Optimization of MgO-GGBS Cementitious Systems Using Thermo-Chemical Approaches
Abstract
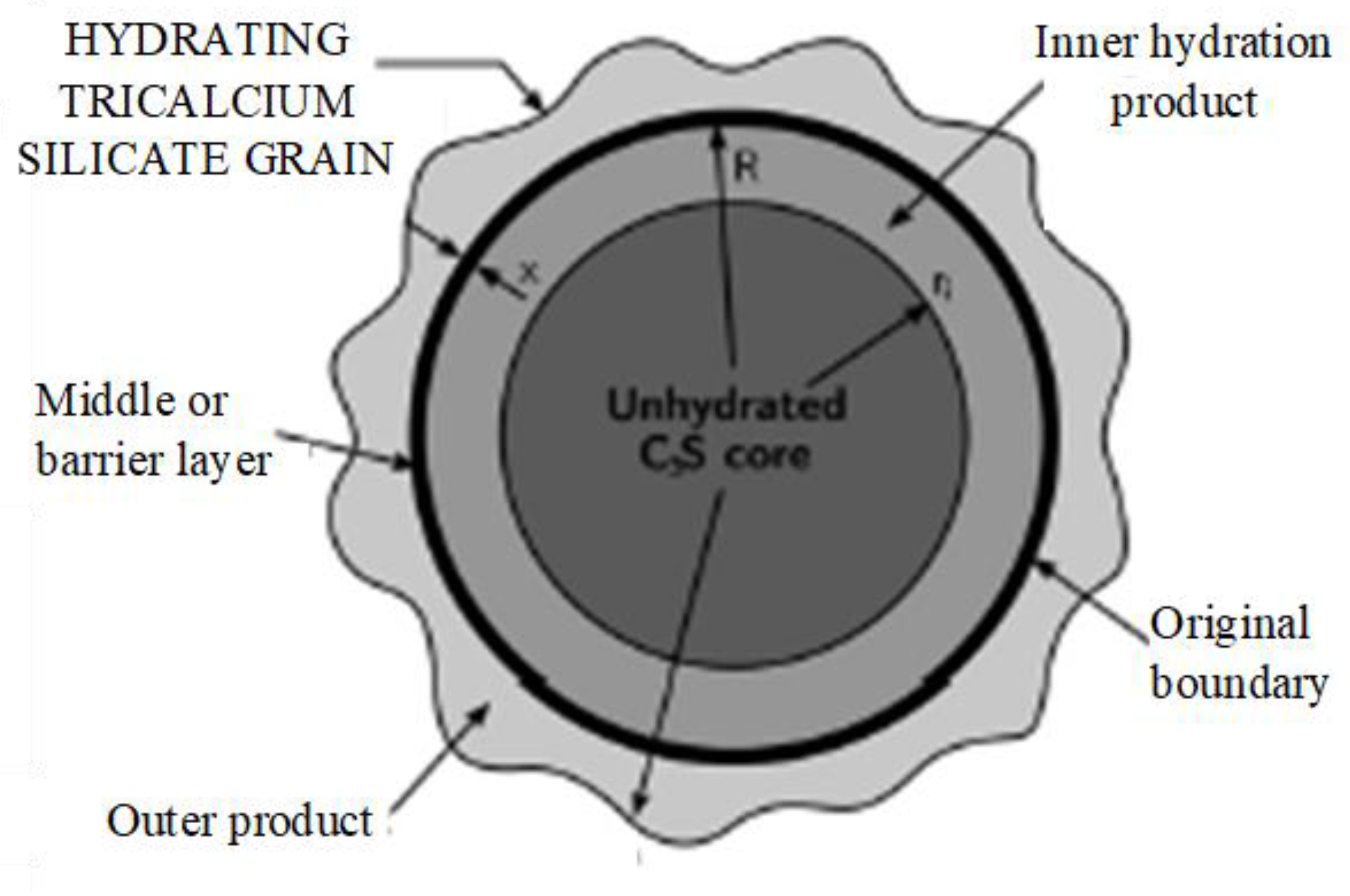
:1. Introduction
2. Materials and Methodology
2.1. Materials
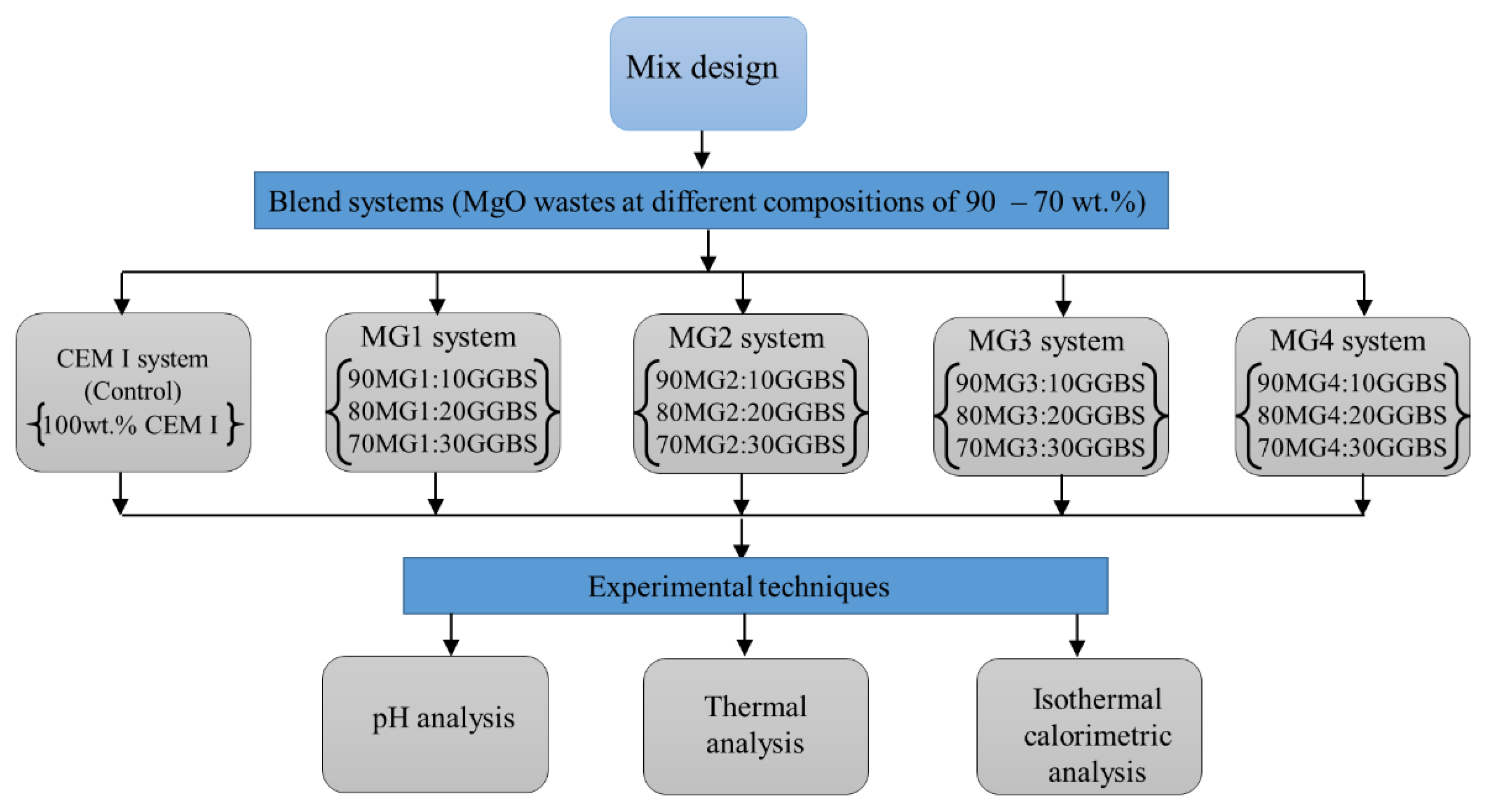
2.2. Mix Design and Sample Preparation
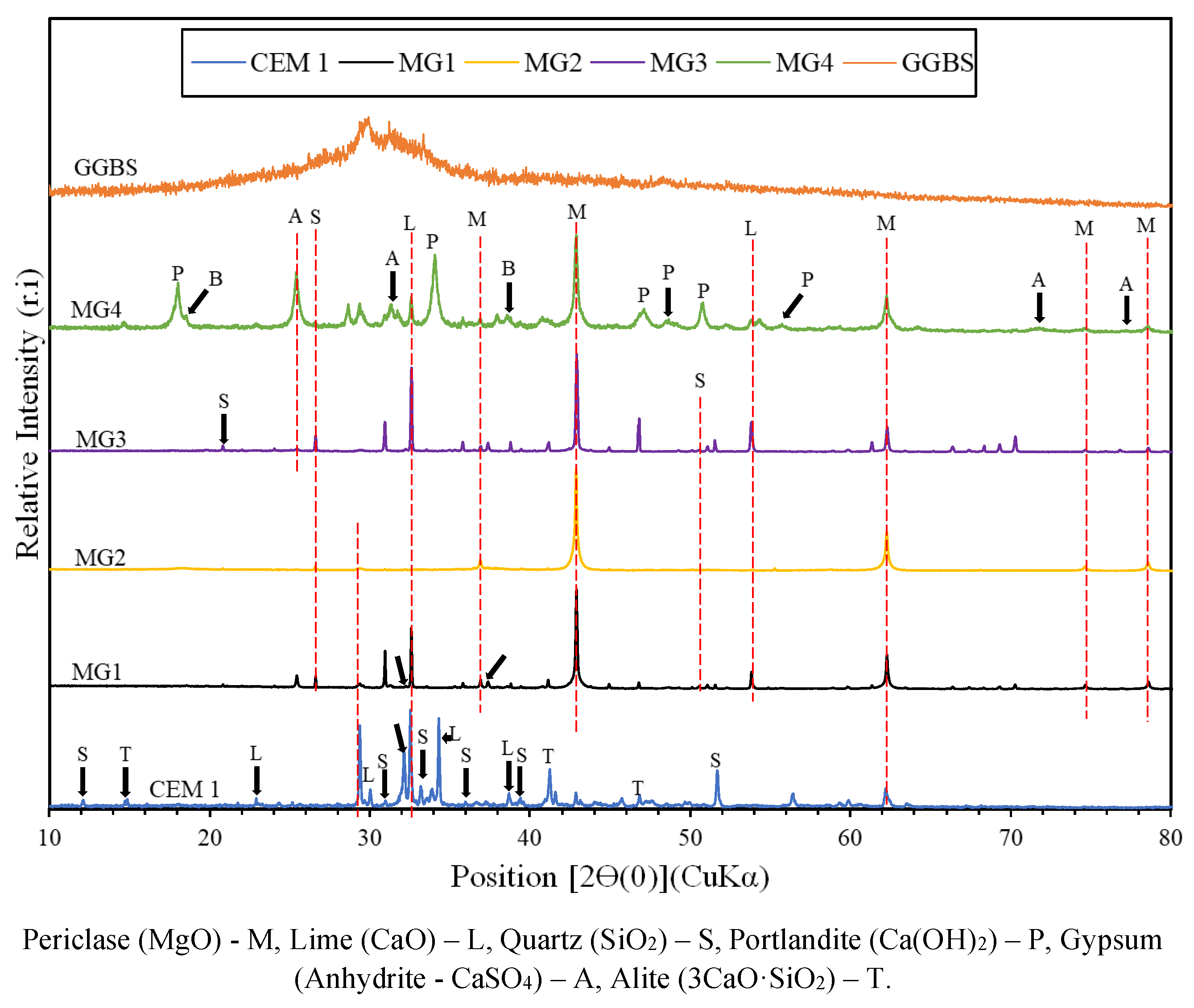
2.3. Experimental Techniques
3. Results
3.1. pH Analysis
3.2. Thermal Analysis
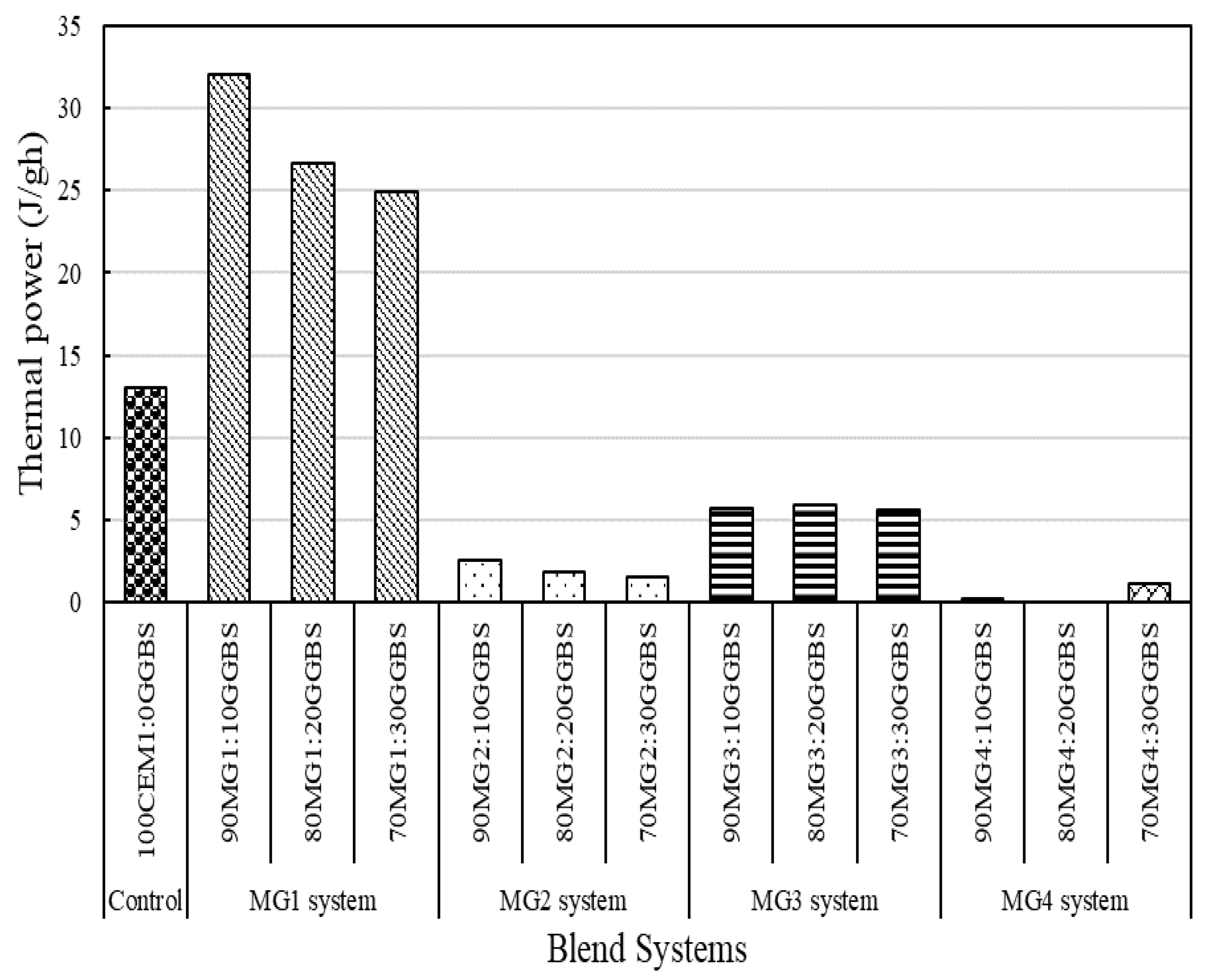
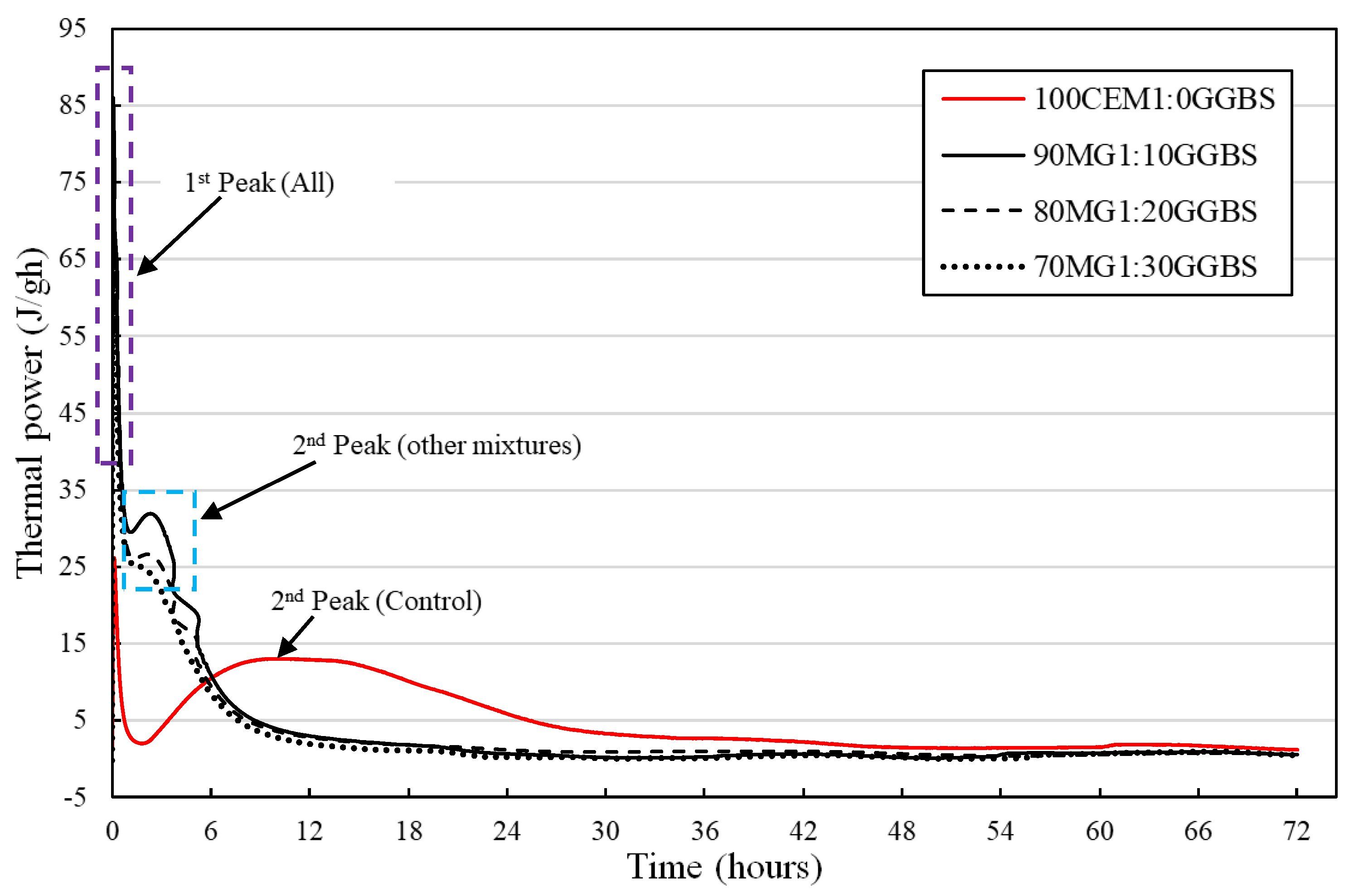
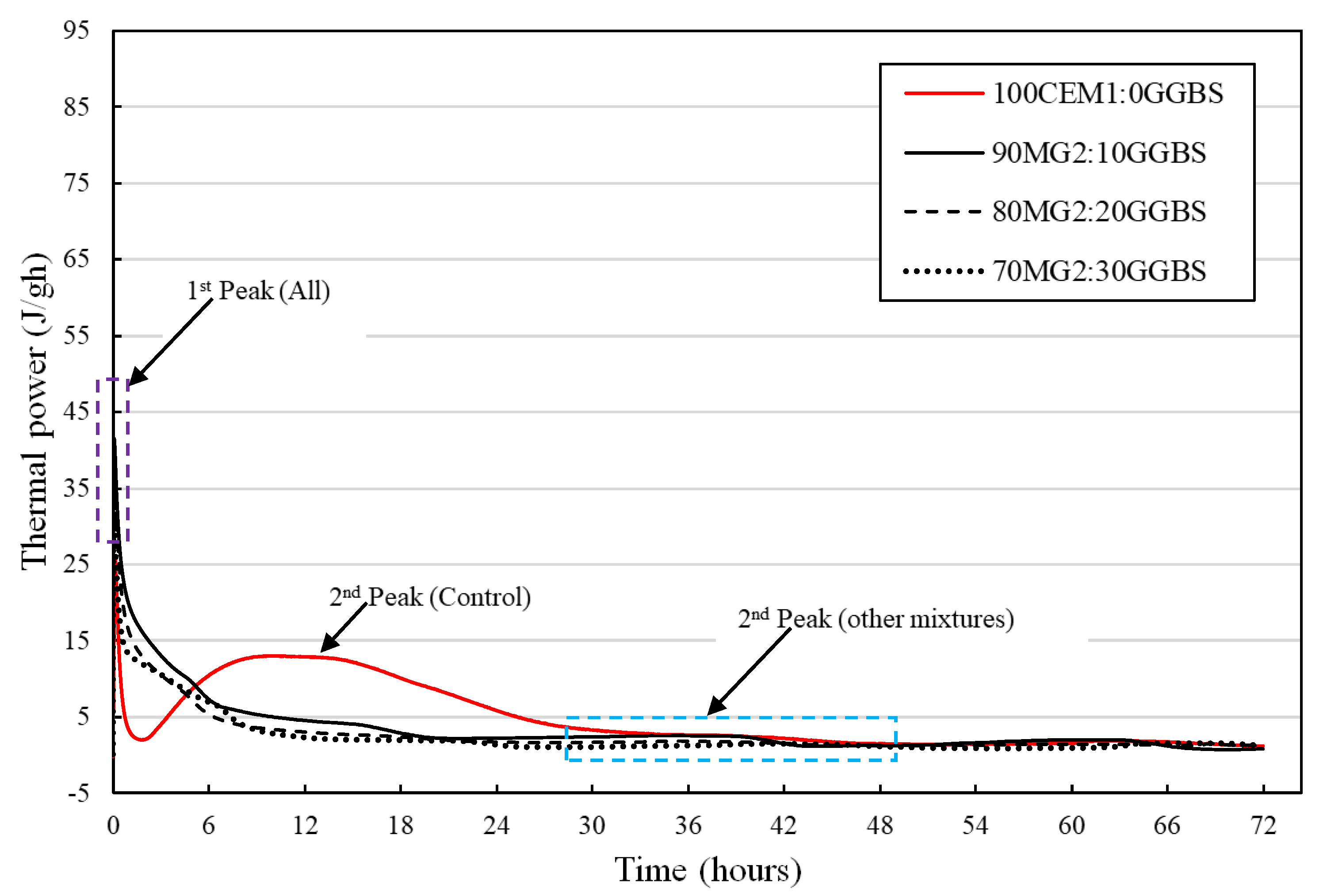
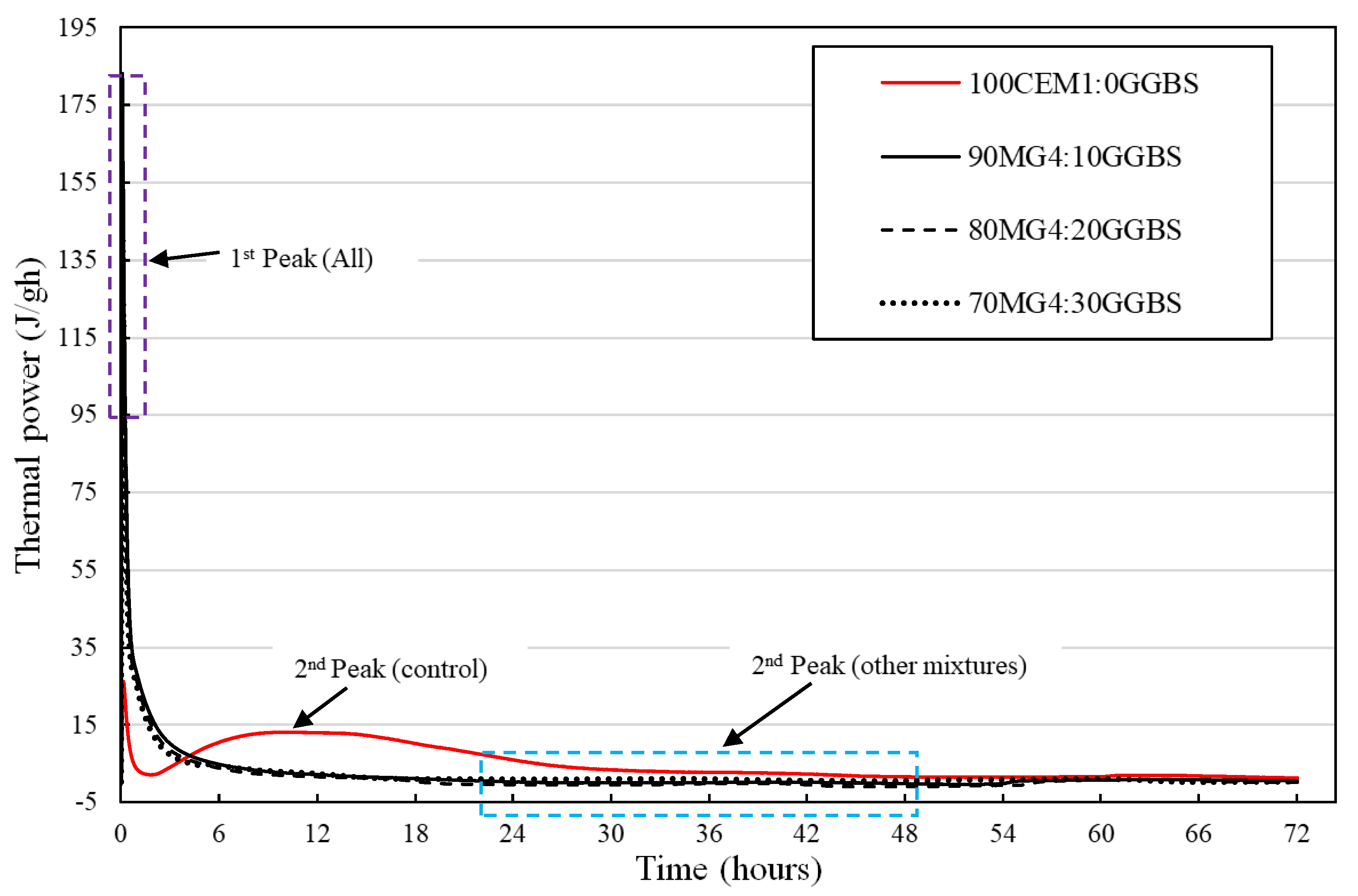
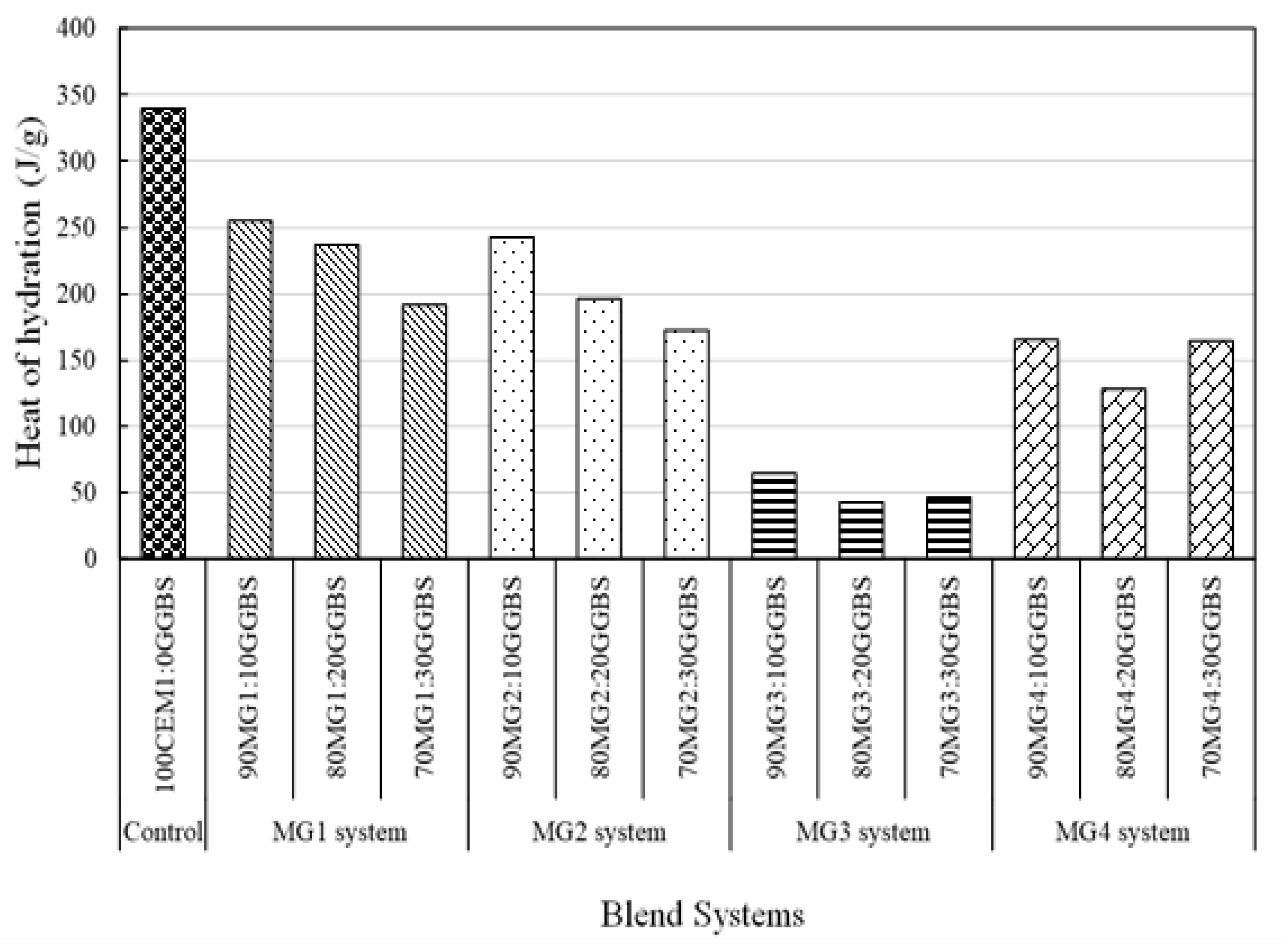
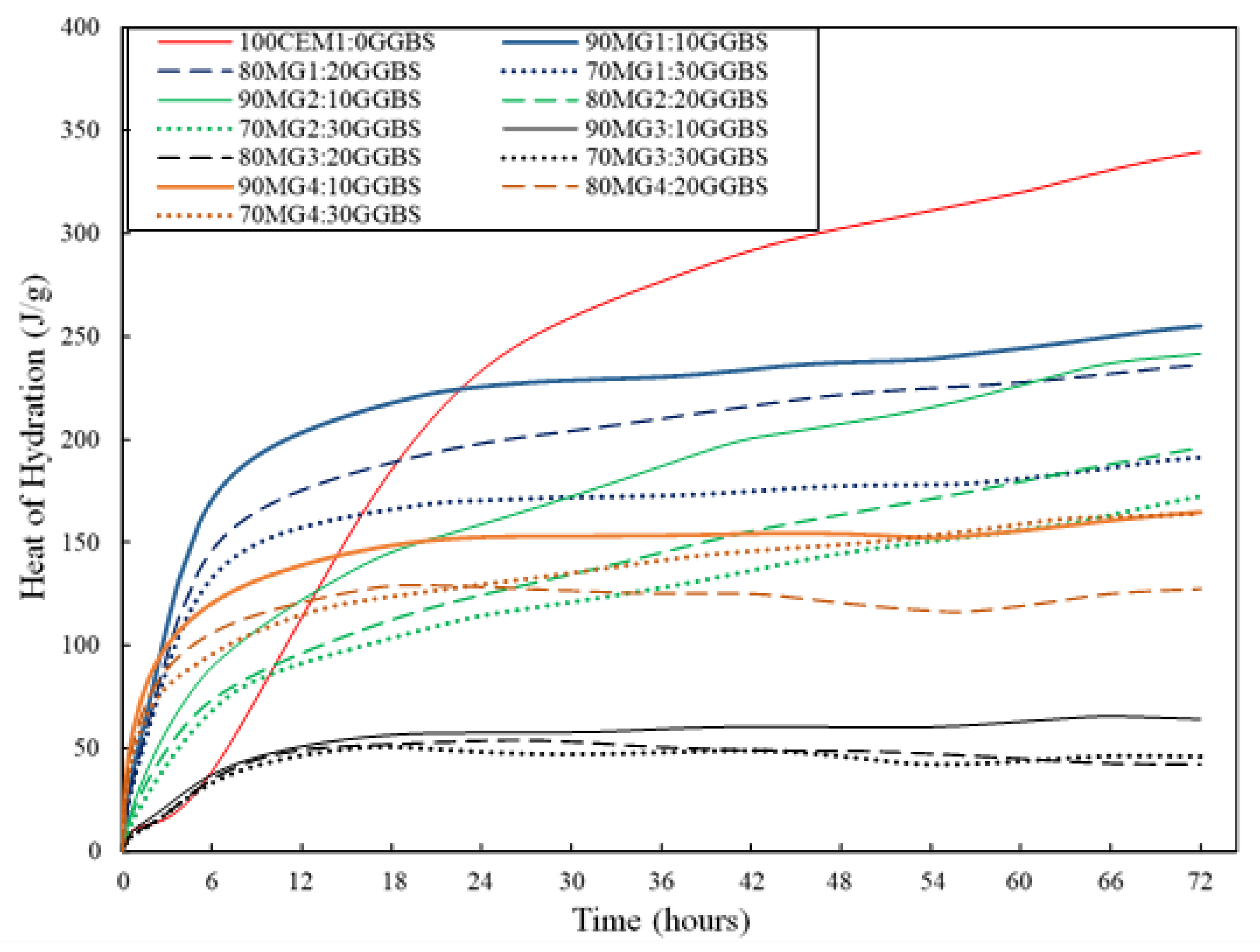
3.3. Isothermal Calorimetric Analysis
4. Discussion
4.1. pH Investigation
4.2. Thermal Investigation
4.3. Isothermal Calorimetric Investigation
5. Conclusions
- 1
- The thermochemical optimisation approach revealed the potentials of MgO waste materials to effectively replace PC up to 90 wt.% within a MgO-GGBS cementitious system and can describe the chemical and thermal performance of the investigated MgO:GGBS compositions with a set design criteria well.
- 2
