Iron and Aluminium Production Wastes as Exclusive Components of Alkali Activated Binders—Towards a Sustainable Alternative
Abstract
:1. Introduction
2. Experimental Plan
2.1. Materials
2.2. Preparation and Mechanical Testing
3. Results and Discussion
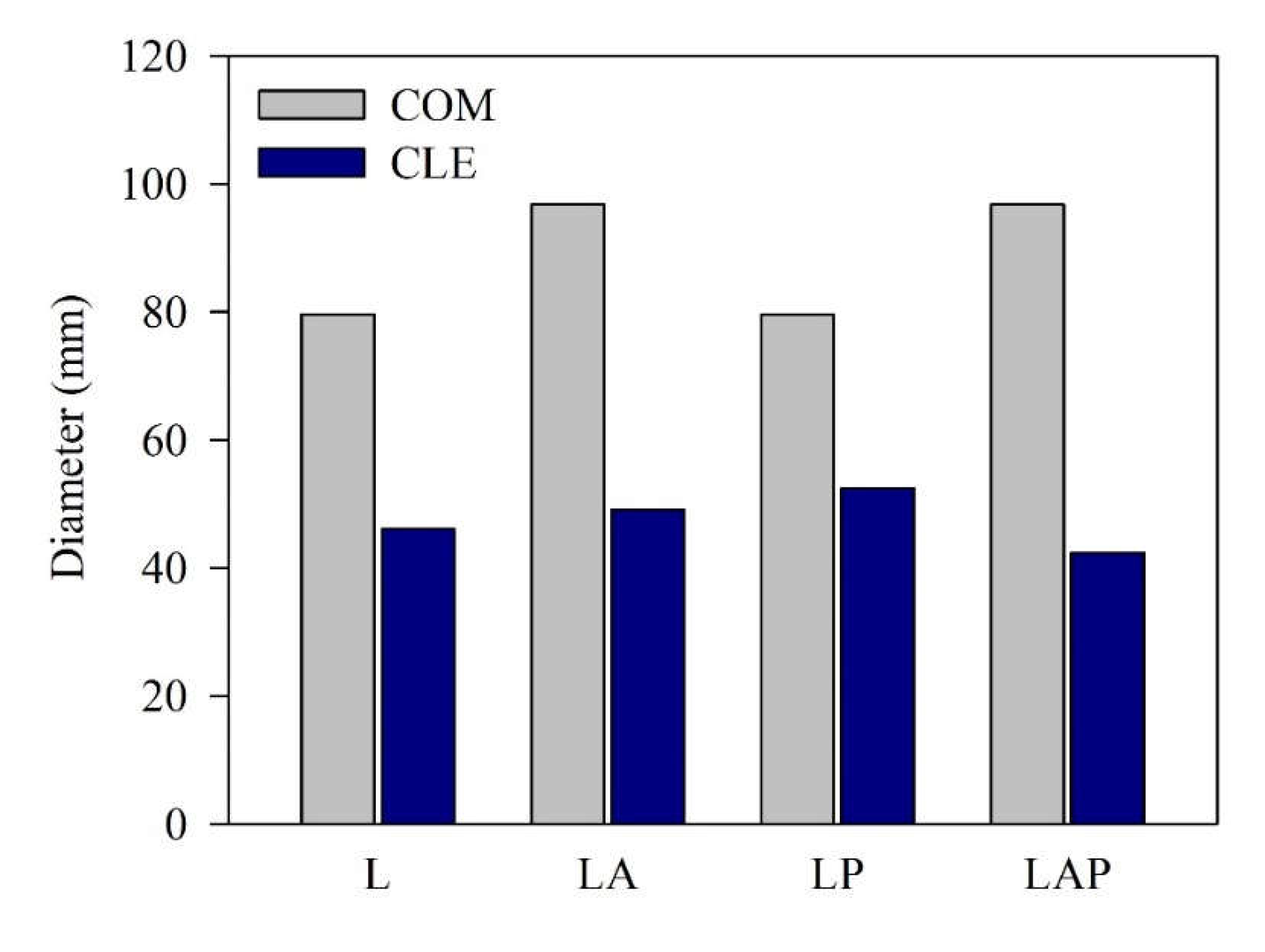
3.1. Workability
3.2. Heat of Hydration
3.2.1. Pastes Fabricated with COM
