How Relevant Are Direct Emissions of Microplastics into Freshwater from an LCA Perspective?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Interim and Simplified Characterization Factor (CF) for Microplastics
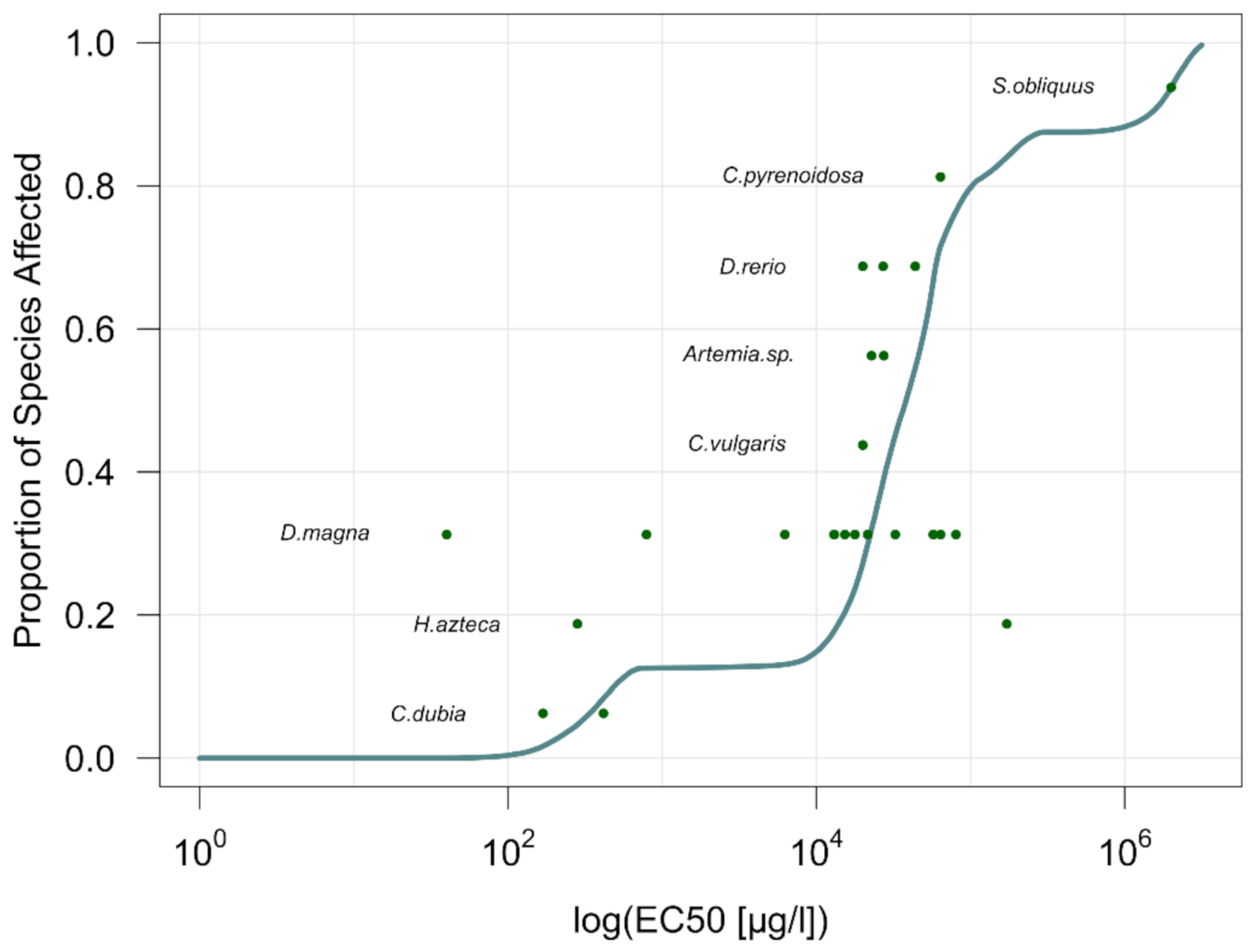
2.1.1. Effect Factor Calculation
2.1.2. Fate Factor Calculation
2.2. Life Cycle Assessment of a Polyester T-Shirt and a Shower Gel Containing Microplastics
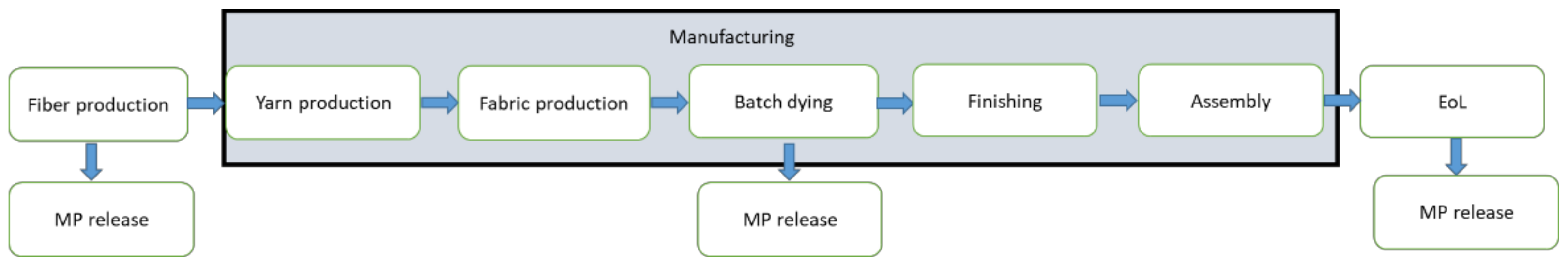
2.2.1. Quantification of Microplastic Releases from Textile Products (Case Study 1)
2.2.2. Quantification of Microplastics Releases from Personal Care Products (Case Study 2)
3. Results
3.1. Interim and Simplified Characterization Factor for Microplastics
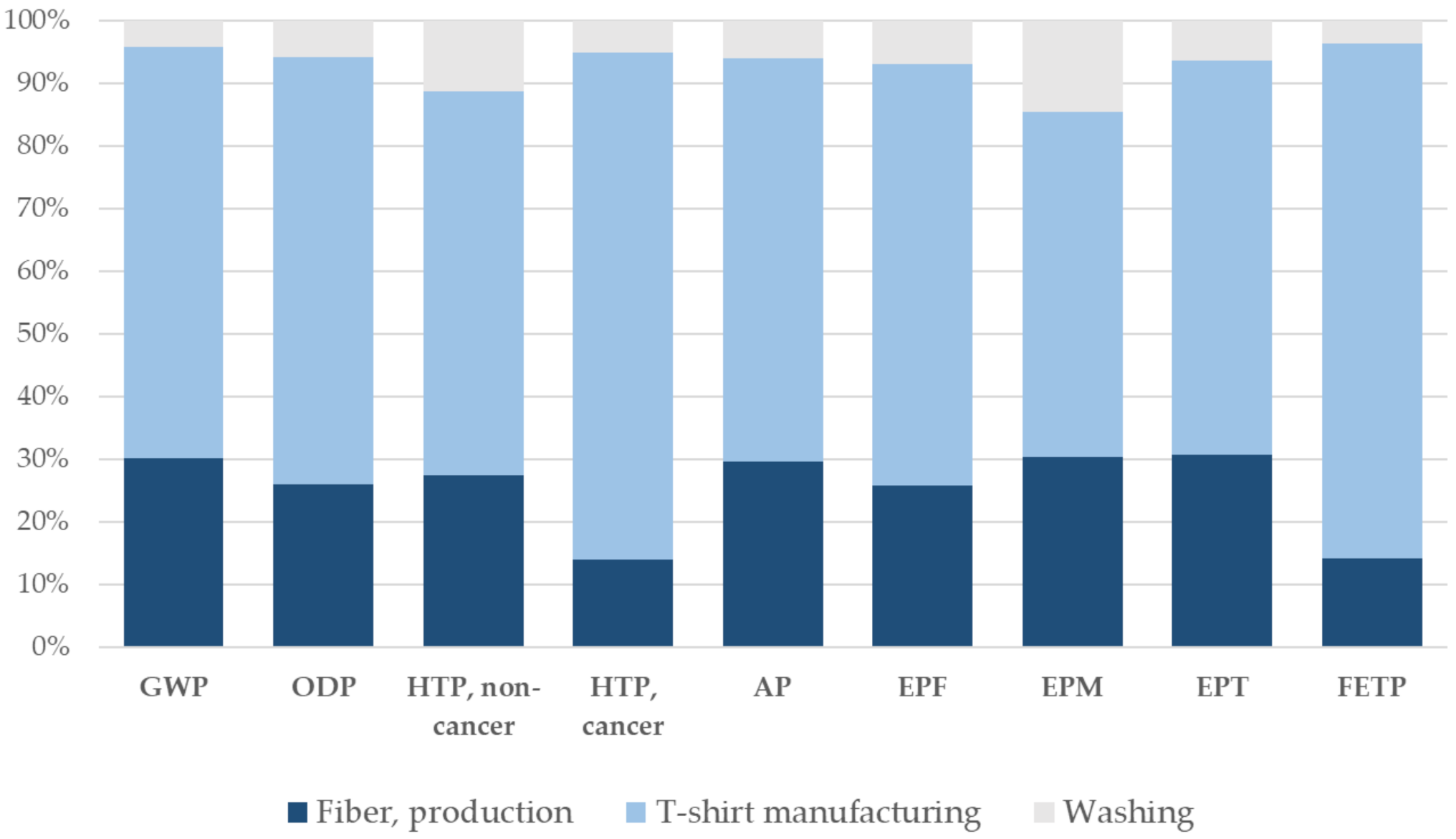
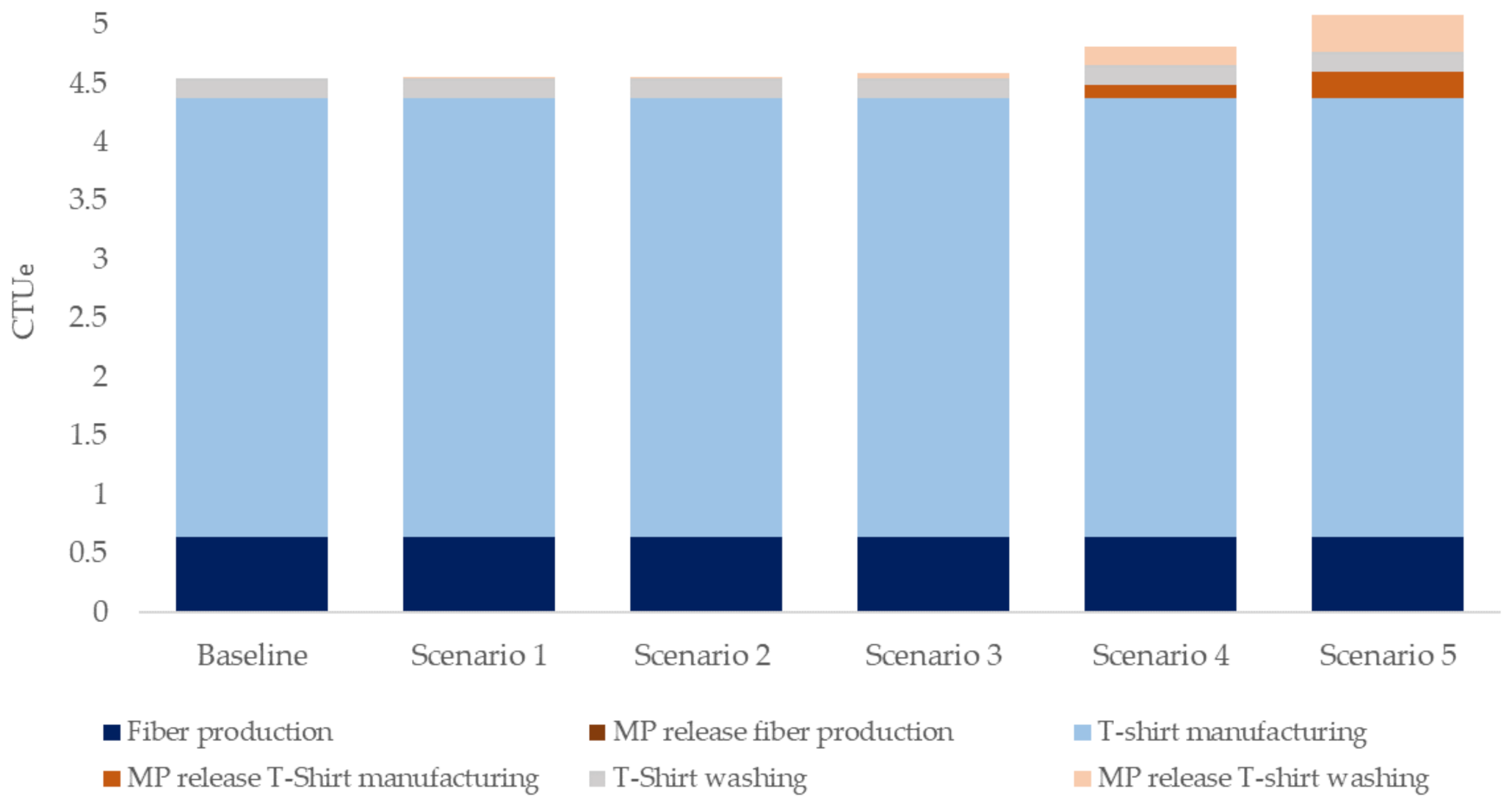
3.2. Life Cycle of a Polyester T-Shirt
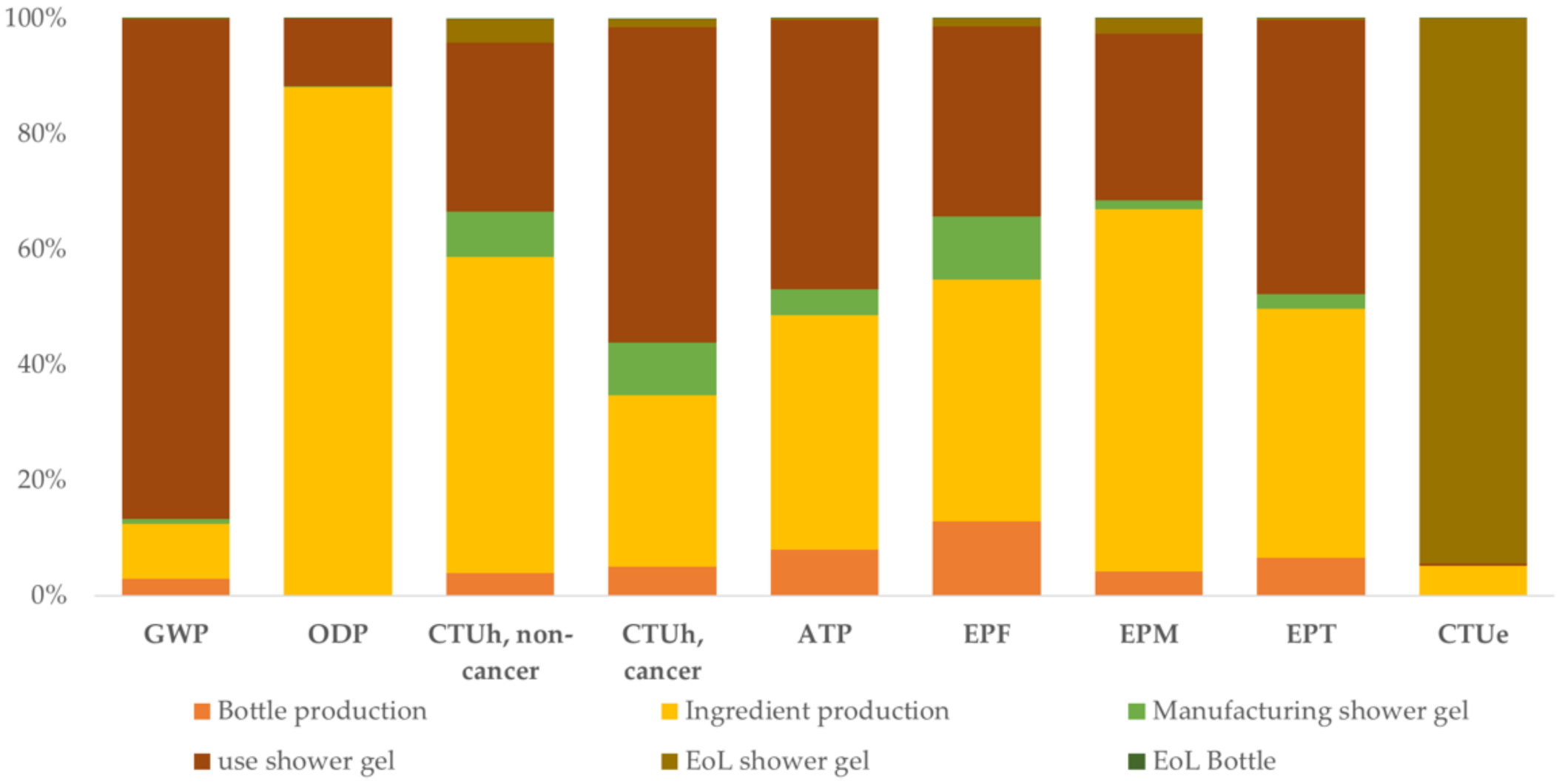
3.3. Life Cycle of a Shower Gel Containing Microplastics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akdogan, Z.; Guven, B. Microplastics in the environment: A critical review of current understanding and identification of future research needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawecki, D.; Nowack, B. Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics As Macro- and Microplastics. Environ. Sci. Technol. 2019, 53, 9664–9676. [Google Scholar] [CrossRef]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Su, L.; Craig, N.J.; Du, F.; Wu, C.; Shi, H. Comparison of microplastic pollution in different water bodies from urban creeks to coastal waters. Environ. Pollut. 2019, 246, 174–182. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of microplastics in freshwater systems: A review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Closing the Loop-An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Postlethwaite, D. Recent Developments in LCA and Critical Issues. In Environmental Toxicology and Risk Assessment: Third Volume; Hughes, J.S., Biddinger, G.R., Mones, E., Eds.; ASTM International: West Conshohocken, PA, USA, 1995; pp. 332–347. ISBN 978-0-8031-5292-2. [Google Scholar]
- Boustead, I. Eco-Balance Methodology for Commodity Thermoplastics; Association of Plastics Manufacturers in Europe: Brussels, Belgium, 1992. [Google Scholar]
- Cordella, M.; Bauer, I.; Lehmann, A.; Schulz, M.; Wolf, O. Evolution of disposable baby diapers in Europe: Life cycle assessment of environmental impacts and identification of key areas of improvement. J. Clean. Prod. 2015, 95, 322–331. [Google Scholar] [CrossRef]
- Accorsi, R.; Cascini, A.; Cholette, S.; Manzini, R.; Mora, C. Economic and environmental assessment of reusable plastic containers: A food catering supply chain case study. Int. J. Prod. Econ. 2014, 152, 88–101. [Google Scholar] [CrossRef]
- Accorsi, R.; Versari, L.; Manzini, R. Glass vs. plastic: Life cycle assessment of extra-virgin olive oil bottles across global supply chains. Sustainability 2015, 7, 2818–2840. [Google Scholar] [CrossRef] [Green Version]
- Lazarevic, D.; Aoustin, E.; Buclet, N.; Brandt, N. Plastic waste management in the context of a European recycling society: Comparing results and uncertainties in a life cycle perspective. Resour. Conserv. Recycl. 2010, 55, 246–259. [Google Scholar] [CrossRef]
