Sustainable Indigenous Fishing in the Pre-Contact Caribbean: Evidence and Critical Considerations from Carriacou, Grenada
Abstract
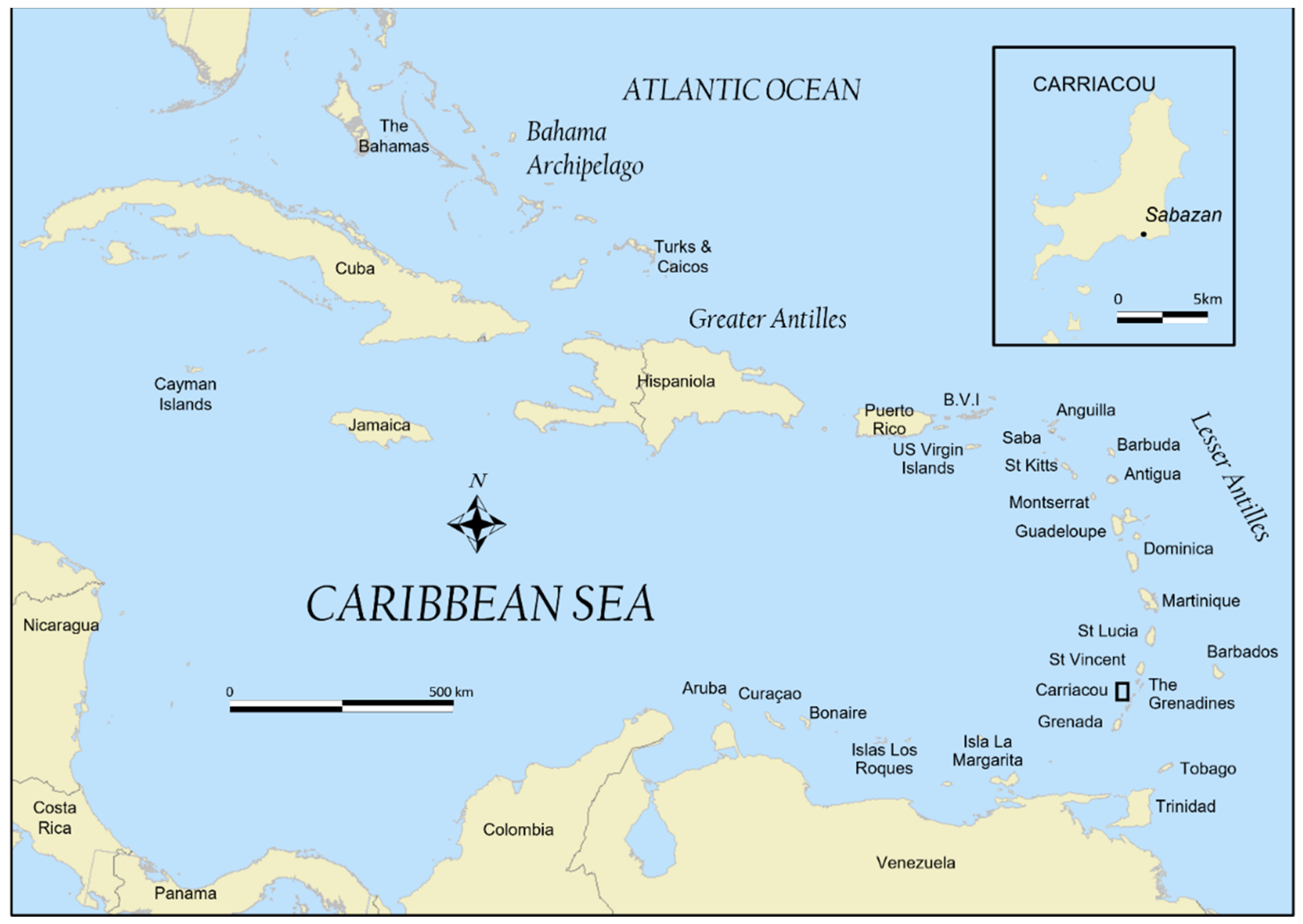
:1. Introduction
2. Background
2.1. Archaeology and Environment
2.2. Forging Theory, Resource Depression, and Abundance Indices
3. Materials and Methods
4. Results
5. Discussion
6. Conclusion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, J.H. An environmental (pre) history of European fishing: Past and future archaeological contributions to sustainable fisheries. J. Fish Biol. 2019, 94, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braje, T.J.; Rick, T.C.; Szpak, P.; Newsome, S.D.; McCain, J.M.; Smith, E.A.; Glassow, M.; Hamilton, S.L. Historical ecology and the conservation of large, hermaphroditic fishes in Pacific Coast kelp forest ecosystems. Sci. Adv. 2017, 3, e1601759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, K.L.; Jackson, J.B.; Donovan, M.K.; Greenstein, B.J.; Korpanty, C.A.; Cook, G.M.; Pandolfi, J.M. Widespread loss of Caribbean acroporid corals was underway before coral bleaching and disease outbreaks. Sci. Adv. 2020, 6, eaax9395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, K.L.; O’Dea, A.; Leonard-Pingel, J.S.; Norris, R.D. Millennial-scale change in the structure of a Caribbean reef ecosystem and the role of human and natural disturbance. Ecography 2020, 43, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.B.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D. Beyond duplicity and ignorance in global fisheries. WIT Trans. Eng. Sci. 2013, 64, 519–535. [Google Scholar]
- Plank, M.J.; Allen, M.S.; Nims, R.; Ladefoged, T.N. Inferring fishing intensity from contemporary and archaeological size-frequency data. J. Archaeol. Sci. 2018, 93, 42–53. [Google Scholar] [CrossRef]
- Baisre, J.A. Setting a baseline for Caribbean fisheries. J. Isl. Coast. Archaeol. 2010, 5, 120–147. [Google Scholar] [CrossRef]
- Grouard, S.; Perdikaris, S.; Espindola Rodrigues, N.E.; Quitmyer, I.R. Size estimation of pre-Columbian Caribbean fish. Int. J. Osteoarchaeol. 2019, 29, 452–468. [Google Scholar] [CrossRef]
- Stager, J.C.; Chen., V. Fossil evidence of shell length decline in queen conch (Strombus gigas L.) at Middleton Cay, Turks and Caicos Islands, British West Indies. Caribb. J. Sci. 1996, 32, 14–20. [Google Scholar]
- Wing, E.S. The sustainability of resources used by Native Americans on four Caribbean islands. Int. J. Osteoarchaeol. 2001, 11, 14–23. [Google Scholar] [CrossRef]
