Effect of Intensification Practices, Lambing Period and Environmental Parameters on Animal Health, and Milk Yield and Quality in Dairy Sheep Production Systems on Crete
Abstract
:1. Introduction
2. Materials and Methods
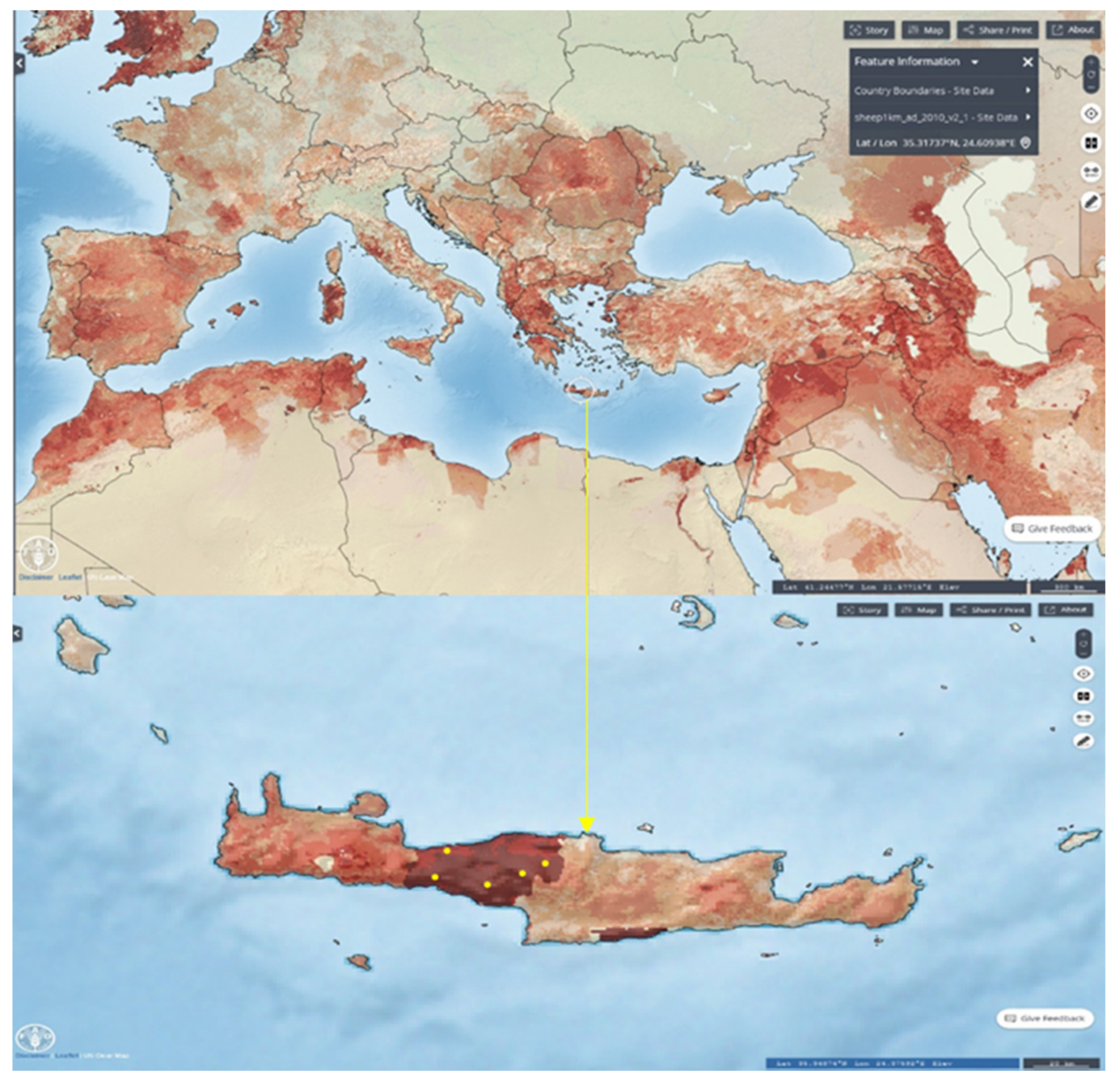
2.1. Study Area, Animals and Systems
2.2. Experimental Design
2.3. Climatic Conditions in the Two Production Season
2.4. Feeding Regimes Used in Extensive and Semi-Intensive Flocks
2.5. Health Management Regimes Used in Semi-Intensive and Extensive Flocks
2.6. Analytical Methods
2.6.1. Milk Samples Analysis of Chemical Composition, Somatic Cell Count and Bacterial Load
2.6.2. Parasitological Examination
2.6.3. Mastitis Related Pathogens Detection
2.6.4. Milk Fatty Acid Analysis
2.7. Statistical Analyses
- Analysis of variance (ANOVA) using linear mixed-effects models, with “management system”, “sampling month”, and “year” as fixed factor sand “flock” as a random factor, for investigating differences on farm level;
- Analysis of variance (ANOVA) using linear mixed-effects models with “management system”, “lambing period” and “production year” as fixed factors and “flock” as a random factor, for investigating differences on farm level for the prevalence of the recorded diseases (as indicated by the farmers and the veterinarian of the flock);
- Analysis of variance (ANOVA) using linear mixed-effects models with “management system”, “lambing period” and “sampling month” as fixed factors and “animal” as a random factor, for investigating differences on animal level. The analysis was conducted separately for the two different sampling years.