- MG1 binder systems were established as the best-performing binder system based on the superior performance of its mix compositions with respect to the set criteria (low pH, increased levels of heat of hydration, and considerable thermal stability (weight loss) after thermal exposure).
- 3
- The thermal investigation (TG/DTG analysis) established that blend compositions within the MG1 binder system developed the necessary cementitious endothermic peaks (loose water, gypsum, brucite, magnesite, and calcite) and demonstrated satisfactory thermal stability that will be beneficial when employed for practical applications (pavement subgrade) in regions with high temperature.
- 4
- The reduction in the evolution of the first exothermic peak immediately after the commencement of the initial hydration reaction of the various MgO:GGBS formulations except MG4 blend systems shows their potential for the reduction of the production of initial ettringite formation, cracking, false strength, and flash setting of the hydrated blend system attributed to the first exothermic peak for in situ applications (mortar, concrete, soil stabilization, etc.).
- 5
- The MgO:GGBS blends resulted in reduced pH levels compared with the control due to the reduced individual pH levels of the blend components (MgO and GGBS). However, MG4 with high sulphate contents and some levels of Ca could not be used in stabilising soils with sulphate contents due to the potential of forming the hydration compound (ettringite), which upon further hydration could result in swelling of the stabilised product.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, Y.; Gu, L.; Liu, S. Microstructural and mechanical properties of marine soft clay stabilized by lime-activated ground granulated blastfurnace slag. Appl. Clay Sci. 2015, 103, 71–76. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S.; Jin, F. Magnesia reactivity on activating efficacy for ground granulated blastfurnace slag for soft clay stabilisation. Appl. Clay Sci. 2016, 126, 57–62. [Google Scholar] [CrossRef]
- Seco, A.; Miqueleiz, L.; Prieto, E.; Marcelino-Sádaba, S.; García, B.; Urmeneta, P. Sulfate soils stabilization with magnesium-based binders. Appl. Clay Sci. 2017, 135, 457–464. [Google Scholar] [CrossRef]