- Wing, E.S.; Wing, S.R. Prehistoric fisheries in the Caribbean. Coral Reefs 2001, 20, 1–8. [Google Scholar]
- Butler, V.L. Seeking balance in “human impacts” research. Comment on Julio Baisre’s “Setting a baseline for Caribbean fisheries”. J. Isl. Coast. Archaeol. 2010, 5, 148–151. [Google Scholar] [CrossRef]
- Reitz, E.J.; Quitmyer, I.R.; Marrinan, R.A. What are we measuring in the zooarchaeological record of prehispanic fishing strategies in the Georgia Bight, USA? J. Isl. Coast. Archaeol. 2009, 4, 2–36. [Google Scholar] [CrossRef]
- Fenberg, P.B.; Roy, K. Ecological and evolutionary consequences of size-selective harvesting: How much do we know? Mol. Ecol. 2008, 17, 209–220. [Google Scholar] [CrossRef]
- Jørgensen, C.; Enberg, K.; Dunlop, E.S.; Arlinghaus, R.; Boukal, B.S.; Brander, K.; Ernande, K.; Gårdmark, A.; Johnston, F.; Matsumura, S.; et al. Managing evolving fish stocks. Science 2007, 318, 1247–1248. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres, F., Jr. Fishing down marine food webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef]
- Charnov, E.L.; Orians, G.H.; Hyatt, K. Ecological implications of resource depression. Am. Nat. 1976, 110, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Broughton, J.M.; Cannon, M.D.; Bayham, F.E.; Byers, D.A. Prey body size and ranking in zooarchaeology: Theory, empirical evidence, and applications from the northern Great Basin. Am. Antiq. 2011, 76, 403–428. [Google Scholar] [CrossRef] [Green Version]
- Byers, D.A.; Broughton, J.M. Holocene environmental change, artiodactyl abundances, and human hunting strategies in the Great Basin. Am. Antiq. 2004, 69, 235–255. [Google Scholar] [CrossRef] [Green Version]
- Codding, B.F.; Bird, D.W.; Bird, R.B. Interpreting abundance indices: Some zooarchaeological implications of Martu foraging. J. Archaeol. Sci. 2010, 37, 3200–3210. [Google Scholar] [CrossRef]
- Denevan, W.M. The pristine myth: The landscape of the Americas in 1492. Ann. Am. Assoc. Geogr. 1992, 82, 369–385. [Google Scholar] [CrossRef]
- Curet, L.A. The archaeological perspective: Comment on Julio Baisre’s “Setting a baseline for Caribbean fisheries. ” J. Isl. Coast. Archaeol. 2010, 5, 152–155. [Google Scholar] [CrossRef]
- McClenachan, L.; Hardt, M.; Jackson, J.; Cooke, R. Mounting evidence for historical overfishing and long-term degradation of Caribbean marine ecosystems: Comment on Julio Baisre’s “Setting a baseline for Caribbean Fisheries”. J. Isl. Coast. Archaeol. 2010, 5, 165–169. [Google Scholar] [CrossRef]