3. Results and Discussion
3.1. Effect of Production System, Lambing Period and Season on Animal Health Parameters
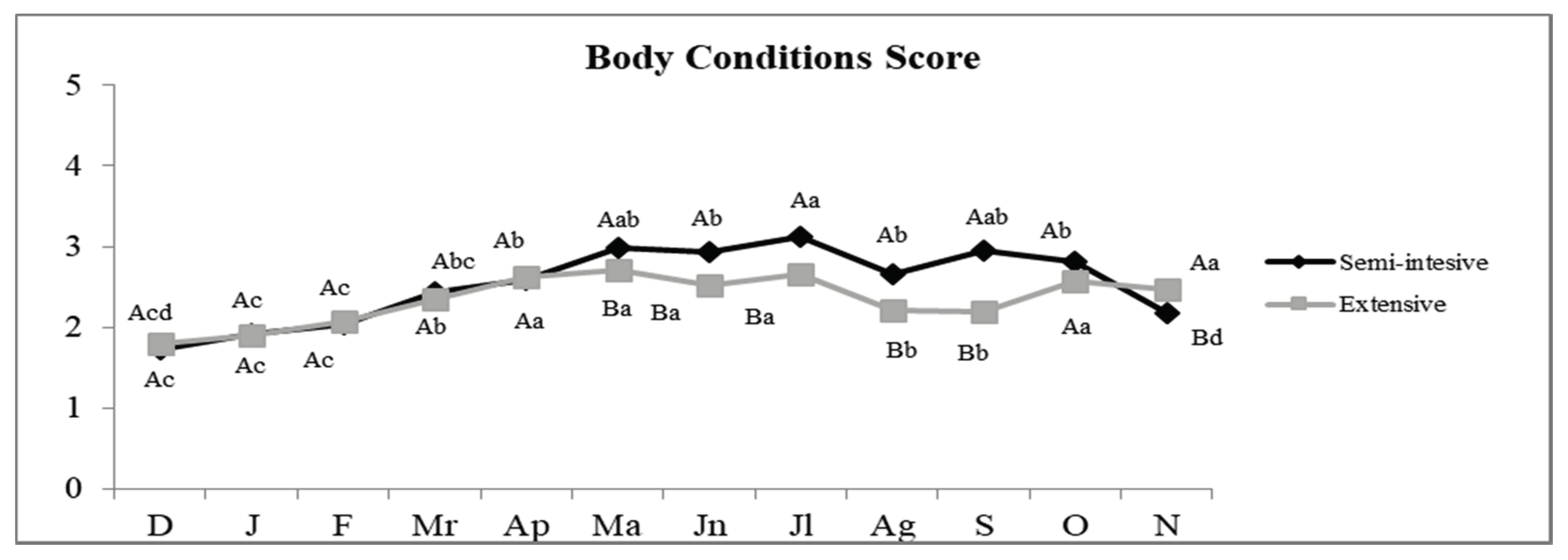
3.1.1. Body Condition Score (BCS)
3.1.2. Faecal Egg Counts (FEC)
3.1.3. Somatic Cell Count (SCC) in Milk
3.1.4. Microbial Load (Colony Forming Units) in Milk
3.1.5. Estimated Incidence Subclinical Mastitis
3.1.6. Incidence of Diseases and Health Related Management Parameters
3.2. Associations between Environmental/Agronomic Factors and Animal Health
3.3. Effect of Production System and Season on Milk Yield and Quality
3.3.1. Milk Yield and Basic Composition, Fat, Protein and Lactose Content
3.3.2. Milk Fatty Acid Profile
3.3.3. Associations between Environmental/Agronomic Factors, and Milk Yield/Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT Database; Rome, I.F. Production/Livestock Primary/Livestock Processed. In Food and Agriculture Organization of the United Nations (FAO); FAO: Rome, Italy, 2021. [Google Scholar]
- Pulina, G.; Milán, M.J.; Lavín, M.P.; Theodoridis, A.; Morin, E.; Capote, J.; Thomas, D.L.; Francesconi, A.H.D.; Caja, G. Current production trends, farm structures, and economics of the dairy sheep and goat sectors. J. Dairy Sci. 2018, 101, 6715–6729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zervas, G.; Tsiplakou, E. The effect of feeding systems on the characteristics of products from small ruminants. Small Rumin. Res. 2011, 101, 140–149. [Google Scholar] [CrossRef]
- OECD. Dairy and Dairy Products; OECD Publishing: Paris, France, 2016. [Google Scholar]
- de Rancourt, M.; Fois, N.; Lavín, M.P.; Tchakérian, E.; Vallerand, F. Mediterranean sheep and goats production: An uncertain future. Small Rumin. Res. 2006, 62, 167–179. [Google Scholar] [CrossRef]
- Hadjigeorgiou, I. Past, present and future of pastoralism in Greece. Pastor. Res. Policy Pract. 2011, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Hoste, H.; Torres-Acosta, J.F.J. Non chemical control of helminths in ruminants: Adapting solutions for changing worms in a changing world. Vet. Parasitol. 2011, 180, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Sierra, D.; Sánchez, A.; Corrales, J.C.; Marco, J.C.; Paape, M.J.; Gonzalo, C. Mastitis in small ruminants. Small Rumin. Res. 2007, 68, 145–153. [Google Scholar] [CrossRef]
- Hadjigeorgiou, I.; Zervas, G. Evaluation of production systems in protected areas: Case studies on the Greek “Natura 2000” network. Options Méditerr. 2009, 91, 101–109. [Google Scholar]
- Casasús, I.; Rogosić, J.; Rosati, A.; Stoković, I.; Gabiña, D. Animal Farming and Environmental Interactions in the Mediterranean Region; EAAP Scientific Series: Ioannina, Greece, 2012; Volume 131. [Google Scholar]
- Volanis, M.; Tzerakis, K. Study of the Sfakia sheep breed. II. Reproductive characteristics of the ewes and growth characteristics of the lambs. Anim. Sci. Rev. 1997, 24, 5–20. [Google Scholar]
- Kairis, O.; Karavitis, C.; Salvati, L.; Kounalaki, A.; Kosmas, K. Exploring the Impact of Overgrazing on Soil Erosion and Land Degradation in a Dry Mediterranean Agro-Forest Landscape (Crete, Greece). Arid Land Res. Manag. 2015, 29, 360–374. [Google Scholar] [CrossRef]
- Stergiadis, S.; Leifert, C.; Seal, C.J.; Eyre, M.D.; Nielsen, J.H.; Larsen, M.K.; Slots, T.; Steinshamn, H.; Butler, G. Effect of feeding intensity and milking system on nutritionally relevant milk components in dairy farming systems in the North East of England. J. Agric. Food Chem. 2012, 60, 7270–7281. [Google Scholar] [CrossRef]
- Stafford, K.; Gregory, N. Implications of intensification of pastoral animal production on animal welfare. N. Z. Vet. J. 2008, 56, 274–280. [Google Scholar] [CrossRef]
- Lean, I.; Westwood, C.; Playford, M. Livestock disease threats associated with intensification of pastoral dairy farming. N. Z. Vet. J. 2008, 56, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Voutzourakis, N.; Tzanidakis, N.; Stergiadis, S.; Rempelos, L.; Eyre, M.; Atsali, I.; Franceschin, E.; Leifert, C.; Stefanakis, A.; Sotiraki, S.; et al. Sustainable Intensification? Increased Production Diminishes Omega-3 Content of Sheep Milk. Sustainability 2020, 12, 1228. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.; Liponi, G.B.; Salari, F. Effect of forage:concentrate ratio on the quality of ewe’s milk, especially on milk fat globules characteristics and fatty acids composition. J. Dairy Res. 2010, 77, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Sevi, A.; Taibi, L.; Albenzio, M.; Muscio, A.; Annicchiarico, G. Effect of parity on milk yield, composition, somatic cell count, renneting parameters and bacteria counts of Comisana ewes. Small Rumin. Res. 2000, 37, 99–107. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; Sotirakoglou, K.; Bosnea, L.; Mataragas, M.; Allouche, L.; Tsiplakou, E.; Pappas, A.C. Farmers Profile and Characterization of Sheep and Goat Dairy Chain in Northwestern Greece. Sustainability 2021, 13, 833. [Google Scholar] [CrossRef]
