A New Process for the Recovery of Ammonia from Ammoniated High-Salinity Brine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermodynamic Evaluation for the Recovery of Ammonium Chloride
2.2. Thermodynamic Evaluation for the Recovery of Ammonium Hydroxide/Carbonate
2.3. The Preparation of the Ammonium Hydroxide/Carbonate Effluent Solution Based on the Magnesium Recovery Process
2.4. The Preparation of the Ammonium Chloride Effluent Solution Based on the Solvay Process
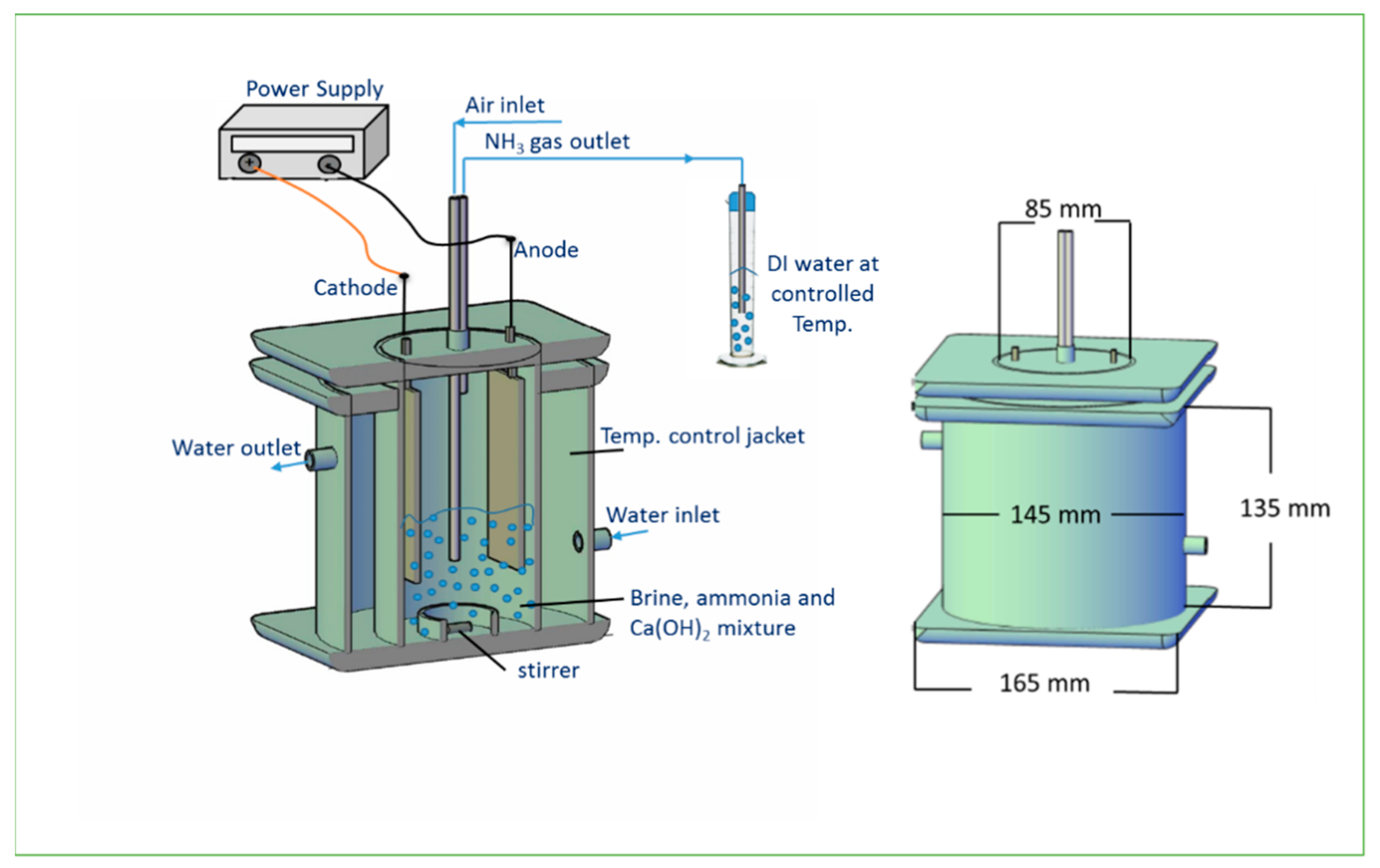
2.5. Experimental Setup
- The anode dissolution, which results in aluminum (metal) electrode ions M+n(aq):M(s) → M+n(aq) + ne−
- Water electrolysis, which results in the generation of hydrogen gas and hydroxide ions:2H2O(aq) + 2e− → H2(g) + 2OH−
- The first step of the coagulant formation in its initial form:M+n(aq) + OH−(aq) → M(OH)2(s)
3. Results and Discussion
3.1. Thermodynamic Analysis for the Recovery of Ammonium Chloride from Ammoniated Brine
3.2. Thermodynamic Analysis for the Recovery of Ammonium Hydroxide/Carbonate
3.3. Ammonia Recovery from the Ammonium Chloride Solution
3.4. Ammonia Recovery from Ammonium Hydroxide/Bicarbonate
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohammad, A.; El-Naas, M.; Al-Marzouqi, A.; Suleiman, M.; Al Musharfy, M. Optimization of magnesium recovery from reject brine for reuse in desalination post-treatment. J. Water Process. Eng. 2019, 31, 100810. [Google Scholar] [CrossRef]
- Idelovitch, E.; Michail, M. CHAPTER 15-Groundwater Recharge for Wastewater Reuse in the Dan Region Project: Summary of Five-Year Experience, 1977–1981. In Artificial Recharge of Groundwater; Asano, T., Ed.; Butterworth-Heinemann: Boston, MA, USA, 1985; pp. 481–507. [Google Scholar]
- Li, W.; Shi, X.; Zhang, S.; Qi, G. Modelling of ammonia recovery from wastewater by air stripping in rotating packed beds. Sci. Total. Environ. 2019, 702, 134971. [Google Scholar] [CrossRef] [PubMed]
- Zarebska, A.; Nieto, D.R.; Christensen, K.; Norddahl, B. Ammonia recovery from agricultural wastes by membrane distillation: Fouling characterization and mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, S.; Khorasani, Z. Ammonia removal from aqueous solutions using hollow-fiber membrane contactors. Chem. Eng. J. 2010, 162, 242–249. [Google Scholar] [CrossRef]
- Xia, Z.; Wang, Q.; She, Z.; Gao, M.; Zhao, Y.; Guo, L.; Jin, C. Nitrogen removal pathway and dynamics of microbial community with the increase of salinity in simultaneous nitrification and denitrification process. Sci. Total. Environ. 2019, 697, 134047. [Google Scholar] [CrossRef]
- Zhao, H.; Mavinic, D.; Oldham, W.; Koch, F. controlling factors for simultaneous nitrification and denitrification in two stage intermittent aeration process treating domestic sweage. Water Res. 1999, 33, 961–970. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Abu Samah, R.; Puteh, M.H.; Ismail, A.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2018, 213, 114–132. [Google Scholar] [CrossRef]
- Hassan, P. Simultaneous Management of Nitrogen and Phosphorus in Dewatered Sludge Liquor by Combining ANAMMOX Process with Struvite Crystallization. UBC Thesis, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, F.M.; Povinelli, J.; Vieira, E.M. Ammonia removal from landfill leachate by air stripping and absorption. Environ. Technol. 2013, 34, 2317–2326. [Google Scholar] [CrossRef]
