Freezing and Heating Tolerance of Pinus nigra Seedlings from Three South to North Balkan Provenances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Frost Hardiness Experiments
2.2.1. Electric Conductivity (EC) Test
2.2.2. Whole-Plant Freeze Testing (WPFT)
2.3. Heat and Drought Tolerance Tests
2.4. Statistical Analysis
3. Results
3.1. Frost Hardiness
3.1.1. Measured by EC Test
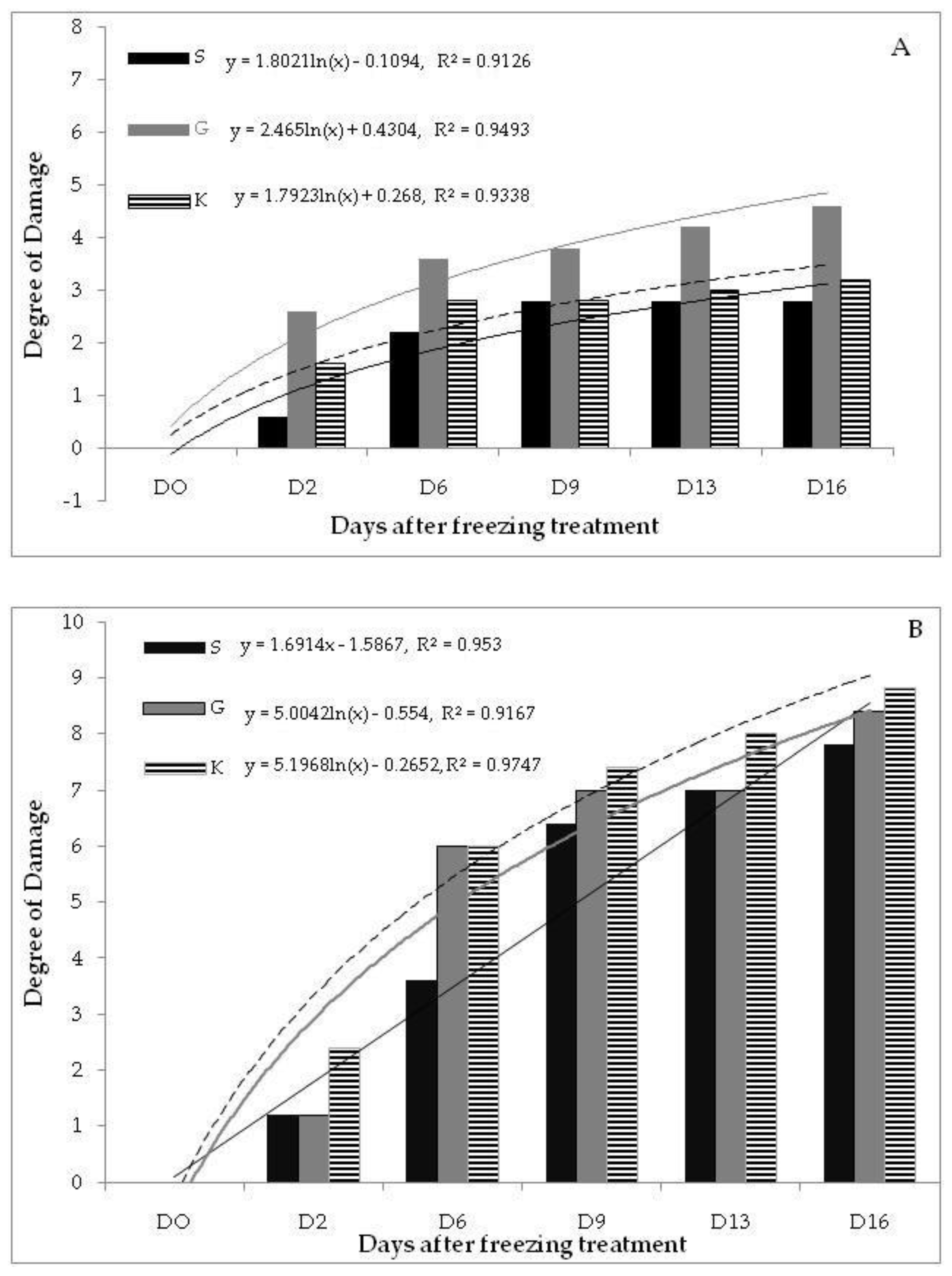
3.1.2. Measured by WPFT
3.2. Heat and Drought Tolerance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piermattei, A.; Renzaglia, F.; Urbinati, C. Recent expansion of Pinus nigra Arn. above the timberline in the central Apennines, Italy. Ann. For. Sci. 2012, 69, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Strimbeck, G.R.; Kjellsen, T.D.; Schaberg, P.G.; Murakami, P.F. Cold in the common garden: Comparative low-temperature tolerance of boreal and temperate conifer foliage. Trees 2007, 21, 557–567. [Google Scholar] [CrossRef]
- Climent, J.; e Silva, F.C.; Chambel, M.R.; Pardos, M.; Almeida, M.H. Freezing injury in primary and secondary needles of Mediterranean pine species of contrasting ecological niches. Ann. For. Sci. 2009, 66, 407. [Google Scholar] [CrossRef] [Green Version]
- Kreyling, J.; Wiesenberg, G.; Thiel, D.; Wohlfart, C.; Huber, G.; Walter, J.; Jentsch, A.; Konnert, M.; Beierkuhnlein, C. Cold hardiness of Pinus nigra Arnold as influenced by geographic origin, warming, and extreme summer drought. Environ. Exp. Bot. 2012, 78, 99–108. [Google Scholar] [CrossRef]
- Marchi, M.; Castaldi, C.; Merlini, P.; Nocentini, S.; Ducci, F. Stand structure and climate influence on the growth trends of a marginal forest population of Pinus nigra spp. Nigra. Ann. Silvic. Res. 2015, 39, 100–110. [Google Scholar] [CrossRef]
- Thiel, D.; Nagy, L.; Beierkuhnlein, C.; Huber, G.; Jentsch, A.; Konnert, M.; Kreyling, J. Uniform drought and warming responses in Pinus nigra provenances despite specific overall performances. For. Ecol. Manag. 2012, 270, 200–208. [Google Scholar] [CrossRef]
- Ivetić, V.; Devetaković, J. Reforestation challenges in Southeast Europe facing climate change. Reforesta 2016, 1, 178–220. [Google Scholar] [CrossRef] [Green Version]
- Martín-Benito, D.; del Rio, M.; Heinrich, I.; Helle, G.; Cañellas, I. Response of climate-growth relationships and water use efficiency to thinning in a Pinus nigra afforestation. For. Ecol. Manag. 2010, 259, 967–975. [Google Scholar] [CrossRef]