3.2.2. Pastes Fabricated with CLE
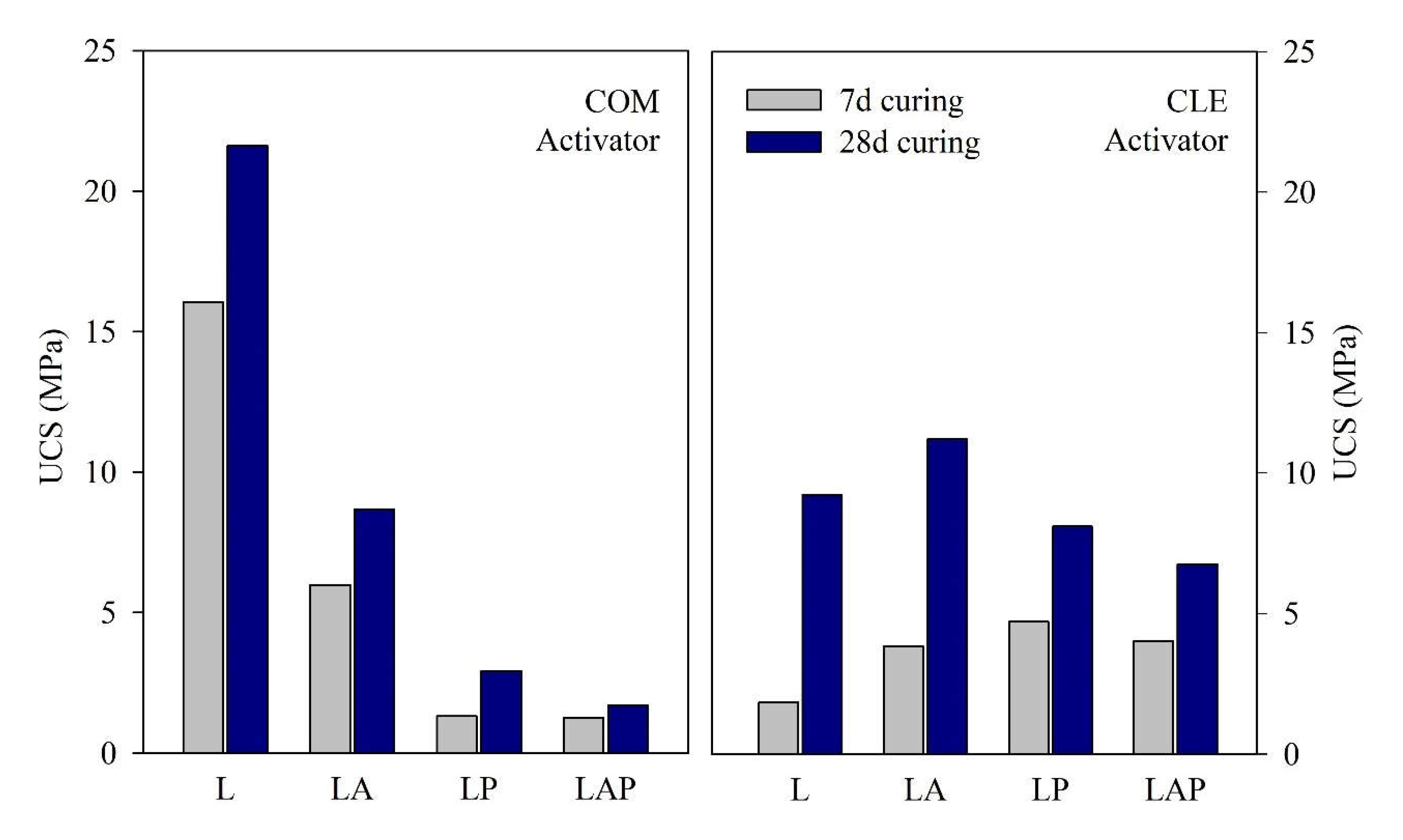
3.3. Uniaxial Compressive Strength
3.4. Microstructure and Mineralogy
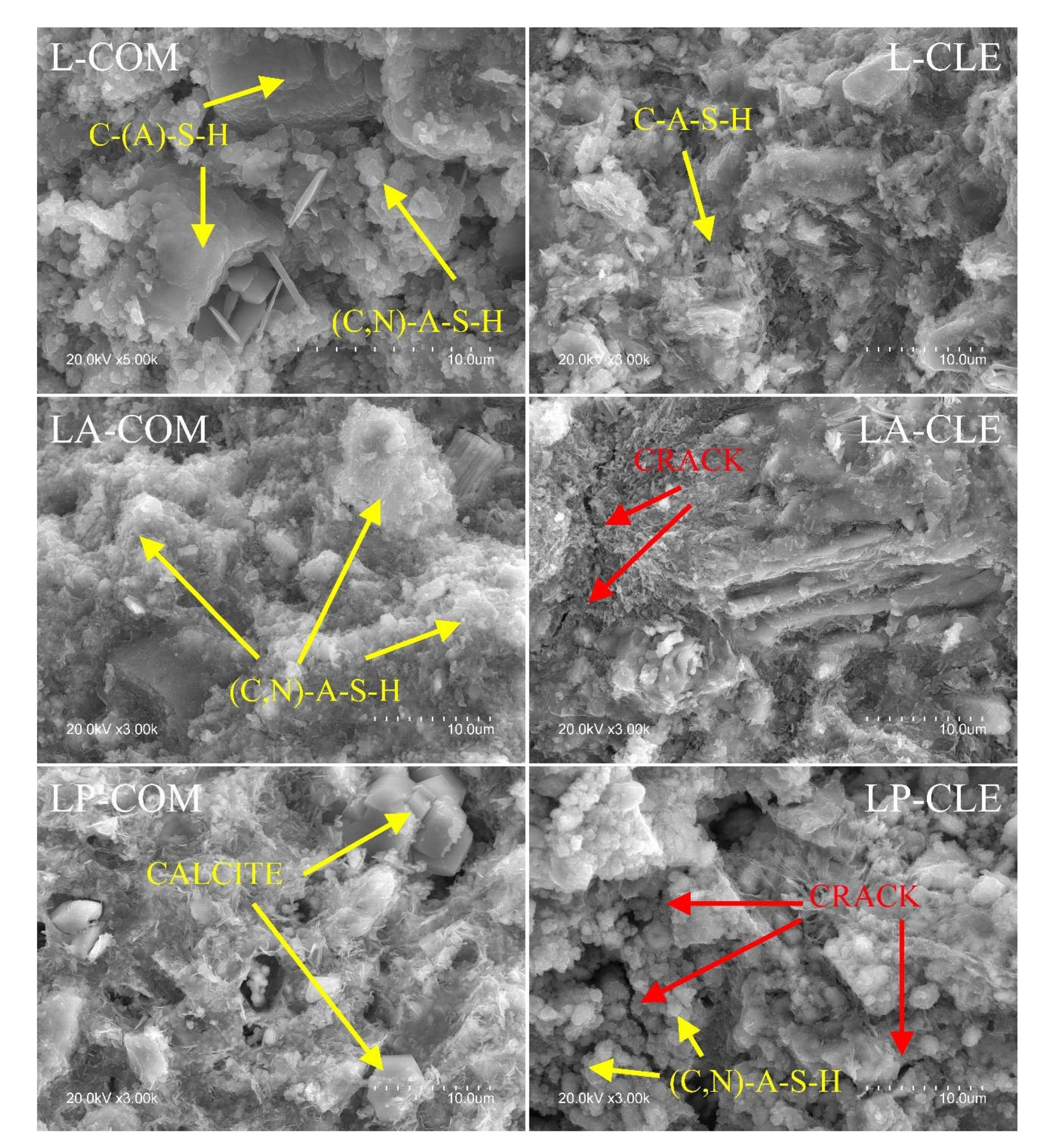
3.4.1. SEM-EDX
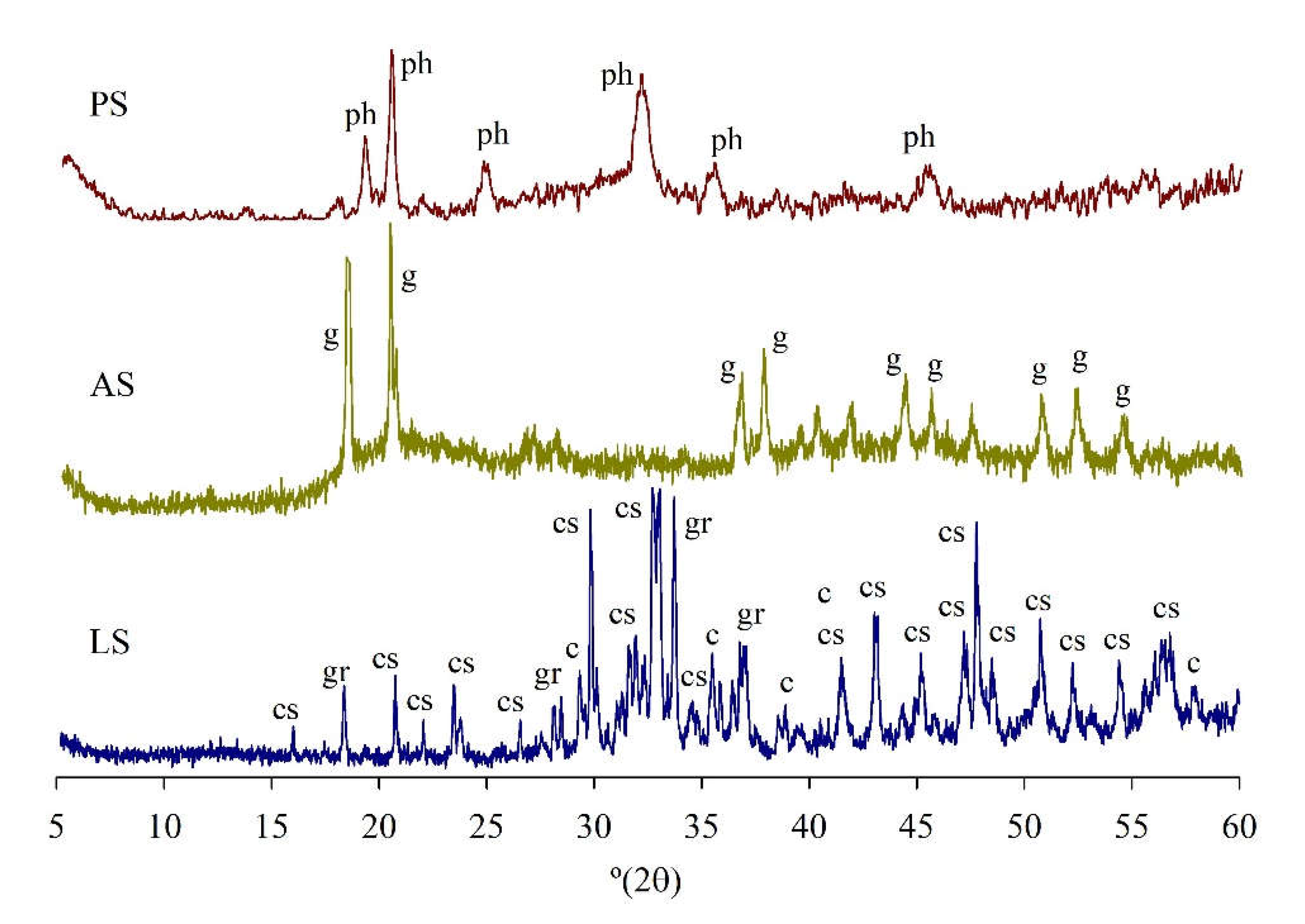
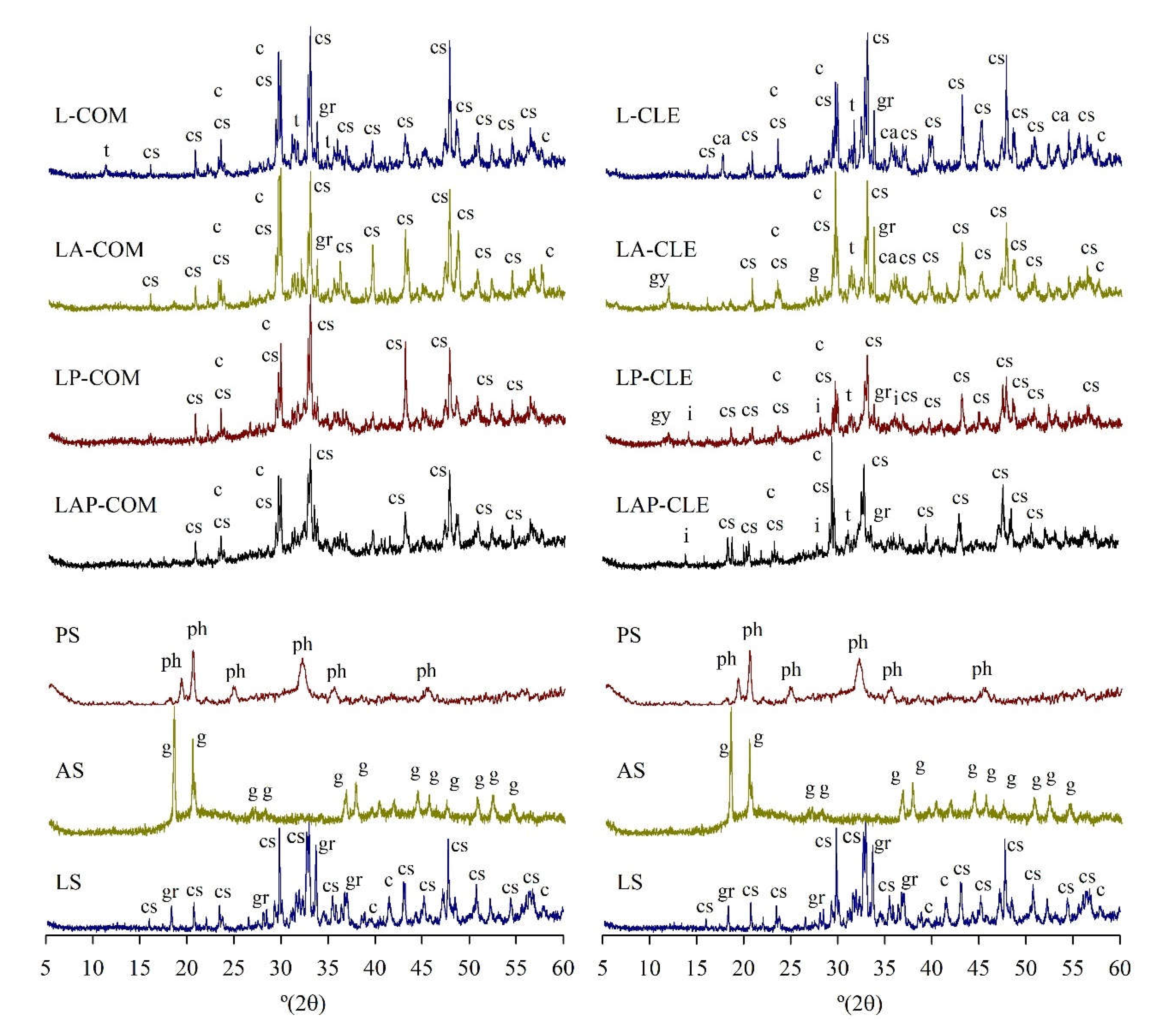
3.4.2. XRD
4. Conclusions
- A similar accumulated heat was obtained for the pastes activated by CLE (60–80 J/g) and COM (65–80 J/g). This suggests that their degree of reaction is close.
- The maximum compressive strength was recorded with the L-COM (25 MPa). The replacement of the LS with AS and PS generally reduced the UCS in the pastes activated with COM, with the PS producing higher strength reductions than the AS. With curing time, there was a shift in the most performing pastes, with the maximum strength activated by the CLE-based formulations (5× higher than the COM pastes).
- Using different alkali activators significantly changed the type of gel obtained with the reference pastes, resulting in a difference of approximately 16 MPa between the L-COM and L-CLE pastes.
- Replacing LS with AS or PS in the CLE systems resulted in microcracks in the matrix.
- The mineralogical results showed that the use of PS or/and AS, or the different alkali activators did not significantly change the crystallography of the pastes.
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. Global Sustainable Development Report 2016 Edition; United Nations: New York, NY, USA, 2016.