- Bochaton, C.; Ephrem, B.; Bérard, B.; Cochard, D.; Gala, M.; Richter, K.K.; Le Lay, A.; Renou, S.; Lenoble, A. The pre-Columbian site of Roseau (Guadeloupe, FWI): Intra-site chronological variability of the subsistence strategies in a Late Ceramic archeological vertebrate assemblage. Archaeol. Anthropol. Sci. 2021, 13, 16. [Google Scholar] [CrossRef]
- Carder, N.; Crock, J.G. A pre-Columbian fisheries baseline from the Caribbean. J. Archaeol. Sci. 2012, 39, 3115–3124. [Google Scholar] [CrossRef]
- Carder, N.; Reitz, E.J.; Crock, J.G. Fish communities and populations during the post-Saladoid period (AD 600/800–1500), Anguilla, Lesser Antilles. J. Archaeol. Sci. 2007, 34, 588–599. [Google Scholar] [CrossRef]
- Giovas, C.M. Though she be but little: Resource resilience, Amerindian foraging, and long-term adaptive strategies in the Grenadines, West Indies. J. Isl. Coast. Archaeol. 2016, 11, 238–263. [Google Scholar] [CrossRef]
- Giovas, C.M.; Clark, M.; Fitzpatrick, S.M.; Stone, J. Intensifying collection and size increase of the tessellated nerite snail (Nerita tessellata) at the Coconut Walk site, Nevis, northern Lesser Antilles, AD 890–1440. J. Archaeol. Sci. 2013, 40, 4024–4038. [Google Scholar] [CrossRef]
- LeFebvre, M.J. Zooarchaeological analysis of prehistoric vertebrate exploitation at the Grand Bay Site, Carriacou, West Indies. Coral Reefs 2007, 26, 931–944. [Google Scholar] [CrossRef]
- Krigbaum, J.; Fitzpatrick, S.M.; Bankaitis, J. Human paleodiet at Grand Bay, Carriacou, Lesser Antilles. J. Isl. Coast. Archaeol. 2013, 8, 210–227. [Google Scholar] [CrossRef]
- Newsom, L.A.; Wing, E.S. On Land and Sea: Native American Uses of Biological Resources in the West Indies; University of Alabama Press: Tuscaloosa, AL, USA, 2004. [Google Scholar]
- Stokes, A.V. A Biogeographic Survey of Prehistoric Human Diet in the West Indies Using Stable Isotopes. Ph.D. Dissertation, University of Florida, Gainesville, FL, USA, 1998. [Google Scholar]
- Keegan, W.F.; Hofman, C.L. The Caribbean Before Columbus; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Fitzpatrick, S.M. Verification of an Archaic age occupation on Barbados, southern Lesser Antilles. Radiocarbon 2011, 53, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Chinique de Armas, Y.; Buhay, W.M.; Suárez, R.R.; Bestel, S.; Smith, D.; Mowat, S.D.; Roksandic, M. Starch analysis and isotopic evidence of consumption of cultigens among fisher–gatherers in Cuba: The archaeological site of Canímar Abajo, Matanzas. J. Archaeol. Sci. 2015, 58, 121–132. [Google Scholar] [CrossRef]