- Rigamonti, L.; Grosso, M.; Møller, J.; Sanchez, V.M.; Magnani, S.; Christensen, T.H. Environmental evaluation of plastic waste management scenarios. Resour. Conserv. Recycl. 2014, 85, 42–53. [Google Scholar] [CrossRef]
- Hou, P.; Xu, Y.; Taiebat, M.; Lastoskie, C.; Miller, S.A.; Xu, M. Life cycle assessment of end-of-life treatments for plastic film waste. J. Clean. Prod. 2018, 201, 1052–1060. [Google Scholar] [CrossRef]
- Henry, B.; Laitala, K.; Klepp, I.G. Microfibres from apparel and home textiles: Prospects for including microplastics in environmental sustainability assessment. Sci. Total Environ. 2019, 652, 483–494. [Google Scholar] [CrossRef]
- Saling, P.; Gyuzeleva, L.; Wittstock, K.; Wessolowski, V.; Griesshammer, R. Life cycle impact assessment of microplastics as one component of marine plastic debris. Int. J. Life Cycle Assess. 2020, 25, 2008–2026. [Google Scholar] [CrossRef]
- Hernandez, E.; Nowack, B.; Mitrano, D.M. Polyester Textiles as a Source of Microplastics from Households: A Mechanistic Study to Understand Microfiber Release during Washing. Environ. Sci. Technol. 2017, 51, 7036–7046. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, T.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. Systematic Study of Microplastic Fiber Release from 12 Different Polyester Textiles during Washing. Environ. Sci. Technol. 2020, 54, 4847–4855. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.D.; Mossotti, R.; Montarsolo, A. Assessment of microplastics release from polyester fabrics: The impact of different washing conditions. Environ. Pollut. 2020, 264, 113960. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.J.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox—the UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Recommendation 2013/179/EU on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations. Off. J. Eur. Union 2013, 124, 210. [Google Scholar]
