Tri-tert-butyl(n-alkyl)phosphonium Ionic Liquids: Structure, Properties and Application as Hybrid Catalyst Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Materials
2.2.1. Typical Procedure for PdNP Preparation
2.2.2. General Procedure for Suzuki Cross-Coupling
3. Results and Discussion
3.1. Synthetic Procedures
3.2. NMR Spectroscopy
3.2.1. 31P NMR Spectral Data
3.2.2. 1H NMR Spectral Data
| Phosphonium Salt | Chemical Shift of α-Protons 1H NMR, ppm | Chemical Shift 31P NMR, ppm | ||
|---|---|---|---|---|
| N | Cation | Anion | ||
| 1a | t-Bu3P+CH3 | I− | 2.11 | 51.3 |
| 1b | BF4− | 1.90 | 51.0 | |
| 2a | t-Bu3P+C3H7 | I− | 2.60 | 50.7 |
| 2b | BF4− | 2.33 | 49.2 | |
| 3a | t-Bu3P+C5H11 | Br− | 2.54 | 48.8 |
| 3b | BF4− | 2.32 | 49.2 | |
| 4a | t-Bu3P+C7H15 | Br− | 2.56 | 49.4 |
| 4b | BF4− | 2.29 | 47.7 | |
| 5a | t-Bu3P+C9H19 | Br− | 2.49 | 49.3 |
| 5b | BF4− | 2.27 | 47.7 | |
| 6a | t-Bu3P+C11H23 | Br− | 2.49 | 50.6 |
| 6b | BF4− | 2.28 | 48.9 | |
| 7a | t-Bu3P+C13H27 | Br− | 2.46 | 50.7 |
| 7b | BF4− | 2.26 | 48.9 | |
| 8a | t-Bu3P+C15H31 | Br− | 2.50 | 50.6 |
| 8b | BF4− | 2.27 | 48.8 | |
| 9a | t-Bu3P+C17H35 | Br− | 2.48 | 50.6 |
| 9b | BF4− | 2.26 | 48.8 | |
3.2.3. 13C{1H} NMR Spectral Data
3.3. Single-Crystal X-ray Diffraction Analysis
3.4. Melting Point
3.5. PdNP Stabilization and TEM Sample Preparation
3.6. PdNP Behavior during Suzuki Reaction
3.7. DLS Data of the Catalytic System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Z.; Crosthwaite, J.M.; Aki, S.N.; Brennecke, J.F. Thermal Stability of Ionic Liquids in Nitrogen and Air Environments. J. Chem. Thermodyn. 2021, 161, 106560. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Łuczak, J.; Paszkiewicz-Gawron, M.; Krukowska, A.; Malankowska, A.; Zaleska-Medynska, A. Ionic liquids for nano- and microstructures preparation. Part 1: Properties and multifunctional role. Adv. Colloid Interface Sci. 2016, 230, 13–28. [Google Scholar] [CrossRef]
- Khazalpour, S.; Yarie, M.; Kianpour, E.; Amani, A.; Asadabadi, S.; Seyf, J.Y.; Rezaeivala, M.; Azizian, S.; Zolfigol, M.A. Applications of phosphonium-based ionic liquids in chemical processes. J. Iran. Chem. Soc. 2020, 17, 1775–1917. [Google Scholar] [CrossRef]
- Egorova, K.; Ananikov, V.P. Fundamental importance of ionic interactions in the liquid phase: A review of recent studies of ionic liquids in biomedical and pharmaceutical applications. J. Mol. Liq. 2018, 272, 271–300. [Google Scholar] [CrossRef]
- Gabriel, S.; Weiner, J. Ueber einige Abkömmlinge des Propylamins. Eur. J. Inorg. Chem. 1888, 21, 2669–2679. [Google Scholar] [CrossRef] [Green Version]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. St. Petersburg 1914, 1800, 405–422. [Google Scholar]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Lawal, I.A.; Klink, M.; Ndungu, P.; Moodley, B. Brief bibliometric analysis of “ionic liquid” applications and its review as a substitute for common adsorbent modifier for the adsorption of organic pollutants. Environ. Res. 2019, 175, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.D.; Hamer, C.K. Ionic liquids—The beginning of the end or the end of the beginning?