- Hofman, C.L.; Hoogland, M.L. Plum Piece: Evidence for archaic seasonal occupation on Saba, northern lesser Antilles around 3300 BP. J. Caribb. Archaeol. 2003, 4, 12–27. [Google Scholar]
- Schultz, C. The Carriacou Hypothesis: Bottomless stacked pots, a study in Amerindian fresh water procurement. In Proceedings of the XVI International Congress for Caribbean Archaeology; International Association for Caribbean Archaeology: Basse Terre, Guadeloupe, 1995; pp. 217–228. [Google Scholar]
- Giovas, C.M. Foraging Variability in the Prehistoric Caribbean: Multiple Foraging Optima, Resource Use, and Anthropogenic Impacts on Carriacou, Grenada. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2013. [Google Scholar]
- Giovas, C.M. Pre-Columbian Amerindian lifeways at the Sabazan site, Carriacou, West Indies. J. Isl. Coast. Archaeol. 2018, 13, 161–190. [Google Scholar] [CrossRef]
- Rabinow, S.; Giovas, C.M. A systematic review of agouti (Dasyproctidae: Dasyprocta) records from the pre-1492 Lesser Antilles: New perspectives on an introduced commensal. Int. J. Osteoarchaeol. 2021. [Google Scholar] [CrossRef]
- Keegan, W.F. The People Who Discovered Columbus: The Prehistory of the Bahamas; University of Florida Press: Gainesville, FL, USA, 1992. [Google Scholar]
- Jones, S. A Long-term perspective on biodiversity and marine resource exploitation in Fiji’s Lau Group. Pac. Sci. 2009, 63, 617–648. [Google Scholar] [CrossRef]
- Broughton, J.M. Widening diet breadth, declining foraging efficiency, and prehistoric harvest pressure: Ichthyofaunal evidence from the Emeryville Shellmound, California. Antiquity 1997, 71, 845–862. [Google Scholar] [CrossRef]
- Lupo, K.D. Evolutionary foraging models in zooarchaeological analysis: Recent applications and future challenges. J. Archaeol. Res. 2007, 15, 143–189. [Google Scholar] [CrossRef]
- Nagaoka, L. Declining foraging efficiency and moa carcass exploitation in southern New Zealand. J. Archaeol. Sci. 2005, 32, 1328–1338. [Google Scholar] [CrossRef]
- Kaplan, H.; Hill, K. The evolutionary ecology of food acquisition. In Evolutionary Ecology and Human Behavior; Smith, E.A., Winterhalder, B., Eds.; Aldine de Gruyter: New York, NY, USA, 1992; pp. 167–201. [Google Scholar]
- Stephens, D.W.; Krebs, J.R. Foraging Theory; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Claassen, C. Shells; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Erlandson, J.M. The role of shellfish in prehistoric economies: A protein perspective. Am. Antiq. 1988, 53, 102–109. [Google Scholar] [CrossRef]