- Ettrup, K.; Kounina, A.; Hansen, S.F.; Meesters, J.A.J.; Vea, E.B.; Laurent, A. Development of Comparative Toxicity Potentials of TiO2 Nanoparticles for Use in Life Cycle Assessment. Environ. Sci. Technol. 2017, 51, 4027–4037. [Google Scholar] [CrossRef] [Green Version]
- Salieri, B.; Hischier, R.; Quik, J.T.K.; Jolliet, O. Fate modelling of nanoparticle releases in LCA: An integrative approach towards “USEtox4Nano”. J. Clean. Prod. 2019, 206, 701–712. [Google Scholar] [CrossRef]
- Salieri, B.; Righi, S.; Pasteris, A.; Olsen, S.I. Freshwater ecotoxicity characterisation factor for metal oxide nanoparticles: A case study on titanium dioxide nanoparticle. Sci. Total Environ. 2015, 505, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Adam, V.; Yang, T.; Nowack, B. Toward an ecotoxicological risk assessment of microplastics: Comparison of available hazard and exposure data in freshwaters. Environ. Toxicol. Chem. 2019, 38, 436–447. [Google Scholar] [CrossRef] [Green Version]
- Fantke, P. USEtox 2.0 Documentation (Version 1); USEtox® International Center: Lyngby, Denmark, 2017. [Google Scholar]
- Eckelman, M.J.; Mauter, M.S.; Isaacs, J.A.; Elimelech, M. New perspectives on nanomaterial aquatic ecotoxicity: Production impacts exceed direct exposure impacts for carbon nanotoubes. Environ. Sci. Technol. 2012, 46, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Schmutz, M.; Hischier, R.; Som, C. Factors allowing users to influence the environmental performance of their T-shirt. Sustainability 2021, 13, 2498. [Google Scholar] [CrossRef]