- European Food Safety Authority. Food safety aspects of dairy cow housing and husbandry systems. EFSA J. 2009, 1189, 1–27. [Google Scholar]
- van Asselt, E.D.; van der Fels-Klerx, H.J.; Marvin, H.J.P.; van Bokhorst-van de Veen, H.; Groot, M.N. Overview of Food Safety Hazards in the European Dairy Supply Chain. Compr. Rev. Food Sci. Food Saf. 2017, 16, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Defra. Code of Recommendations for the Welfare of Livestock: Sheep; Affairs, D.f.E.F.R., Ed.; Defra Publications: London, UK, 2013; pp. 1–27. [Google Scholar]
- Discontools. Available online: http://www.discontools.eu/ (accessed on 22 April 2017).
- Stefanakis, A.; Volanis, M.; Zoiopoulos, P.; Hadjigeorgiou, I. Assessing the potential benefits of technical intervention in evolving the semi-intensive dairy-sheep farms in Crete. Small Rumin. Res. 2007, 72, 66–72. [Google Scholar] [CrossRef]
- Sitzia, M.; Ruiz, F. Dairy Farm Management Systems: Sheep. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- ELSTAT. Holdings and number of animals by kind, region and regional unit. In Livestock Capital and Production; Authority, H.S., Ed.; Hellenic Statistical Authority: Athens, Greece, 2016. [Google Scholar]
- Stefanakis, A.; Laboratory of Parasitology, Veterinary Research Institute, Hellenic Agricultural Organization Demeter, Thessaloniki, Greece. Breed Sheep Population on Crete. Geotechnical Chamber of Greece: Heraklion, Crete, Greece. Personal communication, 2016. [Google Scholar]
- ELSTAT. Milk production, by Region and Regional Unities, 2019. In Livestock Capital and Production; Authority, H.S., Ed.; Hellenic Statistical Authority: Piraeus, Athens, Greece, 2019. [Google Scholar]
- Volanis, M.; Stefanakis, A.; Hadjigeorgiou, I.; Zoiopoulos, P. Supporting the extensive dairy sheep smallholders of the semi-arid region of Crete through technical intervention. Trop. Anim. Health Prod. 2007, 39, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Volanis, M.; Kominakis, A.; Rogdakis, E. Genetic analysis of udder score and milk traits in test day records of Sfakia dairy ewes. Arch. Anim. Breed. 2002, 45, 69–77. [Google Scholar] [CrossRef]
- Vogiatzakis, I.N.; Pungetti, G.; Mannion, A.M. Mediterranean Island Landscapes, 1st ed.; Springer: Dordrecht, The Netherlands, 2008; p. 369. [Google Scholar]
- MAFF. Condition Scoring of Sheep. In Action on Animal Welfare, 2nd ed.; MAFF Publications: London, UK, 1996. [Google Scholar]
- Abecia, J.A.; Garcia, A.; Castillo, L.; Riocerezo, C. The effects of weather on milk production in dairy sheep vary by month of lambing & lactation phase. J. Anim. Behav. Biometeorol. 2017, 5, 56–63. [Google Scholar]
- Peana, I.; Francesconi, A.H.D.; Dimauro, C.; Cannas, A.; Sitzia, M. Effect of winter and spring meteorological conditions on milk production of grazing dairy sheep in the Mediterranean environment. Small Rumin. Res. 2017, 153, 194–208. [Google Scholar] [CrossRef]
- MAFF. Technical Bulletin No. 18, Manual of Veterinary Parasitological Laboratory Techniques; Her Majesty’s Stationery Office: London, UK, 1986. [Google Scholar]
- Hendrix, C.M.; Robinson, E.D. Diagnostic Parasitology for Veterinary Technicians, 4th ed.; Mosby: London, UK, 2014. [Google Scholar]
- Kiossis, E.; Brozos, C.N.; Petridou, E.; Boscos, C. Program for the control of subclinical mastitis in dairy Chios breed ewes during lactation. Small Rumin. Res. 2007, 73, 194–199. [Google Scholar] [CrossRef]
- Butler, G.; Stergiadis, S.; Seal, C.; Eyre, M.; Leifert, C. Fat composition of organic and conventional retail milk in northeast England. J. Dairy Sci. 2011, 94, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000; 528p. [Google Scholar]
- Crawley, M.J. The R Book; Wiley: Chichester, England; Hoboken, NJ, USA, 2007; p. viii. 942p. [Google Scholar]
- ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Biometris: Wageningen, The Netherlands, 2002. [Google Scholar]
- Torres-Acosta, J.F.J.; Hoste, H. Alternative or improved methods to limit gastro-intestinal parasitism in grazing sheep and goats. Small Rumin. Res. 2008, 77, 159–173. [Google Scholar] [CrossRef]
- Giadinis, N.D.; Arsenos, G.; Tsakos, P.; Psychas, V.; Dovas, C.I.; Papadopoulos, E.; Karatzias, H.; Fthenakis, G.C. “Milk-drop syndrome of ewes”: Investigation of the causes in dairy sheep in Greece. Small Rumin. Res. 2012, 106, 33–35. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.P.; Gonzalez-Valerio, T.C.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef] [Green Version]
- Bergonier, D.; de Cremoux, R.; Rupp, R.; Lagriffoul, G.; Berthelot, X. Mastitis of dairy small ruminants. Vet. Res. 2003, 34, 689–716. [Google Scholar] [CrossRef] [Green Version]
- Geurden, T.; Hoste, H.; Jacquiet, P.; Traversa, D.; Sotiraki, S.; Frangipane di Regalbono, A.; Tzanidakis, N.; Kostopoulou, D.; Gaillac, C.; Privat, S.; et al. Anthelmintic resistance and multidrug resistance in sheep gastro-intestinal nematodes in France, Greece and Italy. Vet. Parasitol. 2014, 201, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Rinaldi, L.; Veneziano, V.; Mezzino, L.; Vercruysse, J.; Jackson, F. Evaluation of targeted selective treatments in sheep in Italy: Effects on faecal worm egg count and milk production in four case studies. Vet. Parasitol. 2009, 164, 36–43. [Google Scholar] [CrossRef]
- Jackson, F.; Coop, R.L. Gastrointestinal Helminthosis. In Diseases of Sheep; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2008; pp. 185–195. [Google Scholar]
- Arsenopoulos, K.V.; Fthenakis, G.C.; Katsarou, E.I.; Papadopoulos, E. Haemonchosis: A Challenging Parasitic Infection of Sheep and Goats. Animals 2021, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Krehbiel, C.R. Invited Review: Applied nutrition of ruminants: Fermentation and digestive physiology1. Prof. Anim. Sci. 2014, 30, 129–139. [Google Scholar] [CrossRef]
- Bayne, J.; Edmondson, M. Diseases of the gastrointestinal system. Sheep Goat Cervid Med. 2021, 63–96. [Google Scholar] [CrossRef]
- Lewis, C.J. Control of Important Clostridial Diseases of Sheep. Vet. Clin. Food A 2011, 27, 121–126. [Google Scholar] [CrossRef]
- Westendarp, H. Effects of tannins in animal nutrition. DTW Dtsch. Tierarztl. Wochenschr. 2006, 113, 264–268. [Google Scholar]
- Plant, J.W.; Lewis, C.J. Treatment and Control of Ectoparasites in Sheep. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 203–212. [Google Scholar] [CrossRef]
- Raadsma, H.W.; Egerton, J.R. A review of footrot in sheep: Aetiology, risk factors and control methods. Livest. Sci. 2013, 156, 106–114. [Google Scholar] [CrossRef]
- Brozos, C.; Mavrogianni, V.S.; Fthenakis, G.C. Treatment and control of peri-parturient metabolic diseases: Pregnancy toxemia, hypocalcemia, hypomagnesemia. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 105–113. [Google Scholar] [CrossRef]
- Carson, A. Focus on ovine abortions. Vet. Rec. 2017, 180, 246–247. [Google Scholar]
- Nandi, S.; De, U.K.; Chowdhury, S. Current status of contagious ecthyma or orf disease in goat and sheep—A global perspective. Small Rumin. Res. 2011, 96, 73–82. [Google Scholar] [CrossRef]
- Sevi, A.; Albenzio, M.; Marino, R.; Santillo, A.; Muscio, A. Effects of lambing season and stage of lactation on ewe milk quality. Small Rumin. Res. 2004, 51, 251–259. [Google Scholar] [CrossRef]
- Nudda, A.; Battacone, G.; Boaventura Neto, O.; Cannas, A.; Francesconi, A.H.D.; Atzori, A.S.; Pulina, G. Feeding strategies to design the fatty acid profile of sheep milk and cheese. R. Bras. Zootec 2014, 43, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Astrup, A.; Bradley, B.H.R.; Brenna, J.T.; Delplanque, B.; Ferry, M.; Torres-Gonzalez, M. Regular-Fat Dairy and Human Health: A Synopsis of Symposia Presented in Europe and North America (2014–2015). Nutrients 2016, 8, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hibbeln, J.R.; Nieminen, L.R.; Blasbalg, T.L.; Riggs, J.A.; Lands, W.E. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006, 83 (Suppl. 6), 1483s–1493s. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; U.S. Department of Agriculture, Ed.; Dietary Guidelines Advisory Committee: Washington, DC, USA, 2015.