- Habert, G. Environmental impact of Portland cement production. In Eco-Efficient Concrete; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–25. [Google Scholar] [CrossRef]
- Kurda, R.; Silvestre, J.D.; de Brito, J. Toxicity and environmental and economic performance of fly ash and recycled concrete aggregates use in concrete: A review. Heliyon 2018, 4, e00611. [Google Scholar] [CrossRef]
- Naik, T.R. Sustainability of Concrete Construction. Pract. Period. Struct. Des. Constr. 2008, 13, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.K. Influence of partial replacement of cement by industrial wastes on properties of concrete. In Lecture Notes in Civil Engineering. Springer Science and Business Media Deutschland GmbH; Springer: Singapore, 2021; pp. 693–713. [Google Scholar] [CrossRef]
- Siddique, R.; Kaur, D. Properties of concrete containing ground granulated blast furnace slag (GGBFS) at elevated temperatures. J. Adv. Res. 2012, 3, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Sua-Iam, G.; Makul, N. Utilization of high volumes of unprocessed lignite-coal fly ash and rice husk ash in self-consolidating concrete. J. Clean. Prod. 2014, 78, 184–194. [Google Scholar] [CrossRef]
- Serjun, V.Z.; Mladenovič, A.; Mirtič, B.; Meden, A.; Ščančar, J.; Milačič, R. Recycling of ladle slag in cement composites: Environmental impacts. Waste Manag. 2015, 43, 376–385. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Kannan, D.M.; Aboubakr, S.H.; EL-Dieb, A.S.; Reda Taha, M.M. High performance concrete incorporating ceramic waste powder as large partial replacement of Portland cement. Constr. Build. Mater. 2017, 144, 35–41. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Torres-Carrasco, M.; Rodríguez-Puertas, C.; Del Mar Alonso, M.; Puertas, F. Alkali activated slag cements using waste glass as alternative activators. Rheol. Behav. 2015, 54, 45–57. [Google Scholar]
- Imtiaz, L.; Ur Rehman, S.K.; Memon, S.A.; Khan, M.K.; Javed, M.F. A review of recent developments and advances in eco-friendly geopolymer concrete. Appl. Sci. 2020, 10, 7838. [Google Scholar] [CrossRef]
- Xie, J.; Chen, W.; Wang, J.; Fang, C.; Zhang, B.; Liu, F. Coupling effects of recycled aggregate and GGBS/metakaolin on physicochemical properties of geopolymer concrete. Constr. Build. Mater. 2019, 226, 345–359. [Google Scholar] [CrossRef]
- Kaya-Özkiper, K.; Uzun, A.; Soyer-Uzun, S. Red mud- and metakaolin-based geopolymers for adsorption and photocatalytic degradation of methylene blue: Towards self-cleaning construction materials. J. Clean. Prod. 2021, 288, 125120. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in construction—Recent developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Mymrin, V.; Pedroso, D.E.; Pedroso, C.; Alekseev, K.; Avanci, M.A.; Winter, E.; Cechin, L.; Rolim, P.H.B.; Iarozinski, A.; Catai, R.E. Environmentally clean composites with hazardous aluminum anodizing sludge, concrete waste, and lime production waste. J. Clean. Prod. 2018, 174, 380–388. [Google Scholar] [CrossRef]
- Taki, K.; Mukherjee, S.; Patel, A.K.; Kumar, M. Reappraisal review on geopolymer: A new era of aluminosilicate binder for metal immobilization. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100345. [Google Scholar] [CrossRef]
- El Alouani, M.; Saufi, H.; Moutaoukil, G.; Alehyen, S.; Nematollahi, B.; Belmaghraoui, W.; Taibi, M. Application of geopolymers for treatment of water contaminated with organic and inorganic pollutants: State-of-the-art review. J. Environ. Chem. Eng. 2021, 9, 105095. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Khahro, S.H.; Low, A.; Ayoub, M. Optimization of synthesis of geopolymer adsorbent for the effective removal of anionic surfactant from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 104949. [Google Scholar] [CrossRef]
- Nguyen, H.; Adesanya, E.; Ohenoja, K.; Kriskova, L.; Pontikes, Y.; Kinnunen, P.; Illikainen, M. Byproduct-based ettringite binder—A synergy between ladle slag and gypsum. Constr. Build. Mater. 2019, 197, 143–151. [Google Scholar] [CrossRef]
- Nguyen, H.; Carvelli, V.; Adesanya, E.; Kinnunen, P.; Illikainen, M. High performance cementitious composite from alkali-activated ladle slag reinforced with polypropylene fibers. Cem. Concr. Compos. 2018, 90, 150–160. [Google Scholar] [CrossRef]
- Wang, W.C.; Wang, H.Y.; Tsai, H.C. Study on engineering properties of alkali-activated ladle furnace slag geopolymer. Constr. Build. Mater. 2016, 123, 800–805. [Google Scholar] [CrossRef]
- International Aluminium Institute World Aluminium: Primary Aluminium Production. Available online: https://www.world-aluminium.org/statistics/ (accessed on 20 February 2021).
