–168. [Google Scholar] [CrossRef]
- Rojas, T.; Fuentes Campos, M.E.; Contreras-López, E.; Gómez, S.; Muñoz, A.M. Extracción asistida por ultrasonido de compuestos fenólicos de la cáscara de sanky (Corryocactus brevistylus). Rev. Soc. Quím. Perú 2019, 85, 258–267. [Google Scholar] [CrossRef]
- Contreras-López, E.; Muñoz, A.M.; Salvá-Ruiz, B.K. Evaluación del extracto de cáscara de sanky en la estabilidad de carne de llama. Rev. Investig. Altoandin 2020, 22, 123–134. [Google Scholar] [CrossRef]
- Rodríguez, N.O.; Cruz, A.; Collantes, I. Estudio de la Composición de los Ácidos Grasos Presentes en la Semilla, Cáscara y Pulpa del Corryocactus Brevistylus Subsp. Puquiensis; Congreso Iberoamericano de Química: Lima, Perú, 2018. [Google Scholar]
- Municipalidad-Distrital-de-Saisa. Aprovechamiento Industrial del Sanky (Corryocactus brevistylus), de los Bosques Naturales en la Comunidad de Saisa, Distrito de Saisa, Provincia de Lucanas, Departamento Ayacucho; Municipalidad-Distrital-de-Saisa: Pasañe, Peru, 2009. [Google Scholar]
- Chaves Ávila, R.; Monzón Campos, J.L. La economía social ante los paradigmas económicos emergentes: Innovación social, economía colaborativa, economía circular, responsabilidad social empresarial, economía del bien común, empresa social y economía solidaria. CIRIEC Esp. Rev. Econ. Publica Soc. Coop. 2018, 93, 5–50. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.; Kapur, M.; Kumar, P.; Mondal, M.K. Adsorptive removal of phosphate from aqueous solution using rice husk and fruit juice residue. Process. Saf. Environ. Prot. 2015, 94, 402–409. [Google Scholar] [CrossRef]
- Londoño Carvajal, A.G.; Gómez, G.; Gutiérrez Gallego, A. Métodos Analíticos para la Evaluación de la Calidad Fisicoquímica del Agua; Universidad Nacional de Colombia: Manizales, Colombia, 2010; Available online: https://repositorio.unal.edu.co/handle/unal/54604 (accessed on 5 June 2021).
- Cooney, D.O. Adsorption Design for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Lapham, D.P.; Lapham, J.L. Gas adsorption on commercial magnesium stearate: The origin of atypical isotherms and BET transform data. Powder Technol. 2019, 342, 676–689. [Google Scholar] [CrossRef]
- ASTM. Standard Test. Methods for Moisture in Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. Standard Test. Method for Total Ash Content of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ASTM. Standard Test. Method for pH of Activated Carbon; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Faria, P.; Orfao, J.; Pereira, M. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Romero Cano, L.A. Preparación y Caracterización de Materiales Adsorbentes a Partir de Cáscaras de Frutas para su Uso en la Remoción de Metales y Aplicación a Procesos Ambientales; Universidad de Granada: Granada, Spain, 2018; Available online: https://digibug.ugr.es/handle/10481/49626 (accessed on 18 June 2021).