- Erlandson, J.M. The archaeology of aquatic adaptations: Paradigms for a new millennium. J. Archaeol. Res. 2001, 9, 287–350. [Google Scholar] [CrossRef]
- Ugan, A. Does size matter? Body size, mass collecting, and their implications for understanding prehistoric foraging behavior. Am. Antiq. 2005, 70, 75–89. [Google Scholar] [CrossRef]
- Blick, J. Pre-Columbian impact on terrestrial, intertidal, and marine resources, San Salvador, Bahamas (A.D. 950–1500). J. Nat. Conserv. 2007, 15, 174–183. [Google Scholar] [CrossRef]
- Erlandson, J.M.; Rick, T.C.; Braje, T.J. Fishing up the food web?: 12,000 years of maritime subsistence and adaptive adjustments on California’s Channel Islands. Pac. Sci. 2009, 63, 711–724. [Google Scholar] [CrossRef] [Green Version]
- Bayham, F.E. Factors influencing the Archaic pattern of animal utilization. Kiva 1979, 44, 219–235. [Google Scholar] [CrossRef]
- Ugan, A.; Bright, J. Measuring foraging efficiency with archaeological faunas: The relationship between relative abundance indices and foraging returns. J. Archaeol. Sci. 2001, 28, 1309–1321. [Google Scholar] [CrossRef]
- Nagaoka, L. The effects of resource depression on foraging efficiency, diet breadth, and patch use in southern New Zealand. J. Anthropol. Archaeol. 2002, 21, 419–442. [Google Scholar] [CrossRef]
- Steadman, D.W.; Jones, S. Long-term trends in prehistoric fishing and hunting on Tobago, West Indies. Lat. Am. Antiq. 2006, 17, 316–334. [Google Scholar] [CrossRef]
- Morrison, A.; Addison, D.J. Examining causes and trends in marine trophic level change: 1500 years of fish exploitation at Fatu-ma-Futi, Tutuila Island, American Sāmoa. J. Isl. Coast. Archaeol. 2009, 4, 177–194. [Google Scholar] [CrossRef]
- Reitz, E.J. Fishing down the food web: A case study from St. Augustine, Florida, USA. Am. Antiq. 2004, 69, 63–83. [Google Scholar] [CrossRef]
- Hardt, M.J. Lessons from the past: The collapse of Jamaican coral reefs. Fish Fish. 2009, 10, 143–158. [Google Scholar] [CrossRef]
- Abbott, R.T.; Morris, P.A. Shells of the Atlantic and Gulf Coasts and the West Indies; Houghton Mifflin: Boston, MA, USA, 1995. [Google Scholar]
- Bellwood, D.R. A phylogenetic study of the parrotfishes family Scaridae (Pisces: Labroidei), with a revision of genera. Rec. Aust. Mus. 1994, 20, 1–86. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Version (12/2020). 2020. Available online: www.fishbase.org (accessed on 13 June 2021).