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Harris, W.S.; Klurfeld, D.M. Twentieth-century trends in essential fatty acid intakes and the predicted omega-3 index: Evidence versus estimates. Am. J. Clin. Nutr. 2011, 93, 907–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Średnicka-Tober, D.; Barański, M.; Seal, C.J. Higher PUFA and n-3 PUFA, conjugated linoleic acid, α-tocopherol and iron, but lower iodine and selenium concentrations in organic milk: A systematic literature review and meta- and redundancy analyses. Br. J. Nutr. 2016, 115, 1043–1060. [Google Scholar] [CrossRef] [Green Version]
- Stergiadis, S.; Bieber, A.; Chatzidimitriou, E.; Franceschin, E.; Isensee, A.; Rempelos, L.; Baranski, M.; Maurer, V.; Cozzi, G.; Bapst, B.; et al. Impact of US Brown Swiss genetics on milk quality from low-input herds in Switzerland: Interactions with season. Food Chem. 2018, 251, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, G.; Nielsen, J.H.; Slots, T.; Seal, C.; Eyre, M.D.; Sanderson, R.; Leifert, C. Fatty acid and fat-soluble antioxidant concentrations in milk from high- and low-input conventional and organic systems: Seasonal variation. J. Sci. Food Agric. 2008, 88, 1431–1441. [Google Scholar] [CrossRef]
- Butler, G.; Stergiadis, S.; Chatzidimitriou, E.; Franceschin, E.; Davis, H.R.; Leifert, C.; Steinshamn, H. Differing responses in milk composition from introducing rapeseed and naked oats to conventional and organic dairy diets. Sci. Rep. 2019, 9, 8115. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.; Chatzidimitriou, E.; Leifert, C.; Butler, G. Evidence That Forage-Fed Cows Can Enhance Milk Quality. Sustainability 2020, 12, 3688. [Google Scholar] [CrossRef]
- Soják, L.; Blaško, J.; Kubinec, R.; Górová, R.; Addová, G.; Ostrovský, I.; Margetín, M. Variation among individuals, breeds, parities and milk fatty acid profile and milk yield of ewes grazed on pasture. Small Rumin. Res. 2013, 109, 173–181. [Google Scholar] [CrossRef]




| Somatic Cell Counts (SCC) (×103/100 mL−1 Milk) | Colony Forming Units (CFU) (×103/100 mL−1 Milk) | |||
|---|---|---|---|---|
| Factor | Year 1 (n = 400) | Year 2 (n = 400) | Year 1 (n = 400) | Year 2 (n = 400) |
| management system | ||||
| semi-intensive | 182 ± 5 | 162 ± 6 | 15.5 ± 1.0 | 14.1 ± 1.1 |
| extensive | 138 ± 4 | 191 ± 6 | 12.6 ± 1.0 | 15.8 ± 1.0 |
| lambing period | ||||
| early | 209 ± 6 | 214 ± 6 | 15.1 ± 1.0 | 15.1 ± 1.0 |
| late | 123 ± 4 | 145 ± 4 | 12.9 ± 1.0 | 14.5 ± 1.1 |
| sampling months | ||||
| March | 135 ± 6 b | 140 ± 5 b | 13.8 ± 1.0 | 20.0 ± 1.3 a |
| April | 148 ± 4 b | 145 ± 4 b | 12.3 ± 1.0 | 15.8 ± 1.0 b |
| May | 151 ± 7 b | 135 ± 2 b | 13.8 ± 1.1 | 11.0 ± 1.0 c |
| June | 186 ± 4 a | 309 ± 6 a | 14.8 ± 1.1 | 13.8 ± 1.1 bc |
| ANOVA (p values) | ||||
| Main effects | ||||
| management systems (MS) | *** | * | *** | NS |
| lambing period (LP) | *** | *** | *** | NS |
| sampling season (SS) | ** | *** | T | ** |
| Interactions | ||||
| MS × SS | ***1 | *1 | T | *1 |
| MS × LP | NS | NS | NS | NS |
| LP × SS | NS | **2 | NS | ***2 |
| MS × LP × SS | NS | NS | NS | NS |
| % of Samples from Ewes with Subclinical Mastitis that Tested Positive for | |||||
|---|---|---|---|---|---|
| Factor | % of Ewes with Subclinical Mastitis 1 | GRAM Positive Pathogens 2 | GRAM Negative Pathogen 3 | GRAM Positive and Negative Pathogens | Non- Pathogenic Micro- Organisms |
| management system | |||||
| semi-intensive (n = 400) | 30 ± 3 | 39 ± 5 | 13 ± 2 | 5 ± 1 | 42 ± 4 |
| extensive (n = 400) | 31 ± 3 | 46 ± 5 | 11 ± 2 | 4 ± 1 | 38 ± 4 |