- Elovich, S.Y.; Larionov, O. Theory of adsorption from nonelectrolyte solutions on solid adsorbents. Russ. Chem. Bull. 1962, 11, 191–197. [Google Scholar] [CrossRef]
- Chien, S.; Clayton, W. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Nuñez, J.E.; Colpas, F.; Taron, A. Aprovechamiento de residuos maderosos para la obtencion de resinas de intercambio iónico. Temas Agrarios 2017, 22, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Israel, U.; Eduok, U. Biosorption of zinc from aqueous solution using coconut (Cocos nucifera L.) coir dust. Arch. Appl. Sci. Res. 2012, 4, 809–819. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Moreno Marenco, A.R. Estudio de Diferentes Bioadsorbentes Como Posibles Retenedores de Fosfatos en Aguas; Universidad Nacional de Colombia: Bogotá, Colombia, 2013. [Google Scholar]
- El-Peruano. Aprueban las Listas de Insumos Químicos, Productos y sus Subproductos o Derivados que son Objeto de Control, y Definen los Bienes Fiscalizados Considerados de Uso Doméstico y Artesanal, Conforme lo Establecido en los Artículos 5 y 16 del Decreto Legislativo N° 1126. DECRETO SUPREMO N° 268-2019-EF. 2019; pp. 13–16. Available online: https://busquedas.elperuano.pe/normaslegales/aprueban-las-listas-de-insumos-quimicos-productos-y-sus-sub-decreto-supremo-n-268-2019-ef-1799604-5/ (accessed on 15 June 2021).
- Mosoarca, G.; Vancea, C.; Popa, S.; Gheju, M.; Boran, S. Syringa vulgaris leaves powder a novel low-cost adsorbent for methylene blue removal: Isotherms, kinetics, thermodynamic and optimization by Taguchi method. Sci. Rep. 2020, 10, 17676. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K.; Nasar, A. Walnut shell powder as a low-cost adsorbent for methylene blue dye: Isotherm, kinetics, thermodynamic, desorption and response surface methodology examinations. Sci. Rep. 2020, 10, 7983. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water urification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Kumar, P.S.; Korving, L.; van Loosdrecht, M.C.M.; Witkamp, G.-J. Adsorption as a technology to achieve ultra-low concentrations of phosphate: Research gaps and economic analysis. Water Res. X 2019, 4, 100029. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.; Lv, S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate. Bioresour. Technol. 2011, 102, 716–723. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Meenakshi, S. Fabrication of hybrid chitosan encapsulated magnetic-kaolin beads for adsorption of phosphate and nitrate ions from aqueous solutions. Int. J. Biol. Macromol. 2021, 168, 750–759. [Google Scholar] [CrossRef] [PubMed]