- Li, W.; Ni, P.; Yi, Y. Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci. Total. Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yi, Y.; Puppala, A.J. Suppressing Ettringite-Induced Swelling of Gypseous Soil by Using Magnesia-Activated Ground Granulated Blast-Furnace Slag. J. Geotech. Geoenviron. Eng. 2020, 146, 6020008. [Google Scholar] [CrossRef]
- Qomi, M.J.A.; Krakowiak, K.; Bauchy, M.; Stewart, K.; Shahsavari, R.; Jagannathan, D.; Brommer, D.; Baronnet, A.; Buehler, M.; Yip, S.; et al. Combinatorial molecular optimization of cement hydrates. Nat. Commun. 2014, 5, 4960. [Google Scholar] [CrossRef] [PubMed]
- Sonat, C.; Unluer, C. Investigation of the performance and thermal decomposition of MgO and MgO-SiO2 formulations. Thermochim. Acta 2017, 655, 251–261. [Google Scholar] [CrossRef]
- Liua, H.; Dua, T.; Krishnan, N.M.A.; Li, H.; Bauchy, M. Study on the optimization of some cement based mixing binders’ characteristics. Cem. Concr. Compos. 2019, 101, 5–14. [Google Scholar]
- Roy, R.; Hinduja, S.; Teti, R. Recent advances in engineering design optimisation: Challenges and future trends. CIRP Ann. 2008, 57, 697–715. [Google Scholar] [CrossRef]
- Peyronnard, O.; Benzaazoua, M. Alternative by-product based binders for cemented mine backfill: Recipes optimisation using Taguchi method. Miner. Eng. 2012, 29, 28–38. [Google Scholar] [CrossRef]