- Sánchez-Salguero, R.; Camarero, J.J.; Dobbertin, M.; Fernández-Cancio, A.; Vilà-Cabrera, A.; Manzanedo, R.D.; Zavala, M.A.; Navarro-Cerrillo, R.M. Contrasting vulnerability and resilience to drought-induced decline of densely planted vs. natural rear-edge Pinus nigra forests. For. Ecol. Manag. 2013, 310, 956–967. [Google Scholar] [CrossRef]
- Prentice, I.C.; Cramer, W.; Harrison, S.; Leemans, R.; Monserud, R.A.; Solomon, A.M. Special Paper: A Global Biome Model Based on Plant Physiology and Dominance, Soil Properties and Climate. J. Biogeogr. 1992, 19, 117. [Google Scholar] [CrossRef]
- Glerum, C. Frost hardiness of coniferous seedlings: Principles and applications. In Proceedings of the Evaluating Seedling Quality: Principles, Procedures and Predictive Abilities of Major Tests, Workshop, Corvallis, OR, USA, 16–18 October 1984; Duryea, M.L., Ed.; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1985. [Google Scholar]
- Ganatsas, P. Forest characteristics of Black pine ecosystems and restoration of burned stands. In New Approaches to the Restoration of Black Pine Forests; Kakouros, P., Chrysopolitou, V., Eds.; Management Body of Mount Parnonas and Moustos Wetland: Sparta, Greece, 2010; p. 7. [Google Scholar]
- Ivetić, V.; Grossnickle, S.; Škorić, M. Forecasting the field performance of Austrian pine seedlings using morphological attributes. iFor. Biogeosci. For. 2017, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Stilinović, S. Afforestation; Naučna Knjiga: Belgrade, Serbia, 1991; 274p. (In Serbian) [Google Scholar]
- Critchfield, W.B.; Little, E.L. Geographic Distribution of The Pines of The World; U.S.D.A. Forest Service Miscellaneous Publication 991: Washington, DC, USA, 1966. [Google Scholar]
- Ivetić, V. Šumski Reproduktivni Materijal—Biologija i Tehnologija Proizvodnje; Univerzitet u Beogradu–Šumarski Fakultet: Beograd, Srbija, 2020; 304p, ISBN 978-86-7299-307-3. [Google Scholar]
- Ivetić, V.; Maksimović, Z.; Kerkez, I.; Devetaković, J.R.; Srbijašume, P.; University of Belgrade–Faculty of Forestry. Seedling Quality in Serbia–Results from a Three-Year Survey. Reforesta 2017, 27–53. [Google Scholar] [CrossRef]
- Hydrometeorological Service of Serbia. 2019. Available online: http://www.hidmet.gov.rs/data/klimatologija/latin/2018.pdf (accessed on 3 August 2021).