—A look at the life of ionic liquids through patent claims. Sep. Purif. Technol. 2018, 196, 3–9. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [Green Version]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [Green Version]
- Osada, I.; De Vries, H.; Scrosati, B.; Passerini, S. Ionic-Liquid-Based Polymer Electrolytes for Battery Applications. Angew. Chem. Int. Ed. 2016, 55, 500–513. [Google Scholar] [CrossRef]
- Lecce, D.D.; Hassoun, J. Lithium Metal Battery Using LiFe0.5Mn0.5PO4 Olivine Cathode and Pyrrolidinium-Based Ionic Liquid Electrolyte. ACS Omega 2018, 3, 8583–8588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodiumion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Chagas, L.G.; Jeong, S.; Hasa, I.; Passerini, S. Ionic Liquid-Based Electrolytes for Sodium-Ion Batteries: Tuning Properties to Enhance the Electrochemical Performance of Manganese-Based Layered Oxide Cathode. ACS Appl. Mater. Interfaces 2019, 11, 22278–22289. [Google Scholar] [CrossRef]
- Opallo, M.; Lesniewski, A. A review on electrodes modified with ionic liquids. J. Electroanal. Chem. 2011, 656, 2–16. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Shekurov, R.; Miluykov, V.; Gilmanova, L.; Kataeva, O.; Yamaleeva, Z.; Gerasimova, T.; Ermolaev, V.; Gubaidullin, A.; Laskin, A.; et al. Excellent supercapacitor and sensor performance of robust cobalt phosphinate ferrocenyl organic framework materials achieved by intrinsic redox and structure properties. Dalton Trans. 2019, 48, 16986–16992. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Claus, J.; Sommer, F.O.; Kragl, U. Ionic liquids in biotechnology and beyond. Solid State Ionics 2018, 314, 119–128. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial Polymeric Materials with Quaternary Ammonium and Phosphonium Salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraz, R.; Costa-Rodrigues, J.; Fernandes, M.H.; Santos, M.; Marrucho, I.; Rebelo, L.P.; Prudêncio, C.; Noronha, J.P.; Željko, P.; Branco, L.C. Antitumor Activity of Ionic Liquids Based on Ampicillin. ChemMedChem 2015, 10, 1480–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamshina, J.L.; Rogers, R.D. Are Myths and Preconceptions Preventing us from Applying Ionic Liquid Forms of Antiviral Medicines to the Current Health Crisis? Int. J. Mol. Sci. 2020, 21, 6002. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Yue, Z.; Zeng, X.-A.; Cheng, J.-H.; Aadil, R.M. Ionic liquid as an effective solvent for cell wall deconstructing through astaxanthin extraction from Haematococcus pluvialis. Int. J. Food Sci. Technol. 2018, 54, 583–590. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Pharm. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; Macfarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Li, J.; Wang, H.; Zhang, C.; Liu, Y.; Luo, J. Macroscale superlubricity under extreme pressure enabled by the combination of graphene-oxide nanosheets with ionic liquid. Carbon 2019, 151, 76–83. [Google Scholar] [CrossRef]