- Golchin, B.; Hamzah, M.O.; Hasan, M.R.M. Optimization in producing warm mix asphalt with polymer modified binder and surfactant-wax additive. Constr. Build. Mater. 2017, 141, 578–588. [Google Scholar] [CrossRef]
- Bayiha, B.N. Optimizing the Choice of Limestone Deposits for the Production of Portland Cement in Cameroon. Int. J. Mater. Sci. Appl. 2018, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Optimization of brick waste-based geopolymer binders at ambient temperature and pre-targeted chemical parameters. J. Clean. Prod. 2020, 268, 122285. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Development of optimized binary ceramic tile and concrete wastes geopolymer binders for in-situ applications. J. Build. Eng. 2021, 43, 102906. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Jin, F.; Shen, Z.; Al-Tabbaa, A. Three-year performance of in-situ mass stabilised contaminated site soils using MgO-bearing binders. J. Hazard. Mater. 2016, 318, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of reactive magnesia- and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Hu, J.; Ge, Z.; Wang, K. Influence of cement fineness and water-to-cement ratio on mortar early-age heat of hydration and set times. Constr. Build. Mater. 2014, 50, 657–663. [Google Scholar] [CrossRef]
- Sedaghat, A.; Zayed, A.; Sandberg, P. Measurement and Prediction of Heat of Hydration of Portland Cement Using Isothermal Conduction Calorimetry. J. Test. Eval. 2013, 41. [Google Scholar] [CrossRef] [Green Version]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; Taylor & Francis Group: Danvers, MA, USA, 2016. [Google Scholar]
- Mostafa, N.; Brown, P. Heat of hydration of high reactive pozzolans in blended cements: Isothermal conduction calorimetry. Thermochim. Acta 2005, 435, 162–167. [Google Scholar] [CrossRef]
- Schöler, A.; Lothenbach, B.; Winnefeld, F.; Ben Haha, M.; Zajac, M.; Ludwig, H.-M. Early hydration of SCM-blended Portland cements: A pore solution and isothermal calorimetry study. Cem. Concr. Res. 2017, 93, 71–82. [Google Scholar] [CrossRef]
- BS EN 197-1:2011; Cement, Part 1: Composition, Specifications and Conformity Criteria for Common Cements; British Standards Institution: London, UK, 2011.