- AWWA. B604-18 AWWA Standard Granular Activated Carbon; American Water Works Association: Denver, CO, USA, 2018. [Google Scholar]
- Budianto, A.; Kusdarini, E.; Effendi, S.S.W.; Aziz, M. The production of activated carbon from Indonesian mangrove charcoal. IOP Conf. Ser. Mater. Sci. Eng. 2019, 462, 012006. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef] [Green Version]
- Johansson, L.; Gustafsson, J.P. Phosphate removal using blast furnace slags and opoka-mechanisms. Water Res. 2000, 34, 259–265. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Otero-Calvis, A.; Falcón-Hernández, J.; Yperman, Y. Características fisicoquímicas del carbón activado de conchas de coco modificado con HNO3. Rev. Cuba. Quím. 2017, 29, 26–38. [Google Scholar]
- Moreno-Piraján, J.C.; Navarrete, L.F.; Giraldo, L.; García, V. Adsorción de fenol y 3-cloro fenol sobre carbones activados mediante calorimetría de inmersión. Inf. Tecnol. 2007, 18, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Valdés, H.; Zaror, C.A. Influencia de la composición química superficial del carbón activado en la adsorción de benzotiazoles. Rev. Chi. Ing. 2010, 18, 38–43. [Google Scholar] [CrossRef]
- Jiménez Ramos, I.; Rondon, W.; Astudillo, L.R. Síntesis de carbón activado a partir de epicarpio de Attalea macrolepis y su aplicación en la remoción de Pb2+ en soluciones acuosas. Rev. Int. Contam. Ambient. 2017, 33, 303–316. [Google Scholar] [CrossRef] [Green Version]
- Anee, T.K.; Meenakshi Sundaram, N.; Arivuoli, D.; Ramasamy, P.; Narayana Kalkura, S. Influence of an organic and an inorganic additive on the crystallization of dicalcium phosphate dihydrate. J. Cryst. Growth 2005, 285, 380–387. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, M.; Fang, W.; Wu, D. One-step synthesis of magnetite core/zirconia shell nanocomposite for high efficiency removal of phosphate from water. Appl. Surf. Sci. 2016, 366, 67–77. [Google Scholar] [CrossRef]
- Trinh, V.T.; Nguyen, T.M.P.; Van, H.T.; Hoang, L.P.; Nguyen, T.V.; Ha, L.T.; Vu, X.H.; Pham, T.T.; Nguyen, T.N.; Quang, N.V.; et al. Phosphate adsorption by silver nanoparticles-loaded activated carbon derived from tea residue. Sci. Rep. 2020, 10, 3634. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Ramón de los Santos, C.; Barajas Fernández, J.; Pérez Hernández, G.; Díaz Flores, L.L. Adsorción de cobre (II) y cadmio (II) en suspensiones acuosas de CaCO3 biogénico nanoestructurado. Bol. Soc. Esp. Ceram. V 2019, 58, 2–13. [Google Scholar] [CrossRef]
- Ahmad, M.; Akanji, M.A.; Usman, A.R.A.; Al-Farraj, A.S.F.; Tsang, Y.F.; Al-Wabel, M.I. Turning date palm waste into carbon nanodots and nano zerovalent iron composites for excellent removal of methylthioninium chloride from water. Sci. Rep. 2020, 10, 16125. [Google Scholar] [CrossRef]