- Avilés, M.; Sánchez, C.; Pamies, R.; Sanes, J.; Bermúdez, M. Ionic Liquid Crystals in Tribology. Lubricants 2019, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Hejazifar, M.; Lanaridi, O.; Bica-Schröder, K. Ionic liquid based microemulsions: A review. J. Mol. Liq. 2020, 303, 112264. [Google Scholar] [CrossRef]
- Kissoudi, M.; Samanidou, V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules 2018, 23, 1437. [Google Scholar] [CrossRef] [Green Version]
- Wassershied, P.; Keim, W. Ionic Liquids-New "Solutions" for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Pensado, A.S.; Padua, A. Solvation and Stabilization of Metallic Nanoparticles in Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 8683–8687. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon−Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Scholten, J.D.; Leal, B.C.; Dupont, J. Transition Metal Nanoparticle Catalysis in Ionic Liquids. ACS Catal. 2011, 2, 184–200. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.-Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef] [Green Version]
- Parajó, J.J.; Vallet, P.; Fernádez-Míguez, L.; Villanueva, M.; Salgado, J. Ecotoxicity of Mixtures of IL and Lithium Salt. Cells 2020, 3, 84. [Google Scholar] [CrossRef]
- Fojtášková, J.; Koutník, I.; Vráblová, M.; Sezimová, H.; Maxa, M.; Obalová, L.; Pánek, P. Antibacterial, Antifungal and Ecotoxic Effects of Ammonium and Imidazolium Ionic Liquids Synthesized in Microwaves. Molecules 2020, 25, 5181. [Google Scholar] [CrossRef]
- Dupont, J. From Molten Salts to Ionic Liquids: A “Nano” Journey. Acc. Chem. Res. 2011, 44, 1223–1231. [Google Scholar] [CrossRef]
- Kar, M.; Plechkova, N.V.; Seddon, K.R.; Pringle, J.M.; Macfarlane, D.R. Ionic Liquids—Further Progress on the Fundamental Issues. Aust. J. Chem. 2019, 72, 3. [Google Scholar] [CrossRef] [Green Version]
- Mano, B.; Jesus, F.; Gonçalves, F.J.M.; Ventura, S.P.M.; Pereira, J.L. Applicability of heuristic rules defining structure–ecotoxicity relationships of ionic liquids: An integrative assessment using species sensitivity distributions (SSD). Green Chem. 2020, 22, 6176–6186. [Google Scholar] [CrossRef]
- Pernak, J.; Łęgosz, B.; Walkiewicz, F.; Klejdysz, T.; Borkowski, A.; Chrzanowski, Ł. Ammonium ionic liquids with anions of natural origin. RSC Adv. 2015, 5, 65471–65480. [Google Scholar] [CrossRef]
- Mezzetta, A.; Łuczak, J.; Woch, J.; Chiappe, C.; Nowicki, J.; Guazzelli, L. Surface active fatty acid ILs: Influence of the hydrophobic tail and/or the imidazolium hydroxyl functionalization on aggregates formation. J. Mol. Liq. 2019, 289, 111155. [Google Scholar] [CrossRef]