- Chambers, K. 1000 Years of Tuna (Scombridae: Thunnini) Exploitation at Sabazan, Carriacou, West Indies: Size Change and Sustainability through Time. Bachelor’s Thesis, School of Social Science, University of Queensland,, Brisbane St. Lucia, Australia, 2017. [Google Scholar]
- Keegan, W.F. The ecology of Lucayan Arawak fishing practices. Am. Antiq. 1986, 51, 816–825. [Google Scholar] [CrossRef]
- Fritz, S.C.; Björck, S.; Rigsby, C.A.; Baker, P.A.; Calder-Church, A.; Conley, D.J. Caribbean hydrological variability during the Holocene as reconstructed from crater lakes on the island of Grenada. J. Quat. Sci. 2011, 26, 829–838. [Google Scholar] [CrossRef]
- Gischler, E.; Shinn, E.A.; Oschmann, W.; Fiebig, J.; Buster, N.A. A 1500-Year Holocene Caribbean climate archive from the Blue Hole, Lighthouse Reef, Belize. J. Coast. Res. 2008, 246, 1495–1505. [Google Scholar] [CrossRef]
- Malaizé, B.; Bertran, P.; Carbonel, P.; Bonnissent, D.; Charlier, K.; Galop, D.; Imbert, D.; Serrand, N.; Stouvenot, C.; Pujol, C. Hurricanes and climate in the Caribbean during the past 3700 years BP. Holocene 2011, 21, 911–924. [Google Scholar] [CrossRef] [Green Version]
- Richey, J.N.; Poore, R.Z.; Flower, B.P.; Quinn, T.M. 1400 yr multiproxy record of climate variability from the northern Gulf of Mexico. Geology 2007, 35, 423–426. [Google Scholar] [CrossRef] [Green Version]
- Cobb, K.M.; Charles, C.D.; Cheng, H.; Edwards, R.L. El Niño/Southern Oscillation and tropical Pacific climate during the last millennium. Nature 2003, 424, 271–276. [Google Scholar] [CrossRef]
- Eakin, C.M.; Morgan, J.A.; Heron, S.F.; Smith, T.B.; Liu, G.; Alvarez-Filip, L.; Baca, B.; Bartels, E.; Bastidas, C.; Bouchon, C.; et al. Caribbean corals in crisis: Record thermal stress, bleaching, and mortality in 2005. PLoS ONE 2010, e13969. [Google Scholar] [CrossRef] [Green Version]
- Emanuel, K. Increasing destructiveness of tropical cyclones over the past 30 years. Nature 2005, 436, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.A.; Watkinson, A.R.; McWilliams, J.P.; Côté, I.M. Opposing forces of aerosol cooling and El Niño drive coral bleaching on Caribbean reefs. Proc. Natl. Acad. Sci. USA 2006, 103, 18870–18873. [Google Scholar] [CrossRef] [Green Version]
- Saunders, M.A.; Lea, A.S. Large contribution of sea surface warming to recent increase in Atlantic hurricane activity. Nature 2008, 451, 557–560. [Google Scholar] [CrossRef]
- Grouard, S. Faunal remains associated with Late Saladoïd and Post- Saladoïd occupations at Anse à la Gourde, Guadeloupe, West Indies: Preliminary results. Archaeofauna 2001, 10, 71–98. [Google Scholar]
- Lambrides, A.B.; McNiven, I.J.; Ulm, S. Meta-analysis of Queensland’s coastal Indigenous fisheries: Examining the archaeological evidence for geographic and temporal patterning. J. Archaeol. Sci. Rep. 2019, 28, 102057. [Google Scholar] [CrossRef]
- McKechnie, I.; Moss, M.L. Meta-analysis in zooarchaeology expands perspectives on Indigenous fisheries of the Northwest Coast of North America. J. Archaeol. Sci. Rep. 2016, 8, 470–485. [Google Scholar] [CrossRef] [Green Version]
- McKechnie, I. Investigating the complexities of sustainable fishing at a prehistoric village on western Vancouver Island, British Columbia, Canada. J. Nat. Conserv. 2007, 15, 208–222. [Google Scholar] [CrossRef]
- Olsson, L.; Jerneck, A.; Thoren, H.; Persson, J.; O’Byrne, D. Why resilience is unappealing to social science: Theoretical and empirical investigations of the scientific use of resilience. Sci. Adv. 2015, 1, e1400217. [Google Scholar] [CrossRef] [Green Version]
- Marshall, K.N.; Levin, P.S. When “sustainable” fishing isn’t. In Effective Conservation Science: Data Not Dogma; Kareiva, P., Marvier, M., Silliman, B., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 110–114. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Guénette, S.; Pitcher, T.J.; Sumaila, U.R.; Walters, C.J.; Watson, R.; Zeller, D. Towards sustainability in world fisheries. Nature 2002, 418, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Salas, S.; Chuenpagdee, R.; Charles, A.; Seijo, J.C. (Eds.) Coastal Fisheries of Latin America and the Caribbean; FAO Fisheries and Aquaculture Technical Paper No. 544; FAO: Rome, Italy, 2011. [Google Scholar]
- Boivin, N.; Crowther, A. Mobilizing the past to shape a better Anthropocene. Nat. Ecol. Evol. 2021, 5, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, C. Embracing change: How cultural resilience is increased through cultural heritage. World Archaeol. 2018, 50, 639–650. [Google Scholar] [CrossRef]
- Chislett, G.R. Comparative Aspects of the Ecology of Three Nerita (Molluska: Gastropoda), Species from Different Locations in Barbados. Master’s Thesis, McGill University, Montreal, QC, Canada, 1969. [Google Scholar]
- Food an Agricultural Organization. The Sustainable Intensification of Caribbean Fisheries and Aquaculture; I3932E/1/07.14; Food an Agricultural Organization of the United Nations, 2014; Available online: http://www.fao.org/3/i3932e/i3932e.pdf (accessed on 13 June 2021).