- Semerci, A.; Imal, B.; Gonzalez-Benecke, C.A. Intraspecific variability in cold tolerance in Pinus brutia sampled from two contrasting provenance trials. New For. 2020, 52, 621–637. [Google Scholar] [CrossRef]
- Flint, H.L.; Boyce, B.R.; Beattie, D.J. Index of injury—A useful expression of freezing injury to plant tissues as determined by the electrolytic method. Can. J. Plant Sci. 1967, 47, 229–230. [Google Scholar] [CrossRef] [Green Version]
- Colombo, S.J.; Webb, D.P.; Glerum, C.C. Frost Hardiness Testing: An Operational Manual for Use with Extended Greenhouse Culture; Ontario Ministry of Natural Resources: Peterborough, ON, Canada, 1984; 14p. [Google Scholar]
- Ivetić, V. Praktikum iz Semenarstva Rasadničarstva i Pošumljavanja; Univerzitet u Beogradu–Šumarski fakultet: Beograd, Srbija, 2013; 213p, ISBN 978-86-7299-211-3. (In Serbian) [Google Scholar]
- Burr, K.E.; Hawkings, C.D.B.; L’Hirondelle, S.; Binder, W.D.; George, M.F.; Repo, T. Methods for measuring cold hardiness of conifers. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 369–401. [Google Scholar]
- Savolainen, O.; Bokma, F.; Garcıa-Gil, R.; Komulainen, P.; Repo, T. Genetic variation in cessation of growth and frost hardiness and consequences for adaptation of Pinus sylvestris to climatic changes. For. Ecol. Manag. 2004, 197, 79–89. [Google Scholar] [CrossRef]
- Sarsu, F. Screening Protocols for Heat Tolerance in Rice at the Seedling and Reproductive Stages. In Pre-Field Screening Protocols for Heat-Tolerant Mutants in Rice; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef] [Green Version]
- Hays, K.; Barber, J.; Kenna, M.; Mccollum, T. Drought Avoidance Mechanisms of Selected Bermudagrass Genotypes. HortScience 1991, 26, 180–182. [Google Scholar] [CrossRef]
- Tsakaldimi, M.; Ganatsas, P.; Jacobs, D. Prediction of planted seedling survival of five Mediterranean species based on initial seedling morphology. New For. 2012, 44, 327–339. [Google Scholar] [CrossRef]
- Lu, P.; Joyce, D.G.; Sinclair, R.W. Geographic variation in cold hardiness among eastern white pine (Pinus strobus L.) provenances in Ontario. For. Ecol. Manag. 2003, 178, 329–340. [Google Scholar] [CrossRef]
- Isajev, V.; Fady, B.; Semerci, H.; Andonovski, V. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Black Pine (Pinus nigra); International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Repo, T.; Zhang, G.; Ryyppö, A.; Rikala, R.; Vuorinen, M. The relation between growth cessation and frost hardening in Scots pines of different origins. Trees 2000, 14, 456–464. [Google Scholar] [CrossRef]
- Taïbi, K.; del Campo, A.; Aguado, A.; Mulet, J. Early establishment response of different Pinus nigra ssp. salzmanii seed sources on contrasting environments: Implications for future reforestation programs and assisted population migration. J. Environ. Manag. 2016, 171, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, G.; Landis, T. Seedling quality tests: Cold hardiness. For. Nurs. Notes 2003, 21–27. Available online: https://rngr.net/publications/fnn/2003-summer/articles/seedling-quality-tests-cold-hardiness (accessed on 3 August 2021).