- Reátegui-Romero, W.; Cadenas-Vásquez, W.J.; King-Santos, M.E.; Zaldivar Alvarez, W.F.; Posadas, R.A.Y. Evaluation of Pb (II) adsorption from aqueous solutions using Brassica nigra as a biosorbent. Open Biotechnol. J. 2019, 13, 77–92. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Stylianou, M.A.; Gkantzou, D.; Loizidou, M.D. Removal of Pb (II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 2007, 210, 248–256. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M.; Cıtak, M.; Soylak, M. Adsorption characteristics of Cu (II) and Pb (II) onto expanded perlite from aqueous solution. J. Hazard. Mater. 2007, 148, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, H.; Liu, W.; Su, H.; Lu, Y.; Li, J. Study on regeneration of waste powder activated carbon through pyrolysis and its adsorption capacity of phosphorus. Sci. Rep. 2018, 8, 778. [Google Scholar] [CrossRef] [Green Version]
- Xiong, W.; Tong, J.; Yang, Z.; Zeng, G.; Zhou, Y.; Wang, D.; Song, P.; Xua, R.; Zhang, C.; Cheng, M. Adsorption of phosphate from aqueous solution using iron-zirconium modified activated carbon nanofiber: Performance and mechanism. J. Colloid Interface Sci. 2017, 493, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Baoqing, S.; Wenzhong, T. Preparation of powder activated carbon by rice husks and its adsorption capacity to phosphorus. Chin. J. Environ. Eng. 2013, 7, 1024–1028. [Google Scholar]
- Rashid, M.; Price, N.T.; Gracia Pinilla, M.A.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef] [PubMed]
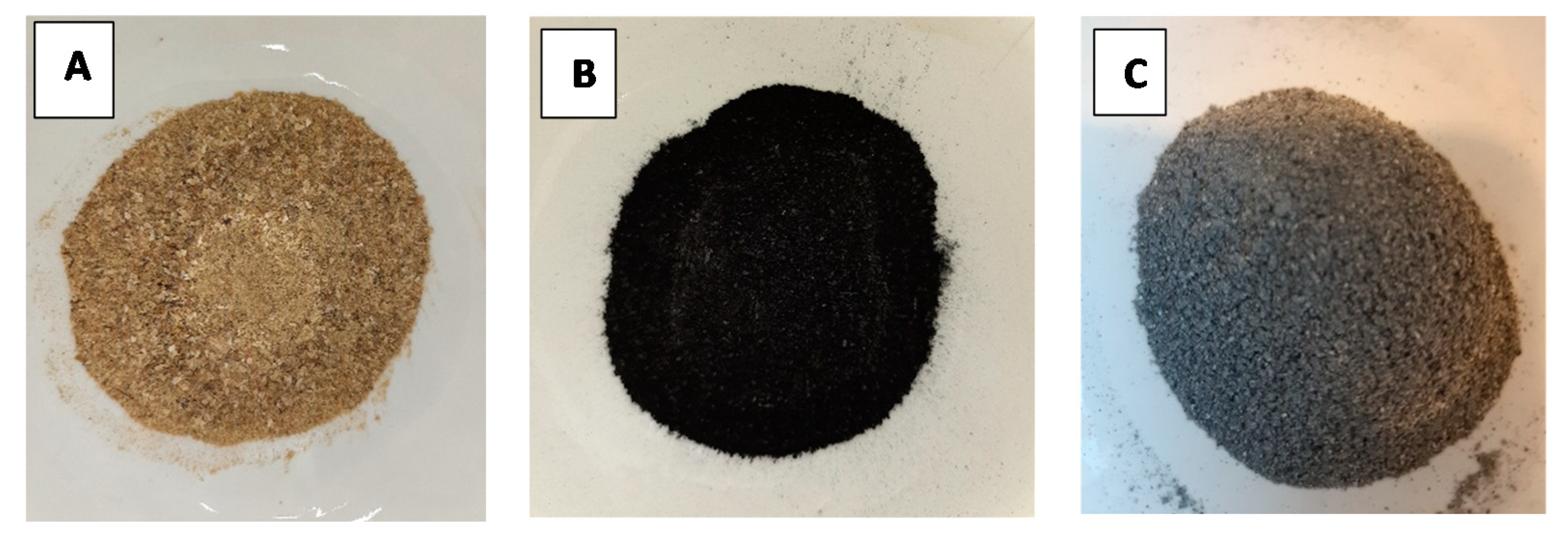



| Parameter | S01 | S02 | S03 |
|---|---|---|---|
| Yield (%) | 76.64 ±1.55 | 28.01 ± 1.55 | 5.64 ± 1.55 |
| Cost (US$/kg) | 2.49 | 13.04 | 45.72 |
| Phosphate removal efficiency (%) | −72.20 ± 2.95 | 61.10 ± 2.95 | 96.83 ± 2.95 |
| Phosphate adsorption capacity (mg/g) | −18.05 ± 0.39 | 15.28 ± 0.39 | 24.21 ± 0.39 |
| Humidity (%) | 10.61 ± 0.81 | 5.38 ± 0.81 | 4.61 ± 0.81 |
| Total ash (dry basis) (%) | 16.55 ± 1.86 | 5.11 ± 1.86 | 4.64 ± 1.86 |
| pH (mass/volume = 1/20) | 3.34 ± 0.03 | 10.39 ± 0.03 | 10.26 ± 0.03 |
| Sample | SBET (m2/g) | Smi (m2/g) | Smi/SBET (%) | Sext (m2/g) | Vmi (cm3/g) | VT cm3/g | Vmi/VT (%) | Dp (nm) |
|---|---|---|---|---|---|---|---|---|
| S01 | −0.41 | - | - | - | - | - | - | - |
| S02 | 9.32 | 4.97 | 53 | 4.36 | 0.0025 | 0.268 | 10 | 7.73 |
| S03 | 14.92 | 3.27 | 22 | 11.65 | 0.0014 | 0.016 | 9 | 4.21 |
| Model | pH | ||
|---|---|---|---|
| 4.0 | 6.5 | 9.0 | |
| Langmuir | |||
| Freundlich | |||
| Dubinin–Radushkevich | |||
| Model | Parameter | pH | ||
|---|---|---|---|---|
| 4.0 | 6.5 | 9.0 | ||