- Golsteijn, L.; Lessard, L.; Campion, J.F.; Capelli, A.; D’Enfert, V.; King, H.; Kremer, J.; Krugman, M.; Orliac, H.; Furnemont, S.R.; et al. Developing Product Environmental Footprint Category Rules (PEFCR) for shampoos: The basis for comparable life cycle assessment. Integr. Environ. Assess. Manag. 2018, 14, 649–659. [Google Scholar] [CrossRef] [PubMed]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Environ. Sci. Pollut. Res. 2017, 24, 19313–19321. [Google Scholar] [CrossRef]
- Lehmann, A.; Roffeis, M.; Finkbeiner, M. Ökobilanzielle Bewertung des Lebensweges Eines Handelsüblichen weißen Baumwolle T-Shirts in Deutschland; Technische Universität Berlin: Berlin, Germany, 2019. [Google Scholar]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Schmiedgruber, M.; Hufenus, R.; Mitrano, D.M. Mechanistic understanding of microplastic fiber fate and sampling strategies: Synthesis and utility of metal doped polyester fibers. Water Res. 2019, 155, 423–430. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Hunt, C.F.; Lin, W.H.; Voulvoulis, N. Evaluating alternatives to plastic microbeads in cosmetics. Nat. Sustain. 2020, 4, 366–372. [Google Scholar] [CrossRef]
- Gouin, T.; Avalos, J.; Brunning, I.; Brzuska, K.; De Graaf, J.; Kaumanns, J.; Koning, T.; Meyberg, M.; Rettinger, K.; Schlatter, H.; et al. Use of Micro-Plastic Beads in Cosmetic Products in Europe and Their Estimated Emissions to the North Sea Environment. SOFW J. 2015, 141, 40–46. [Google Scholar]