- South, D.B. Freeze injury to southern pine seedlings. In Proceedings of the 13th Biennial Southern Silvicultural Research Conference; Connor, K.F., Ed.; Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 441–447. [Google Scholar]
- Lavender, D.P. Plant physiology and nursery environment: Interactions affecting seedling growth. In Forest Nursery Manual: Production of Bareroot Seedlings; Duryea, M.L., Landis, T.D., Eds.; MartinusNijhoff/Dr W. Junk, The Hague/Boston/Lancaster for Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1984; pp. 133–141. [Google Scholar]
- Johnson, D.M.; McCulloh, K.A.; Reinhardt, K. The earliest stages of tree growth: Development, physiology and impacts of microclimate. In Size-and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 65–87. [Google Scholar]
- Janssen, E.; Kint, V.; Bontemps, J.-D.; Özkan, K.; Mert, A.; Köse, N.; Icel, B.; Muys, B. Recent growth trends of black pine ( Pinus nigra J.F. Arnold) in the eastern mediterranean. For. Ecol. Manag. 2018, 412, 21–28. [Google Scholar] [CrossRef]
- Móricz, N.; Garamszegi, B.; Rasztovits, E.; Bidló, A.; Horváth, A.; Jagicza, A.; Illés, G.; Vekerdy, Z.; Somogyi, Z.; Gálos, B. Recent Drought-Induced Vitality Decline of Black Pine (Pinus nigra Arn.) in South-West Hungary—Is This Drought-Resistant Species under Threat by Climate Change? Forests 2018, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Tíscar, P.A.; Lucas-Borja, M.E.; Candel-Pérez, D. Lack of local adaptation to the establishment conditions limits assisted migration to adapt drought-prone Pinus nigra populations to climate change. For. Ecol. Manag. 2018, 409, 719–728. [Google Scholar] [CrossRef]
- Santini, F.; Serrano, L.; Kefauver, S.; Abdullah-Al, M.; Aguilera, M.; Sin, E.; Voltas, J. Morpho-physiological variability of Pinus nigra populations reveals climate-driven local adaptation but weak water use differentiation. Environ. Exp. Bot. 2019, 166. [Google Scholar] [CrossRef]
- Mataruga, M.; Haase, D.; Isajev, V.; Orlovic, S. Growth, survival, and genetic variability of Austrian pine (Pinus nigra Arnold) seedlings in response to water deficit. New For. 2012, 43, 791–804. [Google Scholar] [CrossRef]