| lambing period | |||||
| early (n = 400) | 39 ± 3 | 43 ± 4 | 13 ± 2 | 5 ± 1 | 39 ± 3 |
| late (n = 400) | 22 ± 2 | 42 ± 5 | 12 ± 2 | 5 ± 1 | 41 ± 5 |
| production year | |||||
| year 1 (n = 400) | 31 ± 3 | 61 ± 4 | 4 ± 1 | 1 ± 1 | 34 ± 4 |
| year 2 (n = 400) | 30 ± 2 | 23 ± 3 | 22 ± 2 | 8 ± 1 | 46 ± 4 |
| ANOVA (p values) | |||||
| Main effects | |||||
| management systems (MS) | NS | NS | NS | NS | NS |
| lambing period (LP) | *** | *** | *** | NS | *** |
| production year (Y) | NS | *** | *** | *** | T |
| Interactions | NS | ||||
| MS × LP | NS | NS | NS | NS | NS |
| MS × Y | * | T | NS | NS | NS |
| LP × Y | NS | T | * | NS | NS |
| MS × LP × Y | NS | NS | NS | * | NS |
| Milk Yield (l day−1 ewe−1) | Fat Content (g 100 mL−1 Milk) | |||
|---|---|---|---|---|
| Factor | Year 1 (n = 400) | Year 2 (n = 400) | Year 1 (n = 400) | Year 2 (n = 400) |
| management system | ||||
| semi-intensive | 0.89 ± 0.01 | 0.67 ± 0.01 | 5.51 ± 0.04 | 5.22 ± 0.06 |
| extensive | 0.58 ± 0.01 | 0.44 ± 0.01 | 5.97 ± 0.05 | 5.58 ± 0.06 |
| lambing period | ||||
| Early | 0.65 ± 0.01 | 0.50 ± 0.01 | 6.03 ± 0.04 | 6.57 ± 0.06 |
| Late | 0.82 ± 0.02 | 0.62 ± 0.01 | 5.45 ± 0.05 | 5.20 ± 0.06 |
| sampling months | ||||
| March | 0.87 ± 0.02 a | 0.80 ± 0.02 a | 5.58 ± 0.08 b | 4.80 ± 0.08 b |
| April | 0.77 ± 0.02 b | 0.66 ± 0.02 b | 5.92 ± 0.07 a | 4.85 ± 0.08 b |
| May | 0.72 ± 0.02 b | 0.48 ± 0.01 c | 5.47 ± 0.07 b | 4.80 ± 0.09 b |
| June | 0.66 ± 0.03 c | 0.41 ± 0.01 d | 5.85 ± 0.08 a | 6.12 ± 0.08 a |
| ANOVA (p values) | ||||
| Main effects | ||||
| management systems (MS) | *** | *** | *** | *** |
| lambing period (LP) | *** | *** | *** | *** |
| sampling season (SS) | *** | *** | *** | *** |
| Interactions | ||||
| MS × SS | ***1 | *1 | ***1 | ***1 |
| MS × LP | NS | T | *** | T |
| LP × SS | ***2 | ***2 | ***2 | ***2 |
| MS × LP × SS | T | NS | ** | NS |
| Protein Content (g 100 mL−1 milk) | Lactose Content (g 100 mL−1 milk) | Non-Fat Solids Content (g 100 mL−1 milk) | ||||
|---|---|---|---|---|---|---|
| Factor | Year 1 (n = 400) | Year 2 (n = 400) | Year 1 (n = 400) | Year 2 (n = 400) | Year 1 (n = 400) | Year 2 (n = 400) |
| management system | ||||||
| semi-intensive | 5.14 ± 0.02 | 5.25 ± 0.02 | 4.70 ± 0.02 | 4.53 ± 0.03 | 10.60 ± 0.03 | 10.62 ± 0.03 |
| extensive | 5.18 ± 0.03 | 5.49 ± 0.03 | 4.52 ± 0.03 | 4.44 ± 0.03 | 10.43 ± 0.03 | 10.76 ± 0.04 |
| lambing period | ||||||
| Early | 5.36 ± 0.02 | 5.49 ± 0.03 | 4.40 ± 0.03 | 4.25 ± 0.03 | 10.49 ± 0.03 | 10.61 ± 0.05 |
| Late | 4.97 ± 0.02 | 5.23 ± 0.03 | 4.81 ± 0.02 | 4.71 ± 0.03 | 10.54 ± 0.02 | 10.76 ± 0.03 |
| sampling months | ||||||
| March | 5.02 ± 0.03 c | 5.38 ± 0.04 | 4.93 ± 0.03 a | 4.90 ± 0.03 | 10.67 ± 0.03 | 11.04 ± 0.04 a |
| April | 5.16 ± 0.03 b | 5.40 ± 0.03 | 4.80 ± 0.03 b | 4.70 ± 0.03 | 10.66 ± 0.04 | 10.95 ± 0.04 a |
| May | 5.23 ± 0.04 a | 5.29 ± 0.03 | 4.67 ± 0.04 c | 4.55 ± 0.04 | 10.62 ± 0.05 | 10.72 ± 0.05 b |
| June | 5.20 ± 0.04 a | 5.17 ± 0.04 | 4.31 ± 0.05 d | 4.32 ± 0.05 | 10.29 ± 0.06 | 10.34 ± 0.06 c |
| ANOVA (p values) | ||||||
| Main effects | ||||||
| management systems (MS) | NS | ** | *** | * | *** | *** |
| lambing period (LP) | *** | *** | *** | *** | T | *** |
| sampling season (SS) | *** | T | *** | *** | NS | *** |
| Interactions | ||||||
| MS × SS | ***1 | ***1 | ***1 | ***1 | NS | ***1 |
| MS × LP | NS | NS | NS | ***2 | NS | ***2 |
| LP × SS | ***3 | ***3 | NS | NS | ***3 | ***3 |
| MS × LP × SS | Ns | NS | * | NS | NS | NS |