- Bouchon, C.; Portillo, P.; Bouchon-Navaro, Y.; Louis, M.; Hoetjes, P.; De Meyer, K.; Macrae, D.; Harding, S.; Mallela, J.; Parkinson, R.; et al. Status of coral reefs of the Lesser Antilles: The French West Indies, The Netherlands Antilles, Anguilla, Antigua, Grenada, Trinidad and Tobago. In Status of Coral Reefs of the World; Wilkinson, C., Ed.; Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre: Townsville, Australia, 2008; pp. 265–280. [Google Scholar]
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, V.V. (Eds.) Status and Trends of Caribbean Coral Reefs: 1970–2012; Global Coral Reef Monitoring Network, IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Morris, J.A., Jr. (Ed.) Invasive Lionfish: A Guide to Control and Management; Gulf and Caribbean Fisheries Institute Special Publication Series Number 1: Marathon, FL, USA, 2012. [Google Scholar] [CrossRef]
- van Tussenbroek, B.I.; Arana, H.A.H.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; González-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; San Martin-Chicas, J.; Myton, J. Transitioning to co-management in Caribbean reef fisheries: Tela Bay case study. Sustain. Sci. 2021, 16, 1233–1250. [Google Scholar] [CrossRef]

| Early Period | Middle Period | Late Period | Final Period | Site Total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | Common Name | NISP | % NISP | MNI | % MNI | NISP | % NISP | MNI | % MNI | NISP | % NISP | MNI | % MNI | NISP | % NISP | MNI | % MNI | NISP | % NISP | MNI | % MNI |
| Actinopterygii | bony ray-finned fishes | 30 | 14.2 | 10 | 30.3 | 668 | 29.0 | 106 | 35.6 | 151 | 14.7 | 31 | 30.7 | 156 | 11.5 | 33 | 20.6 | 1005 | 20.5 | 180 | 30.4 |
| Aves | birds | 3 | 0.1 | 2 | 0.7 | 1 | 0.1 | 1 | 0.6 | 4 | 0.1 | 3 | 0.5 | ||||||||
| Mammalia | mammals | 16 | 7.6 | 4 | 12.1 | 90 | 3.9 | 12 | 4.0 | 9 | 0.9 | 3 | 3.0 | 9 | 0.7 | 2 | 1.3 | 124 | 2.5 | 21 | 3.5 |
| Reptilia | reptiles | 4 | 1.9 | 2 | 6.1 | 55 | 2.4 | 6 | 2.0 | 16 | 1.6 | 3 | 3.0 | 52 | 3.8 | 2 | 1.3 | 127 | 2.6 | 13 | 2.2 |
| Echinoidea | sea urchins | 13 | 6.2 | 1 | 3.0 | 329 | 14.3 | 8 | 2.7 | 332 | 32.3 | 5 | 5.0 | 596 | 44.1 | 6 | 3.8 | 1270 | 26.0 | 20 | 3.4 |
| Bivalvia | clams, oysters | 1 | 0.5 | 1 | 3.0 | 68 | 3.0 | 20 | 6.7 | 7 | 0.7 | 3 | 3.0 | 18 | 1.3 | 8 | 5.0 | 94 | 1.9 | 32 | 5.4 |