- Breed, M.F.; Stead, M.G.; Ottewell, K.; Gardner, M.; Lowe, A.J. Which provenance and where? Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Prober, S.M.; Byrne, M.; McLean, E.H.; Steane, D.A.; Potts, B.M.; Vaillancourt, R.E.; Stock, W.D. Climate-adjusted provenancing: A strategy for climate-resilient ecological restoration. Front. Ecol. Evol. 2015, 3, 65. [Google Scholar] [CrossRef] [Green Version]
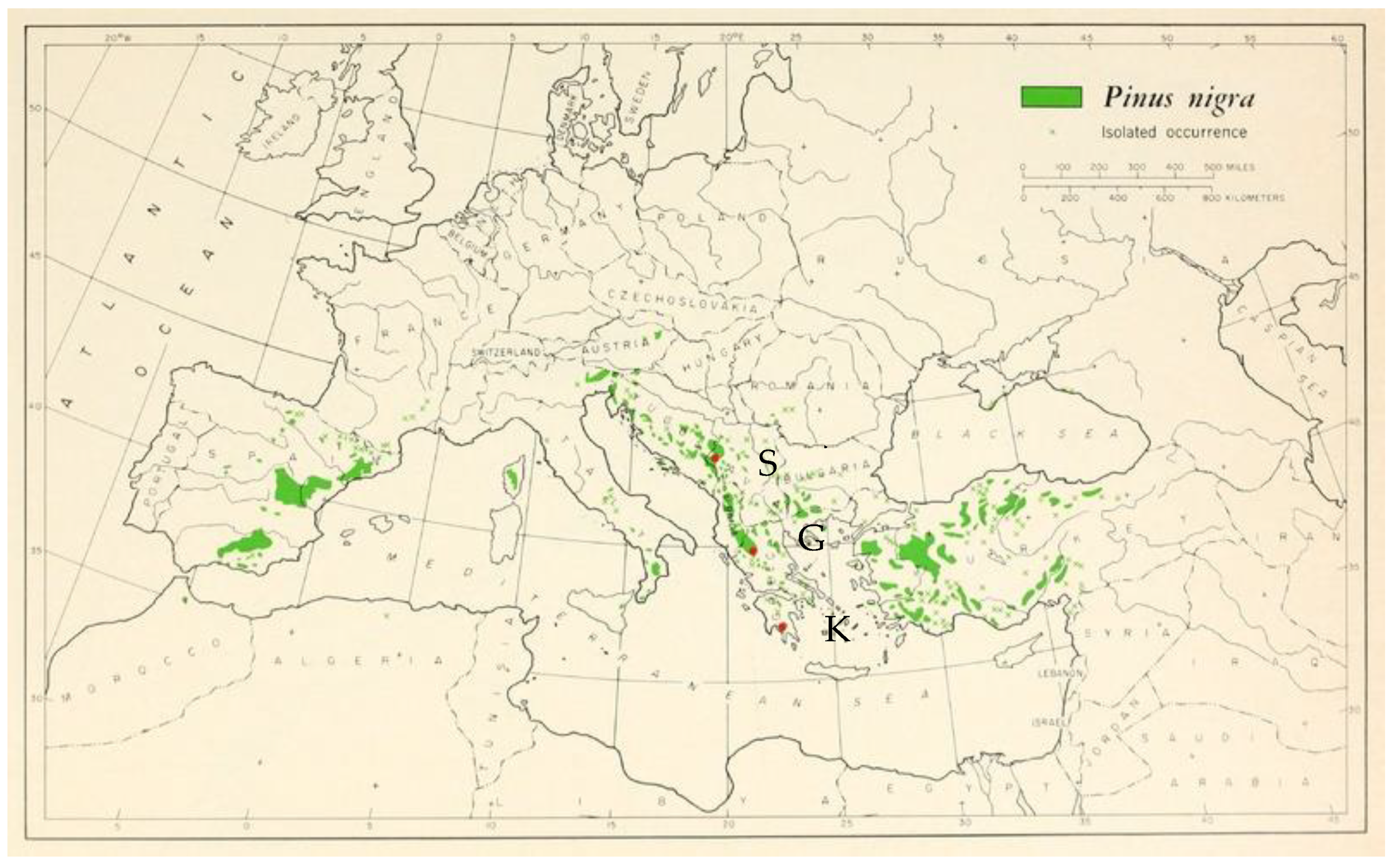
| Provenance | Grevena (Krania) | Kalamata (Taygetos) | Šargan |
|---|---|---|---|
| Country | Greece | Greece | Serbia |
| Coordinates | 39.951169 N; 21.210922 E | 36.954413 N; 22.264140 E | 43.816944 N; 19.481944 E |
| Altitude (m a.s.l.) | 1100 | 1100 | 955 |
| Reg. number | - | - | RS-2-2-pni-41a-089 |
| Subspecies | pallasiana | pallasiana | nigra |
| Climate [18] | |||
| Yearly average (°C) | 10.5 | 11.7 | 7.7 |
| Yearly average max. (°C) | 15.1 | 16.2 | 12.8 |
| Yearly average min. (°C) | 3.7 | 8.3 | 3.9 |
| Absolute maximum (°C) | 35.5 (July) | 32.3 (July) | 35.8 (July) |
| Mean temperature of warmest month (°C) | 21.2 (July) | 20.5 (August) | 17.5 (August) |
| Absolute minimum (°C) | −16.9 (January) | −7.6 (January) | −19.8 (January) |
| Mean temperature of coldest month (°C) | 0.7 (January) | 3.2 (January) | −2.0 (January) |
| Average number of frost days | 96 | 21 | 116 |
| Average number of tropical days | 12 | 6 | 4 |
| Annual precipitation (mm) | 1062 | 885.6 | 1017.3 |
| RCc | RCF | Ii | |
|---|---|---|---|
| −5 °C | |||
| S | 25.59 (2.47) a | 41.48 (4.55) a | 21.35 (1.88) a |
| G | 20.17 (1.77) b | 25.14 (5.63) b | 6.22 (0.71) b |
| K | 17.20 (1.72) c | 24.91 (2.06) b | 9.31 (0.94) b |
| F | 4.92 | ||
| p | 0.0257 | ||
| −18 °C | |||
| S | 25.59 (2.47) a | 65.31 (2.47) | 53.37 (2.22) |
| G | 20.17 (1.77) b | 59.18 (1.78) | 48.87 (2.01) |
| K | 17.20 (1.72) c | 62.34 (1.72) | 54.52 (1.69) |
| F | 0.26 | ||
| p | 0.7756 | ||
| −5 °C | |||||
| Level of Factor | D2 | D6 | D9 | D13 | D16 |
| S | 9.40 (0.24) | 7.80 (0.37) | 7.20 (0.50) | 7.20 (1.10) | 7.20 (0.49) |
| G | 7.40 (1.21) | 6.40 (1.21) | 6.20 (1.11) | 5.80 (1.36) | 5.40 (1.21) |
| K | 8.40 (0.68) | 7.20 (0.97) | 7.20 (0.97) | 7.00 (0.58) | 6.80 (1.16) |