| Total Saturated Fatty Acids (SFA) | Oleic Acid (C18:1 cis9) | Total Monounsaturated Fatty Acids (MUFA) | Total Polyunsaturated Fatty Acids (PUFA) | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 |
| management system (MS) | ||||||||
| SI (n = 200) | 69.0 ± 0.3 | 66.1 ± 0.2 | 16.4 ± 0.2 | 18.8 ± 0.2 | 24.3 ± 0.2 | 27.2 ± 0.2 | 6.8 ± 0.1 | 6.8 ± 0.1 |
| EX (n = 200) | 66.0 ± 0.3 | 63.6 ± 0.3 | 19.1 ± 0.2 | 19.7 ± 0.2 | 27.0 ± 0.2 | 28.6 ± 0.2 | 7.0 ± 0.1 | 7.9 ± 0.1 |
| lambing period (LP) | ||||||||
| early (n = 200) | 68.5 ± 0.3 | 65.0 ± 0.3 | 17.4 ± 0.2 | 19.6 ± 0.2 | 24.9 ± 0.2 | 27.9 ± 0.2 | 6.6 ± 0.1 | 7.1 ± 0.1 |
| late (n = 200) | 66.6 ± 0.3 | 64.6 ± 0.2 | 18.1 ± 0.2 | 18.9 ± 0.2 | 26.3 ± 0.2 | 27.9 ± 0.2 | 7.2 ± 0.1 | 7.5 ± 0.1 |
| Sampling month (M) | ||||||||
| March (n = 200) | 69.2 ± 0.3 | 66.4 ± 0.2 | 16.9 ± 0.2 | 17.7 ± 0.2 | 24.3 ± 0.2 | 26.5 ± 0.2 | 6.5 ± 0.1 | 7.1 ± 0.1 |
| May (n = 200) | 65.8 ± 0.3 | 63.2 ± 0.2 | 18.6 ± 0.2 | 20.9 ± 0.2 | 26.9 ± 0.2 | 29.3 ± 0.2 | 7.3 ± 0.1 | 7.5 ± 0.1 |
| ANOVA (p values) | ||||||||
| Main effects | ||||||||
| MS | *** | *** | *** | *** | *** | *** | * | *** |
| LP | *** | NS | * | *** | *** | NS | *** | *** |
| M | *** | *** | *** | *** | *** | *** | *** | ** |
| Interactions | ||||||||
| MS × LP | NS | NS | NS | NS | NS | NS | NS | NS |
| MS × M | NS | NS | **2 | *2 | NS | *2 | NS | ***2 |
| LP × M | **3 | ***3 | **3 | NS | ***3 | T | ***3 | ***3 |
| MS × LP × M | NS | NS | NS | T | NS | NS | NS | NS |
| Lauric Acid (C12:0) | Myristic Acid (C14:0) | Palmitic Acid (C16:0) | Stearic Acid (C18:0) | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 |
| management system (MS) | ||||||||
| SI (n = 200) | 5.8 ± 0.1 | 4.4 ± 0.1 | 13.0 ± 0.1 | 11.9 ± 0.1 | 26.2 ± 0.2 | 26.0 ± 0.1 | 6.7 ± 0.1 | 9.0 ± 0.1 |
| EX (n = 200) | 4.3 ± 0.1 | 3.7 ± 0.1 | 11.3 ± 0.1 | 10.7 ± 0.1 | 26.0 ± 0.2 | 26.0 ± 0.1 | 8.7 ± 0.2 | 9.2 ± 0.1 |
| lambing period (LP) | ||||||||
| early (n = 200) | 5.2 ± 0.1 | 4.1 ± 0.1 | 12.8 ± 0.1 | 11.7 ± 0.1 | 26.7 ± 0.2 | 26.2 ± 0.1 | 7.4 ± 0.1 | 9.2 ± 0.1 |
| late (n = 200) | 4.9 ± 0.1 | 4.0 ± 0.1 | 11.5 ± 0.1 | 11.0 ± 0.1 | 25.5 ± 0.2 | 25.8 ± 0.1 | 8.0 ± 0.2 | 8.0 ± 0.1 |
| Sampling month (M) | ||||||||
| March (n = 200) | 5.5 ± 0.1 | 4.8 ± 0.1 | 12.0 ± 0.1 | 11.7± 0.1 | 25.7 ± 0.2 | 25.7 ± 0.1 | 7.1 ± 0.2 | 8.0 ± 0.1 |
| May (n = 200) | 4.6 ± 0.1 | 3.3 ± 0.1 | 12.3 ± 0.1 | 11.0 ± 0.1 | 26.5 ± 0.2 | 26.3 ± 0.1 | 8.3 ± 0.1 | 10.2 ± 0.1 |
| ANOVA (p values) | ||||||||
| Main effects | ||||||||
| MS | *** | *** | *** | *** | NS | NS | *** | NS |
| LP | ** | NS | *** | *** | *** | ** | ** | NS |
| M | *** | *** | ** | *** | ** | ** | *** | *** |
| Interactions | ||||||||
| MS × LP | NS | T | NS | NS | NS | NS | NS | NS |
| MS × M | NS | NS | *2 | T | NS | *2 | NS | **2 |
| LP × M | *3 | NS | ***3 | ***3 | *3 | T | NS | NS |
| MS × LP × M | NS | NS | NS | NS | NS | NS | NS | NS |
| Linoleic Acid (LA; C18:2 cis9 cis12) | Total Omega-6 PUFA | Total Omega-3 PUFA | Omega-6/Omega-3 Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 |
| management system (MS) | ||||||||
| SI (n = 200) | 29.5 ± 0.5 | 25.7 ± 0.3 | 38.5 ± 0.6 | 34.8 ± 0.4 | 12.0 ± 0.02 | 13.8 ± 0.3 | 3.6 ± 0.1 | 2.8 ± 0.1 |
| EX (n = 200) | 29.5 ± 0.4 | 28.5 ± 0.1 | 38.2 ± 0.5 | 38.8 ± 0.4 | 14.2 ± 0.04 | 19.4 ± 0.5 | 3.4 ± 0.1 | 2.7 ± 0.1 |
| lambing period (LP) | ||||||||
| early (n = 200) | 27.9 ± 0.5 | 26.5 ± 0.4 | 36.6 ± 0.5 | 36.2 ± 0.4 | 12.3 ± 0.03 | 16.1 ± 0.4 | 3.7 ± 0.1 | 2.8 ± 0.1 |
| late (n = 200) | 31.1 ± 0.5 | 27.6 ± 0.3 | 40.1 ± 0.5 | 37.4 ± 0.4 | 13.9 ± 0.03 | 17.0 ± 0.4 | 3.4 ± 0.1 | 2.7 ± 0.1 |