- Ribeiro, M.J.; Labrincha, J.A. Properties of sintered mullite and cordierite pressed bodies manufactured using Al-rich anodising sludge. Ceram. Int. 2008, 34, 593–597. [Google Scholar] [CrossRef]
- Mymrin, V.; Molinetti, A.; Alekseev, K.; Avanci, M.A.; Klitzke, W.; Silva, D.A.; Ferraz, F.A.; Iarozinski, N.A.; Catai, R.E. Characterization of construction materials on the base of mortar waste, activated by aluminum anodization sludge and lime production waste. Constr. Build. Mater. 2019, 212, 202–209. [Google Scholar] [CrossRef]
- Onutai, S.; Jiemsirilers, S.; Thavorniti, P.; Kobayashi, T. Aluminium hydroxide waste based geopolymer composed of fly ash for sustainable cement materials. Constr. Build. Mater. 2015, 101, 298–308. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, L.; Ramey, D.; Waterman, B.; Ormsby, S. Utilization of aluminum sludge (AS) to enhance mine tailings-based geopolymer. J. Mater. Sci. 2015, 50, 1370–1381. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, Á. Sustainable alkali activated materials: Precursor and activator derived from industrial wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Ogundiran, M.B.; Nugteren, H.W.; Witkamp, G.J. Geopolymerisation of fly ashes with waste aluminium anodising etching solutions. J. Environ. Manag. 2016, 181, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristelo, N.; Fernández-Jiménez, A.; Castro, F.; Fernandes, L.; Tavares, P. Sustainable alkaline activation of fly ash, aluminium anodising sludge and glass powder blends with a recycled alkaline cleaning solution. Constr. Build. Mater. 2019, 204, 609–620. [Google Scholar] [CrossRef]
- Gupta, R.; Singhal, A.; Devi, A.; Verma, S.K. A Study on Application of Pickling Sludge in Pavements Tiles. In Proceedings of the 3rd International Conference of Recent Trends in Environmental Science and Engineering (RTESE’19), Ottawa, ON, Canada, 11–12 June 2019. [Google Scholar] [CrossRef]
- Muliawan, J.; Astutiningsih, S. Preparation and characterization of Phosphate-Sludge kaolin mixture for ceramics bricks. Int. J. Technol. 2018, 9, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.L.; Lin, D.F.; Luo, H.L. Influence of phosphate of the waste sludge on the hydration characteristics of eco-cement. J. Hazard. Mater. 2009, 168, 1105–1110. [Google Scholar] [CrossRef]
- Doǧan, Ö.; Karpuzcu, M. Recovery of Phosphate Sludge as Concrete Supplementary Material. Clean-Soil Air Water 2010, 38, 977–980. [Google Scholar] [CrossRef]
- Bersch, R.; Brehm, F.; Kazmierczak, C. Changes in Ceramic Blocks Porosity with Phosphate Sludge due to Salt Crystallization WASCON 2012 towards Effective, Durable and Sustainable Production and Use of Alternative Materials in Construction; Swedish Geotechnical Institute and The International Society for the Environmental and Technical Implications of Construction with Alternative Materials: Gothenburg, Sweden, 2012. [Google Scholar]
- UNE 80103 UNE 80103:2013—Metodos de Ensayo de Cementos. Ensayos físicos. Determinación de la densidade real. AENOR—Asoc. Española Norm. y Certificación 2013. [Google Scholar]
- Pacheco-Torgal, F.; Castrogomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef] [Green Version]