- Adamová, G.; Gardas, R.; Rebelo, L.P.; Robertson, A.J.; Seddon, K.R. Alkyltrioctylphosphonium chloride ionic liquids: Synthesis and physicochemical properties. Dalton Trans. 2011, 40, 12750–12764. [Google Scholar] [CrossRef]
- Adamová, G.; Gardas, R.L.; Nieuwenhuyzen, M.; Puga, A.V.; Rebelo, L.P.N.; Robertson, A.J.; Seddon, K.R. Alkyltributylphosphonium chloride ionic liquids: Synthesis, physicochemical properties and crystal structure. Dalton Trans. 2012, 41, 8316. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, M.L.; Kubota, F.; Yoshida, W.; Goto, M. Application of a Novel Phosphonium-Based Ionic Liquid to the Separation of Platinum Group Metals from Automobile Catalyst Leach Liquor. Ind. Eng. Chem. Res. 2019, 58, 3845–3852. [Google Scholar] [CrossRef]
- Xun, S.; Ti, Q.; Wu, L.; He, M.; Wang, C.; Chen, L.; Yang, W.; Zhu, L.; Zhu, W.; Li, H. Few Layer g-C3N4 Dispersed Quaternary Phosphonium Ionic Liquid for Highly Efficient Catalytic Oxidative Desulfurization of Fuel. Energy Fuels 2020, 34, 12379–12387. [Google Scholar] [CrossRef]
- Sardar, S.; Mumtaz, A.; Jabeen, E.; Taneez, M.; Wilfred, C.D.; Maqsood, A. Efficient CO2 sorption in phosphonium-based organic salts. J. Mol. Liq. 2020, 318, 114044. [Google Scholar] [CrossRef]
- Goyal, M.; Kumar, S.; Verma, C.; Bahadur, I.; Ebenso, E.E.; Lgaz, H.; Chung, I.-M. Interfacial adsorption behavior of quaternary phosphonium based ionic liquids on metal-electrolyte interface: Electrochemical, surface characterization and computational approaches. J. Mol. Liq. 2020, 298, 111995. [Google Scholar] [CrossRef]
- Ermolaev, V.; Miluykov, V.; Rizvanov, I.; Krivolapov, D.; Zvereva, E.V.; Katsyuba, S.; Sinyashin, O.; Schmutzler, R. Phosphonium ionic liquids based on bulky phosphines: Synthesis, structure and properties. Dalton Trans. 2010, 39, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. Sterically demanding trialkylphosphines for palladium-catalyzed cross coupling reactions—alternatives to PtBu3. Chem. Soc. Rev. 2009, 39, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, D.M.; Ermolaev, V.V.; Miluykov, V.A.; Gubaidullin, A.T.; Islamov, D.R.; Kataeva, O.N.; Ananikov, V.P. Sterically Hindered Phosphonium Salts: Structure, Properties and Palladium Nanoparticle Stabilization. Nanomaterials 2020, 10, 2457. [Google Scholar] [CrossRef]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- CrysAlisPro; Version 1.171.41.106a; Rigaku Oxford Diffraction: Austin, TX, USA, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Hoffmann, H.; Schellenbeck, P. Notiz über die Darstellung von Tri-tert.-butylphosphin. Eur. J. Inorg. Chem. 1967, 100, 692–693. [Google Scholar] [CrossRef]