| Langmuir | qmax (mg/g) | −3.22 | −1.96 | −1.84 |
| kL (L/mg) | −2.45 | −0.63 | −0.64 | |
| RL | −0.69 | 2.69 | 2.82 | |
| R2 | 0.793 | 0.996 | 0.996 | |
| R2 ajusted | 0.173 | 0.982 | 0.982 | |
| Freundlich | KF | 13.00 | 63.80 | 64.46 |
| n | −2.12 | 0.34 | 0.19 | |
| R2 | 0.878 | 0.949 | 0.888 | |
| R2 ajusted | 0.512 | 0.796 | 0.550 | |
| Dubinin–Radushkevich | qmax (mg/g) | 1.06 | 6.60 | 6.74 |
| β (mol2 kJ−2) | −0.0017 | 0.0019 | 0.0020 | |
| E (kJ/mol) | 16.2 | 15.8 | ||
| R2 | 0.912 | 0.987 | 0.988 | |
| R2 ajusted | 0.648 | 0.948 | 0.951 | |
| Adsorbent | Doses | pH | Temperature (°C) | qt (mg g−1) | Reference |
|---|---|---|---|---|---|
| Sanky Shell Adsorbent (S02) | 0.05 g adsorbent/25 mL | 6.5 | 25 | 14.44 | This work |
| Powdered Activated Carbon | 0.02 g adsorbent/30 mL | 3.6 | 20 | 16.86 | [73] |
| Zirconium Oxide and Iron Oxide on Activated Carbon Nanofibers | 0.3 g adsorbent/300 mL | 4.0 | 25 | 26.3 | [74] |
| Activated Carbon Derived from Rice Husk | 0.2 g adsorbent/50 mL | 7.0 | 25 | 6.93 | [75] |
| Humic Acid Coated with Magnetite Nanoparticles | 0.05 g adsorbent/50 mL | 6.6 | 25 | 28.9 | [76] |
| Biochar from Grape Stalks Charred at 500 °C | 0.05 g adsorbent/25 mL | 6.5 | 25 | 25.9 | [2] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-López, E.; Miyashiro Kiyan, V.; Porras Cerrón, J.; Muñoz, A.M.; Ramos-Escudero, F.; Portuguez-Maurtua, M.; Yuli-Posadas, R.; Garayar-Tasayco, H. Sanky (Corryocactus brevistylus) Peel as Low-Cost Adsorbent for Removal of Phosphate from Aqueous Solutions. Sustainability 2021, 13, 8994. https://doi.org/10.3390/su13168994
Contreras-López E, Miyashiro Kiyan V, Porras Cerrón J, Muñoz AM, Ramos-Escudero F, Portuguez-Maurtua M, Yuli-Posadas R, Garayar-Tasayco H. Sanky (Corryocactus brevistylus) Peel as Low-Cost Adsorbent for Removal of Phosphate from Aqueous Solutions. Sustainability. 2021; 13(16):8994. https://doi.org/10.3390/su13168994
Chicago/Turabian StyleContreras-López, Eliana, Victor Miyashiro Kiyan, Jaime Porras Cerrón, Ana María Muñoz, Fernando Ramos-Escudero, Marcelo Portuguez-Maurtua, Ricardo Yuli-Posadas, and Humberto Garayar-Tasayco. 2021. "Sanky (Corryocactus brevistylus) Peel as Low-Cost Adsorbent for Removal of Phosphate from Aqueous Solutions" Sustainability 13, no. 16: 8994. https://doi.org/10.3390/su13168994
APA StyleContreras-López, E., Miyashiro Kiyan, V., Porras Cerrón, J., Muñoz, A. M., Ramos-Escudero, F., Portuguez-Maurtua, M., Yuli-Posadas, R., & Garayar-Tasayco, H. (2021). Sanky (Corryocactus brevistylus) Peel as Low-Cost Adsorbent for Removal of Phosphate from Aqueous Solutions. Sustainability, 13(16), 8994. https://doi.org/10.3390/su13168994