- BS EN 15167-1:2006; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout. Part 1: Definitions, Specifications and Conformity Criteria; British Standards Institution: London, UK, 2006.
- BS EN ISO 17892-4:2016; Geotechnical Investigation and Testing—Laboratory Testing of Soil. Part 4: Determination of Particle Size Distribution (ISO 17892-4:2016); British Standards Institution: London, UK, 2016.
- Shand, M.A. The Chemistry and Technology of Magnesia; John Wiley & Sons, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Seco, A.; Ramírez, F.; Miqueleiz, L.; García, B. Stabilization of expansive soils for use in construction. Appl. Clay Sci. 2011, 51, 348–352. [Google Scholar] [CrossRef]
- Espuelas, S.; Omer, J.; Marcelino-Sádaba, S.; Echeverría, A.M.; Seco, A. Magnesium oxide as alternative binder for unfired clay bricks manufacturing. Appl. Clay Sci. 2017, 146, 23–26. [Google Scholar] [CrossRef] [Green Version]
- BS EN ISO 787-9-2019; General Methods of Test for Pigments and Extenders. Part 9: Determination of pH Value of an Aqueous Suspension; British Standards Institution: London, UK, 2019.
- ASTM E2550-17; Standard test method for thermal stability by thermogravimetry. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2017.
- Snelson, D.G.; Kinuthia, J.M. Resistance of mortar containing unprocessed pulverised fuel ash (PFA) to sulphate attack. Cem. Concr. Compos. 2010, 32, 523–531. [Google Scholar] [CrossRef]
- BS EN 196-7:2007; Methods of Testing Cement. Part 7: Methods of Taking and Preparing Samples of Cement; British Standards Institution: London, UK, 2007.
- BS EN 196-11:2018; Methods of Testing Cement. Part 11: Heat of Hydration—Isothermal Conduction Calorimetry Method; British Standards Institution: London, UK, 2018.
- Fernandez, R.; Martirena, F.; Scrivener, K. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzères, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
- Abdalqader, A.; Jin, F.; Al-Tabbaa, A. Characterisation of reactive magnesia and sodium carbonate-activated fly ash/slag paste blends. Constr. Build. Mater. 2015, 93, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Borrachero, M.V.; Paya, J.; Bonilla, M.; Monzó, J. The use of thermogravimetric analysis technique for the characterization of construction materials. J. Therm. Anal. Calorim. 2008, 91, 503–509. [Google Scholar] [CrossRef]
- Collier, N. Transition and Decomposition Temperatures of Cement Phases—A Collection of Thermal Analysis Dat. Ceram. Silik. 2016, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Klein, C.; Hurlbut, J.C. Manual of Minerology; John Wiley and Sons: New York, NY, USA, 1985; Volume 20. [Google Scholar]
- Engbrecht, D.; Hirschfeld, D. Thermal analysis of calcium sulfate dihydrate sources used to manufacture gypsum wallboard. Thermochim. Acta 2016, 639, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Al-Tabbaa, A. Thermogravimetric study on the hydration of reactive magnesia and silica mixture at room temperature. Thermochim. Acta 2013, 566, 162–168. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Oliveira, A.D.S. Chemical phases and microstructural analysis of pastes based on magnesia cement. Constr. Build. Mater. 2018, 188, 615–620. [Google Scholar] [CrossRef]
- Song, H.; Jeong, Y.; Bae, S.; Jun, Y.; Yoon, S.; Oh, J.E. A study of thermal decomposition of phases in cementitious systems using HT-XRD and TG. Constr. Build. Mater. 2018, 169, 648–661. [Google Scholar] [CrossRef]
- Lanas, J.; Alvarez, J. Dolomitic lime: Thermal decomposition of nesquehonite. Thermochim. Acta 2004, 421, 123–132. [Google Scholar] [CrossRef]
- Darko, K.; Branislav, Z. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar]
- Amaral, L.; Oliveira, I.; Salomão, R.; Frollini, E.; Pandolfelli, V. Temperature and common-ion effect on magnesium oxide (MgO) hydration. Ceram. Int. 2010, 36, 1047–1054. [Google Scholar] [CrossRef]
- Kuenzel, C.; Zhang, F.; Ferrándiz-Mas, V.; Cheeseman, C.; Gartner, E. The mechanism of hydration of MgO-hydromagnesite blends. Cem. Concr. Res. 2018, 103, 123–129. [Google Scholar] [CrossRef]
- He, Y.; Lai, Z.; Yan, T.; He, X.; Lu, Z.; Lv, S.; Li, F.; Fan, X.; Zhang, H. Effect of Cd2+ on early hydration process of magnesium phosphate cement and its leaching toxicity properties. Constr. Build. Mater. 2019, 209, 32–40. [Google Scholar] [CrossRef]
- Snelson, D.G.; Wild, S.; O’Farrell, M. Heat of hydration of Portland Cement–Metakaolin–Fly ash (PC–MK–PFA) blends. Cem. Concr. Res. 2008, 38, 832–840. [Google Scholar] [CrossRef]
- Tydlitát, V.; Zákoutský, J.; Schmieder, M.; Černý, R. Application of large-volume calorimetry for monitoring the early-stage hydration heat development in cement-based composites as a function of w/c. Thermochim. Acta 2012, 546, 44–48. [Google Scholar] [CrossRef]