- Al-Otaibi, J.S.; Gogary, T.M.E.; El-Demerdash, S.H. Umbrella inversion and structure of phosphorus-containing compounds: A quantum chemical study. J. Theor. Comput. Chem. 2018, 17, 1850042. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Rogers, R.D.; Mantz, R.A.; Trulove, P.C.; Cocalia, V.A.; Visser, A.E.; Anderson, J.L.; Anthony, J.L.; Brennecke, J.F.; Maginn, E.J.; et al. Physicochemical Properties. In Ionic Liquids in Synthesis, 2nd ed.; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 57–174. [Google Scholar]
- Banerjee, A.; Scott, R.W.J. Optimization of transition metal nanoparticle-phosphonium ionic liquid composite catalytic systems for deep hydrogenation and hydrodeoxygenation reactions. Green Chem. 2014, 17, 1597–1604. [Google Scholar] [CrossRef] [Green Version]
- Chacon, G.; Dupont, J. Arene Hydrogenation by Metal Nanoparticles in Ionic Liquids. ChemCatChem 2019, 11, 333–341. [Google Scholar] [CrossRef]
- Chen, J.; Xie, F.; Li, X.; Chen, L. Ionic liquids for the preparation of biopolymer materials for drug/gene delivery: A review. Green Chem. 2018, 20, 4169–4200. [Google Scholar] [CrossRef] [Green Version]
- Hemmati, M.; Rajabi, M.; Asghari, A. Magnetic nanoparticle based solid-phase extraction of heavy metal ions: A review on recent advances. Microchim. Acta 2018, 185, 160. [Google Scholar] [CrossRef]
- Yi, L.; Wu, W.; Li, T. Crystalline Nanoparticles. In Pharmaceutical Crystals: Science and Engineering, 1st ed.; Li, T., Mattei, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; Volume 11, pp. 463–503. [Google Scholar]
- Saldan, I.; Semenyuk, Y.; Marchuk, I.; Reshetnyak, O. Chemical synthesis and application of palladium nanoparticles. J. Mater. Sci. 2015, 50, 2337–2354. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, N. Towards Rational Design of Nanoparticle Catalysis in Ionic Liquids. Catalysts 2013, 3, 543–562. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Yuan, Y.; Dyson, P.J. Nanometallic chemistry: Deciphering nanoparticle catalysis from the perspective of organometallic chemistry and homogeneous catalysis. Dalton Trans. 2013, 42, 13294–13304. [Google Scholar] [CrossRef]
- Nützenadel, C.; Züttel, A.; Chartouni, D.; Schmid, G.; Schlapbach, L. Critical size and surface effect of the hydrogen interaction of palladium clusters. Eur. Phys. J. D 2000, 8, 245–250. [Google Scholar] [CrossRef]
- Eremin, D.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems. Co-Ord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Gnad, C.; Abram, A.; Urstoeger, A.; Weigl, F.; Schuster, M.; Köhler, K. Leaching Mechanism of Different Palladium Surface Species in Heck Reactions of Aryl Bromides and Chlorides. ACS Catal. 2020, 10, 6030–6041. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azov, V.A.; Egorova, K.; Seitkalieva, M.M.; Kashin, A.; Ananikov, V.P. “Solvent-in-salt” systems for design of new materials in chemistry, biology and energy research. Chem. Soc. Rev. 2018, 47, 1250–1284. [Google Scholar] [CrossRef] [PubMed]


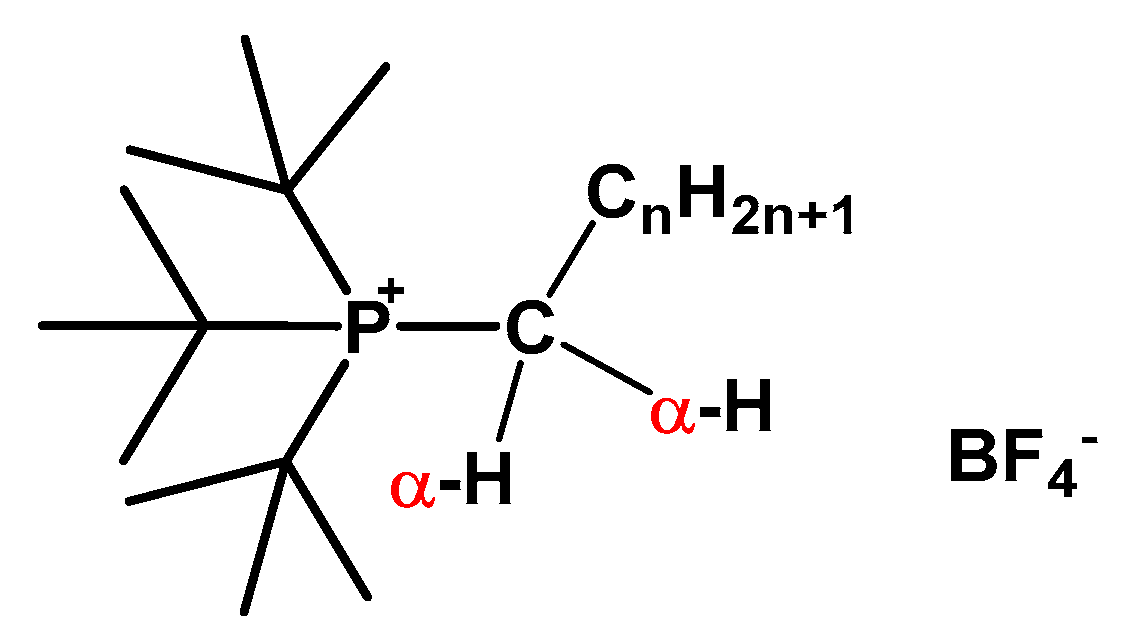










| PIL | Cq | Ct | Ca | Cb | Cc | Cm | Cx | Cy | Cz |
|---|---|---|---|---|---|---|---|---|---|