- European Chemicals Agency. Annex XV Restriction Report—Proposal for a Restriction: Intentionally Added Microplastics; European Chemicals Agency: Helsinki, Finland, 2019.
- Kentin, E.; Kaarto, H. An EU ban on microplastics in cosmetic products and the right to regulate. Rev. Eur. Comp. Int. Environ. Law 2018, 27, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Culliney, K. REACH Microplastics Restriction: Worst-Case and Best-Case Scenarios ‘Already Clear’, Says Cosmetics Europe Chief. Available online: https://www.cosmeticsdesign-europe.com/Article/2020/06/18/ECHA-microplastics-restriction-impact-on-beauty-and-personal-care-clear-says-Cosmetics-Europe (accessed on 11 October 2020).
- Lei, K.; Qiao, F.; Liu, Q.; Wei, Z.; Qi, H.; Cui, S.; Yue, X.; Deng, Y.; An, L. Microplastics releasing from personal care and cosmetic products in China. Mar. Pollut. Bull. 2017, 123, 122–126. [Google Scholar] [CrossRef]
- Aurisano, N.; Albizzati, P.F.; Hauschild, M.; Fantke, P. Extrapolation Factors for Characterizing Freshwater Ecotoxicity Effects. Environ. Toxicol. Chem. 2019, 38, 2568–2582. [Google Scholar] [CrossRef] [PubMed]
- Wigger, H.; Kawecki, D.; Nowack, B.; Adam, V. Systematic Consideration of Parameter Uncertainty and Variability in Probabilistic Species Sensitivity Distributions. Integr. Environ. Assess. Manag. 2020, 16, 211–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Xiao, R.; Yuan, Z. Life cycle assessment of cotton T-shirts in China. Int. J. Life Cycle Assess. 2015, 20, 994–1004. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Adam, V.; von Wyl, A.; Nowack, B. Probabilistic environmental risk assessment of microplastics in marine habitats. Aquat. Toxicol. 