| Gastropoda | sea and land snails | 124 | 58.8 | 12 | 36.4 | 334 | 14.5 | 111 | 37.2 | 159 | 15.5 | 46 | 45.5 | 262 | 19.4 | 92 | 57.5 | 879 | 18.0 | 261 | 44.1 |
| Polyplacophora | chitons | 4 | 1.9 | 2 | 6.1 | 140 | 6.1 | 20 | 6.7 | 44 | 4.3 | 8 | 7.9 | 112 | 8.3 | 13 | 8.1 | 300 | 6.1 | 43 | 7.3 |
| Decapoda | crabs | 19 | 9.0 | 1 | 3.0 | 614 | 26.7 | 12 | 4.0 | 309 | 30.1 | 2 | 2.0 | 147 | 10.9 | 3 | 1.9 | 1089 | 22.3 | 18 | 3.0 |
| Cirripedia | barnacles | 1 | 0.0 | 1 | 0.3 | 1 | 0.0 | 1 | 0.2 | ||||||||||||
| Total | 211 | 33 | 2302 | 298 | 1027 | 101 | 1353 | 160 | 4893 | 592 | |||||||||||
| Sabazan Fish Index | ||||
|---|---|---|---|---|
| Fish MNI | Gastropod MNI | Total Fish and Gastropod MNI | Fish Index | |
| Final | 33 | 92 | 125 | 0.264 |
| Late | 31 | 46 | 77 | 0.403 |
| Middle | 106 | 112 | 218 | 0.486 |
| Total | 170 | 250 | 420 | |
| Cochran–Armitage Test | p | |||
| χ2: | 16.29 | <0.001 | ||
| χ2trend: | 16.09 | <0.001 | ||
| Departure from linearity: χ2: | 0.19 | 0.659 | ||
| Sabazan Large Fish Index | |||||
|---|---|---|---|---|---|
| Period | Large Fish MN | * Stndrd. Large Fish MNI | Small Fish MNI | * Stndrd. Small Fish MNI | Large Fish Index |
| Final | 17 | 0.39 | 32 | 8.51 | 0.044 |
| Late | 10 | 0.20 | 25 | 4.78 | 0.040 |
| Middle | 49 | 0.26 | 106 | 3.72 | 0.065 |
| Total | 76 | 163 | |||
| Sabazan Large Nearshore Fish Index | |||||
|---|---|---|---|---|---|
| Period | Large Nearshore Fish MNI | * Stndrd. Large Nearshore Fish MNI | Small Nearshore Fish MNI | * Stndrd. Small Nearshore Fish MNI | Large Nearshore Fish Index |
| Final | 13 | 0.30 | 16 | 4.25 | 0.065 |
| Late | 8 | 0.16 | 17 | 3.25 | 0.047 |
| Middle | 38 | 0.20 | 78 | 2.74 | 0.068 |
| Total | 59 | 111 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovas, C.M. Sustainable Indigenous Fishing in the Pre-Contact Caribbean: Evidence and Critical Considerations from Carriacou, Grenada. Sustainability 2021, 13, 9152. https://doi.org/10.3390/su13169152
Giovas CM. Sustainable Indigenous Fishing in the Pre-Contact Caribbean: Evidence and Critical Considerations from Carriacou, Grenada. Sustainability. 2021; 13(16):9152. https://doi.org/10.3390/su13169152
Chicago/Turabian StyleGiovas, Christina M. 2021. "Sustainable Indigenous Fishing in the Pre-Contact Caribbean: Evidence and Critical Considerations from Carriacou, Grenada" Sustainability 13, no. 16: 9152. https://doi.org/10.3390/su13169152