| 1b | 37.9 (d, 1JPC = 32.1) | 29.1 | 0.4 (d, 1JPC = 45.8) | - | - | - | - | - | - |
| 2b | 39.2 (d, 1JPC = 29.4) | 29.8 | 20.4 (d, 1JPC = 35.2) | 18.9 (d, 2JPC = 6.4) | 16.3 (d, 3JPC = 14.2) | - | - | - | - |
| 3b | 39.3 (d, 1JPC = 29.1) | 29.8 | 18.5 (d, 1JPC = 35.3) | 24.7 (d, 2JPC = 6.7) | 33.7 (d, 3JPC = 12.6) | - | - | 22.2 | 13.8 |
| 4b | 39.3 (d, 1JPC = 29.2) | 29.8 | 18.5 (d, 1JPC = 35.2) | 25.1 (d, 2JPC = 6.6) | 31.7 (d, 3JPC = 12.7) | 28.8 | 31.5 | 22.6 | 14.0 |
| 5b | 39.3 (d, 1JPC = 29.3) | 29.8 | 18.5 (d, 1JPC = 35.2) | 25.0 (d, 2JPC = 6.6) | 31.7 (d, 3JPC = 12.4) | 29.3; 29.2 | 31.8 | 22.6 | 14.1 |
| 6b | 39.3 (d, 1JPC = 29.1) | 29.8 | 18.5 (d, 1JPC = 34.9) | 25.1 (d, 2JPC = 6.6) | 31.7 (d, 3JPC = 12.6) | 29.6; 29.4; 29.3; 29.2 | 31.9 | 22.7 | 14.1 |
| 7b | 39.3 (d, 1JPC = 29.4) | 29.8 | 18.5 (d, 1JPC = 35.0) | 25.0 (d, 2JPC = 6.7) | 31.7 (d, 3JPC = 12.6) | 29.6; 29.3; 29.2 | 31.9 | 22.7 | 14.1 |
| 8b | 39.3 (d, 1JPC = 29.4) | 29.8 | 18.5 (d, 1JPC = 35.2) | 25.0 (d, 2JPC = 6.7) | 31.7 (d, 3JPC = 12.6) | 29.7–29.5; 29.4; 29.2 | 31.9 | 22.7 | 14.1 |
| 9b | 39.3 (d, 1JPC = 29.6) | 29.8 | 18.5 (d, 1JPC = 35.1) | 25.0 (d, 2JPC = 6.5) | 31.7 (d, 3JPC = 12.5) | 29.7–29.5; 29.4–29.3; 29.2 | 31.9 | 22.7 | 14.1 |
| Entry | PIL | The Average Size of PdNPs, nm | The Average Size of PdNPs after Suzuki Reaction, nm |
|---|---|---|---|
| 1 | 1b | 7.3 ± 1.5 | 3.0 ± 1.2 |
| 2 | 2b | 5.1 ± 3.0 | 5.0 ± 1.6 |
| 3 | 3b | 3.7 ± 0.9 | 4.6 ± 1.3 |
| 4 | 4b | 2.8 ± 0.9 | 2.8 ± 1.0 |
| 5 | 5b | 4.5 ± 3.1 | 5.8 ± 3.0 |
| 6 | 6b | 3.2 ± 0.8 | 2.9 ± 1.0 |
| 7 | 7b | 4.1 ± 1.2 | 3.7 ± 1.1 |
| 8 | 8b | 4.2 ± 1.2 | 3.5 ± 1.0 |
| 9 | 9b | 3.6 ± 1.4 | 2.6 ± 0.9 |
| Entry | PIL | Size of Nanocomposites (nm) |
|---|---|---|
| 1 | 4b | 309 ± 2 |
| 2 | 5b | 371 ± 2 |
| 3 | 6b | 219 ± 3 |
| 4 | 7b | 842 ± 5 |
| 5 | 8b | 304 ± 2 |
| 6 | 9b | 238 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipova, D.M.; Ermolaev, V.V.; Miluykov, V.A.; Valeeva, F.G.; Gaynanova, G.A.; Zakharova, L.Y.; Minyaev, M.E.; Ananikov, V.P. Tri-tert-butyl(n-alkyl)phosphonium Ionic Liquids: Structure, Properties and Application as Hybrid Catalyst Nanomaterials. Sustainability 2021, 13, 9862. https://doi.org/10.3390/su13179862
Arkhipova DM, Ermolaev VV, Miluykov VA, Valeeva FG, Gaynanova GA, Zakharova LY, Minyaev ME, Ananikov VP. Tri-tert-butyl(n-alkyl)phosphonium Ionic Liquids: Structure, Properties and Application as Hybrid Catalyst Nanomaterials. Sustainability. 2021; 13(17):9862. https://doi.org/10.3390/su13179862
Chicago/Turabian StyleArkhipova, Daria M., Vadim V. Ermolaev, Vasili A. Miluykov, Farida G. Valeeva, Gulnara A. Gaynanova, Lucia Ya. Zakharova, Mikhail E. Minyaev, and Valentine P. Ananikov. 2021. "Tri-tert-butyl(n-alkyl)phosphonium Ionic Liquids: Structure, Properties and Application as Hybrid Catalyst Nanomaterials" Sustainability 13, no. 17: 9862. https://doi.org/10.3390/su13179862