2021, 230, 105689. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nowack, B. A Meta-analysis of Ecotoxicological Hazard Data for Nanoplastics in Marine and Freshwater Systems. Environ. Toxicol. Chem. 2020, 39, 2588–2598. [Google Scholar] [CrossRef]
- de Klein, J.J.M.; Quik, J.T.K.; Bäuerlein, P.S.; Koelmans, A.A. Towards validation of the NanoDUFLOW nanoparticle fate model for the river Dommel, The Netherlands. Environ. Sci. Nano 2016, 3, 434–441. [Google Scholar] [CrossRef]
- Sheng, C.; Zhang, S.; Zhang, Y. The influence of different polymer types of microplastics on adsorption, accumulation, and toxicity of triclosan in zebrafish. J. Hazard. Mater. 2021, 402, 123733. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic microfiber emissions to land rival those to waterbodies and are growing. PLoS ONE 2020, 15, e0237839. [Google Scholar] [CrossRef] [PubMed]


| Life Cycle Stage | MP (kg) to WWTP | MP (kg) to Freshwater (WWTP Removal 97.6%) | MP (kg) to Freshwater (WWTP Removal 50%) | MP (kg) to Freshwater (No WWTP) |
|---|---|---|---|---|
| Fiber production of one T-shirt | 2.40 × 10−7 | 5.74 × 10−9 | 1.20 × 10−7 | 2.40 × 10−7 |
| Manufacturing of one T-shirt | 6.91 × 10−5 | 1.65 × 10−6 | 3.46 × 10−5 | 6.91 × 10−5 |
| Washing of one T-shirt (44 cycles) | 6.20 × 10−5 | 1.48 × 10−6 | 3.10 × 10−5 | 6.20 × 10−5 |
| KdegW (s−1) | FF (Days) | EF (PAF m3 kg−1) | XF (-) | CF (PAF m3 d kg−1) | |
|---|---|---|---|---|---|
| Fast degradation | 1.6 × 10-8 | 120 | 22.6 | 1 | 2712 |
| Mean degradation | 5.8 × 10-10 | 142 | 22.6 | 1 | 3209 |
| No degradation | 0 | 143 | 22.6 | 1 | 3231 |