| Sampling month (M) | ||||||||
| March (n = 200) | 29.0 ± 0.5 | 27.4 ± 0.4 | 36.7 ± 0.5 | 36.9 ± 0.5 | 11.3 ± 0.02 | 14.5 ± 0.3 | 3.8 ± 0.1 | 3.1 ± 0.1 |
| May (n = 200) | 30.0 ± 0.4 | 26.7 ± 0.3 | 40.0 ± 0.5 | 36.7 ± 0.3 | 15.0 ± 0.04 | 18.7 ± 0.4 | 3.2 ± 0.1 | 2.4 ± 0.1 |
| ANOVA (p values) | ||||||||
| Main effects | ||||||||
| MS | NS | *** | NS | *** | *** | *** | * | NS |
| LP | *** | * | *** | * | *** | *** | * | NS |
| M | NS | NS | ** | NS | *** | ** | ** | *** |
| Interactions | ||||||||
| MS × LP | NS | NS | NS | NS | NS | **1 | ***1 | NS |
| MS × M | NS | ***2 | NS | NS | ***2 | ***2 | ***2 | ***2 |
| LP × M | T | ***3 | *3 | ***3 | **3 | ***3 | *3 | NS |
| MS × LP × M | NS | T | NS | NS | ** | NS | ** | * |
| α-Linolenic Acid (ALA; C18:3 cis 9.cis 12.cis 15) | Eicosapentaenoic Acid (EPA; C20:5 n-3) | Docosapentaenoic Acid (DPA; C22:5 n-3) | Docosahexaenoic Acid (DHA; C22:6 n-3) | |||||
|---|---|---|---|---|---|---|---|---|
| Factor | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 |
| management system (MS) | ||||||||
| SI (n = 200) | 5.7 ± 0.1 | 7.3 ± 0.1 | 0.54 ± 0.02 | 0.49 ± 0.01 | 1.26 ± 0.03 | 1.09 ± 0.02 | 0.44 ± 0.02 | 0.31 ± 0.01 |
| EX (n = 200) | 8.0 ± 0.2 | 11.5 ± 0.3 | 0.69 ± 0.03 | 0.65 ± 0.01 | 1.29 ± 0.04 | 1.43 ± 0.03 | 0.49 ± 0.02 | 0.42 ± 0.01 |
| lambing period (LP) | ||||||||
| early (n = 200) | 6.5 ± 0.2 | 9.3 ± 0.3 | 0.59 ± 0.02 | 0.55 ± 0.01 | 1.20 ± 0.04 | 1.22 ± 0.03 | 0.43 ± 0.01 | 0.36 ± 0.01 |
| late (n = 200) | 7.2 ± 0.2 | 9.5 ± 0.2 | 0.64 ± 0.02 | 0.59 ± 0.01 | 1.35 ± 0.04 | 1.29 ± 0.03 | 0.51 ± 0.02 | 0.37 ± 0.01 |
| Sampling month (M) | ||||||||
| March (n = 200) | 5.8 ± 0.1 | 7.6 ± 0.2 | 0.48 ± 0.01 | 0.51 ± 0.01 | 0.90 ± 0.02 | 1.05 ± 0.02 | 0.38 ± 0.01 | 0.31 ± 0.01 |
| May (n = 200) | 7.9 ± 0.2 | 11.3 ± 0.3 | 0.75 ± 0.03 | 0.63 ± 0.01 | 1.66 ± 0.04 | 1.47 ± 0.03 | 0.56 ± 0.02 | 0.43 ± 0.01 |
| ANOVA (p values) | ||||||||
| Main effects | ||||||||
| MS | *** | *** | *** | *** | NS | *** | * | *** |
| LP | ** | NS | NS | ** | ** | * | *** | NS |
| M | *** | *** | *** | *** | *** | *** | *** | *** |
| Interactions | ||||||||
| MS × LP | NS | ***1 | NS | NS | NS | NS | NS | NS |
| MS × M | ***2 | ***2 | **2 | ***2 | NS | ***2 | NS | ***2 |
| LP × M | ***3 | ***3 | NS | NS | *3 | ***3 | NS | ***3 |
| MS × LP × M | NS | NS | NS | NS | NS | NS | NS | T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voutzourakis, N.; Stefanakis, A.; Stergiadis, S.; Rempelos, L.; Tzanidakis, N.; Eyre, M.; Butler, G.; Leifert, C.; Sotiraki, S. Effect of Intensification Practices, Lambing Period and Environmental Parameters on Animal Health, and Milk Yield and Quality in Dairy Sheep Production Systems on Crete. Sustainability 2021, 13, 9706. https://doi.org/10.3390/su13179706
Voutzourakis N, Stefanakis A, Stergiadis S, Rempelos L, Tzanidakis N, Eyre M, Butler G, Leifert C, Sotiraki S. Effect of Intensification Practices, Lambing Period and Environmental Parameters on Animal Health, and Milk Yield and Quality in Dairy Sheep Production Systems on Crete. Sustainability. 2021; 13(17):9706. https://doi.org/10.3390/su13179706
Chicago/Turabian StyleVoutzourakis, Nikolaos, Alexandros Stefanakis, Sokratis Stergiadis, Leonidas Rempelos, Nikolaos Tzanidakis, Mick Eyre, Gillian Butler, Carlo Leifert, and Smaragda Sotiraki. 2021. "Effect of Intensification Practices, Lambing Period and Environmental Parameters on Animal Health, and Milk Yield and Quality in Dairy Sheep Production Systems on Crete" Sustainability 13, no. 17: 9706. https://doi.org/10.3390/su13179706