- Puertas, F.; Varga, C.; Alonso, M.M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Achtemichuk, S.; Hubbard, J.; Sluce, R.; Shehata, M.H. The utilization of recycled concrete aggregate to produce controlled low-strength materials without using Portland cement. Cem. Concr. Compos. 2009, 31, 564–569. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; MacPhee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Chung, C.W.; Chun, J.; Wang, G.; Um, W. Effects of iron oxides on the rheological properties of cementitious slurry. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 453, 94–100. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Banthia, N.; Zhang, Y.; Zhang, Z. Interpreting the early-age reaction process of alkali-activated slag by using combined embedded ultrasonic measurement, thermal analysis, XRD, FTIR and SEM. Compos. Part B Eng. 2020, 186, 107840. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]




| Element | LS | AS | PS |
|---|---|---|---|
| Na2O | 0.13 | 2.4 | 3.6 |
| SiO2 | 18.79 | 0.3 | - |
| Al2O3 | 7.39 | - | - |
| Al(OH)3 | - | 94.1 | - |
| MgO | 5.41 | - | - |
| K2O | 0.03 | - | - |
| CaO | 49.48 | - | 1.0 |
| TiO2 | 0.40 | - | - |
| Fe2O3 | 10.07 | - | 35.2 |
| ZnO | 1.25 | - | 12.9 |
| MnO | 1.43 | - | - |
| BaO | 0.10 | - | - |
| P2O5 | - | - | 43.7 |
| SO3 | 3.42 | 3.0 | - |
| P2O5 | 0.07 | - | - |
| Cl | 0.05 | - | 2.4 |
| Cr2O3 | 0.68 | - | - |
| CuO | 0.03 | - | - |
| F | 1.20 | - | - |
| Paste ID | Precursors (Solids) | Activator/Solids (A/S) | ||
|---|---|---|---|---|
| LS | AS | PS | ||
| (wt.%) | (wt.%) | (wt.%) | (wt. Ratio) | |
| L-COM | 100 | 0 | 0 | 0.40 |
| LA-COM | 90 | 10 | 0 | 0.50 |
| LP-COM | 90 | 0 | 10 | 0.75 |
| LAP-COM | 80 | 10 | 10 | 0.55 |
| L-CLE | 100 | 0 | 0 | 0.40 |
| LA-CLE | 90 | 10 | 0 | 0.50 |
| LP-CLE | 90 | 0 | 10 | 0.85 |
| LAP-CLE | 80 | 10 | 10 | 0.85 |
| Paste ID | Atomic Molar Ratios | ||||
|---|---|---|---|---|---|
| Al2O3/SiO2 | Al2O3/Na2O | CaO/SiO2 | Na2O/CaO | MgO/Al2O3 | |
| L-COM | 0.28 | 1.08 | 4.43 | 0.16 | 0.67 |
| LA-COM | 0.63 | 4.25 | 3.47 | 0.19 | 0.18 |
| LP-COM | 0.32 | 0.98 | 5.75 | 0.18 | 0.49 |
| L-CLE | 0.21 | 1.67 | 4.79 | 0.07 | 0.48 |
| LA-CLE | 0.40 | 1.79 | 2.85 | 0.19 | 0.26 |
| LP-CLE | 0.21 | 0.42 | 2.67 | 0.31 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristelo, N.; Castro, F.; Miranda, T.; Abdollahnejad, Z.; Fernández-Jiménez, A. Iron and Aluminium Production Wastes as Exclusive Components of Alkali Activated Binders—Towards a Sustainable Alternative. Sustainability 2021, 13, 9938. https://doi.org/10.3390/su13179938
Cristelo N, Castro F, Miranda T, Abdollahnejad Z, Fernández-Jiménez A. Iron and Aluminium Production Wastes as Exclusive Components of Alkali Activated Binders—Towards a Sustainable Alternative. Sustainability. 2021; 13(17):9938. https://doi.org/10.3390/su13179938
Chicago/Turabian StyleCristelo, Nuno, Fernando Castro, Tiago Miranda, Zahra Abdollahnejad, and Ana Fernández-Jiménez. 2021. "Iron and Aluminium Production Wastes as Exclusive Components of Alkali Activated Binders—Towards a Sustainable Alternative" Sustainability 13, no. 17: 9938. https://doi.org/10.3390/su13179938
APA StyleCristelo, N., Castro, F., Miranda, T., Abdollahnejad, Z., & Fernández-Jiménez, A. (2021). Iron and Aluminium Production Wastes as Exclusive Components of Alkali Activated Binders—Towards a Sustainable Alternative. Sustainability, 13(17), 9938. https://doi.org/10.3390/su13179938