| Scenario | MP Release | WWTP Removal | |
|---|---|---|---|
| Baseline | 0 | 0 | |
| 1 | Mean release | 0.04% | 97.6% |
| 2 | Worst-case release | 0.06% | 97.6% |
| 3 | 1% increase of ecotoxicity impacts | 0.33% | 97.6% |
| 4 | WWTP removal 50% | 0.06% | 50% |
| 5 | No WWTP removal | 0.06% | 0% |
| Baseline | WWTP 0% | WWTP 50% | WWTP 97% | |
|---|---|---|---|---|
| Bottle production | 0.039 | |||
| Ingredients production | 2.48 | |||
| Manufacturing shower gel | 0.054 | |||
| Use shower gel | 0.19 | |||
| EoL shower gel | 46.9 | 61.8 | 54.3 | 47.2 |
| EoL Bottle | 0.005 | |||
| Total | 49.6 | 64.5 | 57.1 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salieri, B.; Stoudmann, N.; Hischier, R.; Som, C.; Nowack, B. How Relevant Are Direct Emissions of Microplastics into Freshwater from an LCA Perspective? Sustainability 2021, 13, 9922. https://doi.org/10.3390/su13179922
Salieri B, Stoudmann N, Hischier R, Som C, Nowack B. How Relevant Are Direct Emissions of Microplastics into Freshwater from an LCA Perspective? Sustainability. 2021; 13(17):9922. https://doi.org/10.3390/su13179922
Chicago/Turabian StyleSalieri, Beatrice, Natasha Stoudmann, Roland Hischier, Claudia Som, and Bernd Nowack. 2021. "How Relevant Are Direct Emissions of Microplastics into Freshwater from an LCA Perspective?" Sustainability 13, no. 17: 9922. https://doi.org/10.3390/su13179922
APA StyleSalieri, B., Stoudmann, N., Hischier, R., Som, C., & Nowack, B. (2021). How Relevant Are Direct Emissions of Microplastics into Freshwater from an LCA Perspective? Sustainability, 13(17), 9922. https://doi.org/10.3390/su13179922