- Moghaddam, F.; Sirivivatnanon, V.; Vessalas, K. The effect of fly ash fineness on heat of hydration, microstructure, flow and compressive strength of blended cement pastes. Case Stud. Constr. Mater. 2019, 10, e00218. [Google Scholar] [CrossRef]
- Bensted, J. Seventh International Congress on the Chemistry of Cement; Editions Septima: Paris, France, 1980; Volume 2, Part II; pp. 1–6. [Google Scholar]
- Taylor, F.W.; Barret, P.; Brown, P.W.; Double, D.D.; Frohnsdorff, G.; Johansen, V.T. Materiaux et Constructions. Mater. Struct. 1984, 17, 457. [Google Scholar] [CrossRef]
- Thomas, J.J.; Biernacki, J.J.; Bullard, J.W.; Bishnoi, S.; Dolado, J.S.; Scherer, G.; Luttge, A. Modeling and simulation of cement hydration kinetics and microstructure development. Cem. Concr. Res. 2011, 41, 1257–1278. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Mineral admixtures in mortars and Quantification of the physical effects of inert materials on short-term hydration. Cem. Concr. Res. 2005, 35, 719–730. [Google Scholar]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]










| Oxide | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| CEM I | MG1 | MG2 | MG3 | MG4 | GGBS | |
| CaO | 61.49 | 9.39 | 3.28 | 6.02 | 32.41 | 37.99 |
| SiO2 | 18.84 | 2.51 | 3.33 | 4.90 | 1.20 | 35.54 |
| Al2O3 | 4.77 | 0.52 | 0.83 | 1.09 | 0.52 | 11.46 |
| MgO | 3.54 | 56.26 | 78.44 | 45.61 | 24.79 | 8.78 |
| Fe2O3 | 2.87 | 2.13 | 2.82 | 2.43 | 1.05 | 0.42 |
| Mn2O3 | 0.05 | 0.15 | 0.27 | 0.16 | 0.08 | 0.43 |
| SO3 | 3.12 | 6.22 | 0.30 | 1.85 | 17.83 | 1.54 |
| TiO2 | 0.26 | 0.01 | <0.01 | 0.04 | <0.01 | 0.70 |
| K2O | 0.57 | 0.18 | 0.10 | 0.10 | 0.23 | 0.43 |
| Na2O | 0.02 | 0.09 | 0.03 | 0.06 | 0.13 | 0.37 |
| P2O5 | 0.10 | 0.06 | 0.06 | 0.06 | 0.05 | 0.02 |
| V2O5 | 0.06 | 0.10 | <0.01 | 0.10 | 0.07 | 0.04 |
| BaO | 0.05 | 0.01 | <0.01 | 0.01 | <0.01 | 0.09 |
| L.O.I | 4.30 | 22.30 | 10.70 | 37.40 | 21.80 | 2.00 |
| Properties | Binders | |||||
|---|---|---|---|---|---|---|
| CEM 1 | MG1 | MG2 | MG3 | MG4 | GGBS | |
| Particle diameter | ||||||
| Median d50 (µm) | 18.86 | 16.24 | 18.72 | 62.74 | 6.52 | 13.69 |
| Physical properties | ||||||
| Colour | Grey | Light-Brown | Very pale brown | Greyish brown | Pale brown | Off-white |
| Bulk density (kg/m3) | - | - | - | - | - | 1200 |
| Particle density/Specific gravity (Mg/m3) | 3.16 | 2.86 | 2.91 | 2.88 | 2.65 | 2.90 |
| Others | ||||||
| pH value | 12.86 | 10.79 | 12.04 | 11.09 | 12.95 | 10.20 |
| Reactivity (m) | - | 30 | 1.8 | >1440 | >1440 | - |
| Mix System | Blend Composition | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|---|
| CEM 1 | MG1 | MG2 | MG3 | MG4 | GGBS | ||
| CEM I system (Control) | 100CEM 1:0GGBS | 100 | - | - | - | - | - |
| MG1 System | 90MG1:10GGBS | - | 90 | - | - | - | 10 |
| 80MG1:20GGBS | - | 80 | - | - | - | 20 | |
| 70MG1:30GGBS | - | 70 | - | - | - | 30 | |
| MG2 system | 90MG2:10GGBS | - | - | 90 | - | - | 10 |
| 80MG2:20GGBS | - | - | 80 | - | - | 20 | |
| 70MG2:30GGBS | - | - | 70 | - | - | 30 | |
| MG3 system | 90MG3:10GGBS | - | - | - | 90 | - | 10 |
| 80MG3:20GGBS | - | - | - | 80 | - | 20 | |
| 70MG3:30GGBS | - | - | - | 70 | - | 30 | |
| MG4 system | 90MG4:10GGBS | - | - | - | - | 90 | 10 |
| 80MG4:20GGBS | - | - | - | - | 80 | 20 | |
| 70MG4:30GGBS | - | - | - | - | 70 | 30 | |
| Blend Systems | Hydrated Cementitious Phases | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Loose Water (H2O) | Gypsum (CaSO4.2H2O-CaSO4) | Brucite (Mg(OH)2) | Magnesite (MgCO3) | Calcium Carbonate (CaCO3) | ||||||
| Peak Location | Temp. (°C) | Peak Location | Temp. (°C) | Peak Location | Temp. (°C) | Peak Location | Temp. (°C) | Peak Location | Temp. (°C) | |
| MG1 | 1 | 105 | - | - | 2 | 371 | 3 | 537 | 4 | 737 |
| MG2 | 1 | 103 | - | - | 2 | 360 | - | - | 3 | 709 |
| MG3 | 1 | 101 | - | - | 2 | 401 | 3 | 658 | 4 | 782 |
| MG4 | - | - | 1,2 | 105–371 | 3 | 446 | - | - | 4 | 703 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleke, B.; Kinuthia, J.; Oti, J. Optimization of MgO-GGBS Cementitious Systems Using Thermo-Chemical Approaches. Sustainability 2021, 13, 9378. https://doi.org/10.3390/su13169378
Adeleke B, Kinuthia J, Oti J. Optimization of MgO-GGBS Cementitious Systems Using Thermo-Chemical Approaches. Sustainability. 2021; 13(16):9378. https://doi.org/10.3390/su13169378
Chicago/Turabian StyleAdeleke, Blessing, John Kinuthia, and Jonathan Oti. 2021. "Optimization of MgO-GGBS Cementitious Systems Using Thermo-Chemical Approaches" Sustainability 13, no. 16: 9378. https://doi.org/10.3390/su13169378
APA StyleAdeleke, B., Kinuthia, J., & Oti, J. (2021). Optimization of MgO-GGBS Cementitious Systems Using Thermo-Chemical Approaches. Sustainability, 13(16), 9378. https://doi.org/10.3390/su13169378