| Average | 8.40 (0.49) | 7.13 (0.52) | 6.87 (0.49) | 6.67 (0.58) | 6.47 (0.58) |
| F | 1.5151 | 0.5827 | 0.4132 | 0.5244 | 0.8816 |
| p | 0.2589 | 0.5734 | 0.6706 | 0.6049 | 0.4393 |
| −18 °C | |||||
| S | 8.80 (0.97) | 6.40 (0.60) a | 3.60 (0.40) | 3.00 (0.45) a | 2.20 (0.37) |
| G | 8.80 (0.20) | 4.00 (0.00) b | 3.00 (0.00) | 3.00 (0.00) a | 1.60 (0.24) |
| K | 7.60 (0.68) | 4.00 (0.63) b | 2.60 (0.24) | 2.00 (0.00) b | 1.20 (0.20) |
| Average | 8.40 (0.40) | 4.80 (0.40) | 3.07 (0.18) | 2.67 (0.19) | 1.67 (0.19) |
| F | 1.0000 | 7.5789 | 3.4545 | 5.0000 | 3.1667 |
| p | 0.3966 | 0.0074 | 0.0653 | 0.0263 | 0.0786 |
| Control | Drought Treatment | Heat and Drought Treatment | |||
|---|---|---|---|---|---|
| Provenance | Both Provenaces | Grevena | Šargan | Grevena | Šargan |
| Days after treatment | |||||
| 1 | 1 (0.00) | 1 (0.00) | 1 (0.00) | 1 (0.00) | 1 (0.00) |
| 4 | 1 (0.00) c | 2 (0.97) b | 1.9 (0.72) b | 2.3 (0.88) a | 2.3 (0.99) a |
| 7 | 1 (0.00) c | 2.8 (0.98) b | 2.9 (0.81) b | 3.3 (0.69) a | 3.4 (0.81) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivetić, V.; Tsakaldimi, M.; Ganatsas, P.; Kerkez Janković, I.; Devetaković, J. Freezing and Heating Tolerance of Pinus nigra Seedlings from Three South to North Balkan Provenances. Sustainability 2021, 13, 9290. https://doi.org/10.3390/su13169290
Ivetić V, Tsakaldimi M, Ganatsas P, Kerkez Janković I, Devetaković J. Freezing and Heating Tolerance of Pinus nigra Seedlings from Three South to North Balkan Provenances. Sustainability. 2021; 13(16):9290. https://doi.org/10.3390/su13169290
Chicago/Turabian StyleIvetić, Vladan, Marianthi Tsakaldimi, Petros Ganatsas, Ivona Kerkez Janković, and Jovana Devetaković. 2021. "Freezing and Heating Tolerance of Pinus nigra Seedlings from Three South to North Balkan Provenances" Sustainability 13, no. 16: 9290. https://doi.org/10.3390/su13169290
APA StyleIvetić, V., Tsakaldimi, M., Ganatsas, P., Kerkez Janković, I., & Devetaković, J. (2021). Freezing and Heating Tolerance of Pinus nigra Seedlings from Three South to North Balkan Provenances. Sustainability, 13(16), 9290. https://doi.org/10.3390/su13169290