- Luther, A.K.; Desloover, J.; Fennell, D.; Rabaey, K. Electrochemically driven extraction and recovery of ammonia from human urine. Water Res. 2015, 87, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Liu, J.; Ding, L. Recovery of phosphate and ammonia nitrogen from the anaerobic digestion su-pernatant of activated sludge by chemical precipitation. J. Clean. Prod. 2015, 102, 437–446. [Google Scholar] [CrossRef]
- Dube, P.; Vanotti, M.; Szogi, A.; González, M.C.G. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Koon, J.H.; Kaufman, W.J. Ammonia removal from municipal wastewaters by ion exchange. J. Water Pollut. Control. Fed. 1975, 47, 448–465. [Google Scholar]
- Zhang, C.; Ma, J.; Waite, T.D. The impact of absorbents on ammonia recovery in a capacitive mem-brane stripping system. Chem. Eng. J. 2020, 382, 122851. [Google Scholar] [CrossRef]
- Ranade, V.V.; Bhandari, V.M. Industrial Wastewater Treatment, Recycling and Reuse; Elsevier Science: Oxford, UK, 2014. [Google Scholar]
- Hasanoğlu, A.; Romero, J.; Pérez, B.; Plaza, A. Ammonia removal from wastewater streams through membrane contactors: Ex-perimental and theoretical analysis of operation parameters and configuration. Chem. Eng. J. 2010, 160, 530–537. [Google Scholar] [CrossRef]
- Plunkett, J.W. The Almanac of American Employers 2008; Plunkett Research Limited: Houston, TX, USA, 2007. [Google Scholar]
- Osman, M.A.; El-Naas, M.; Al-Zuhair, S. Electrocoagulation treatment of reject brine effluent from Solvay process. Desalination Water Treat. 2019, 163, 325–335. [Google Scholar] [CrossRef]
- Studies in Environmental Science: Other volumes in this series. In Studies in Environmental Science; Halling-Sorensen, B.; Jorgensen, S.E. (Eds.) Elsevier: Amsterdam, The Netherlands, 1993; pp. v–vi. [Google Scholar]
- Li, X.; Zhao, Q.; Hao, X. Ammonium removal from landfill leachate by chemical precipitation. Waste Manag. 1999, 19, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, T.; Weatherley, L. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Metcalf, I.; Eddy, G.; Tchobanoglous, F.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Education: New York, NY, USA, 2002. [Google Scholar]
- Sprovieri, J.A.S.; de Souza, T.S.O.; Contrera, R.C. Ammonia removal and recovery from municipal landfill leachates by heating. J. Environ. Manag. 2019, 256, 109947. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Lee, J.-G.; Tan, T.; Yeo, J.; Wong, P.W.; Ghaffour, N.; An, A.K. Enhanced ammonia recovery from wastewater by Nafion membrane with highly porous honeycomb nanostructure and its mechanism in membrane distillation. J. Membr. Sci. 2019, 590, 117265. [Google Scholar] [CrossRef]
- Winkler, M.K.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Larriba, O.; Rovira-Cal, E.; Juznic-Zonta, Z.; Guisasola, A.; Baeza, J.A. Evaluation of the integration of P recovery, polyhydroxy-alkanoate production and short cut nitrogen removal in a mainstream wastewater treatment process. Water Res. 2020, 172, 115474. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R. Chapter 8—Metallurgical Slags, Dust and Fumes. In Waste Management Series; Rao, S.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 269–327. [Google Scholar]
- Fassbender, A.G. ThermoEnergy Ammonia Recovery Process for Municipal and Agricultural Wastes. Sci. World J. 2001, 1 (Suppl. S2), 908–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, F.; El-Naas, M.H.; Suleiman, M.I.; Al Musharfy, M. Optimization of a Solvay-Based Approach for CO2 Capture. Int. J. Chem. Eng. Appl. 2016, 7, 230–234. [Google Scholar] [CrossRef] [Green Version]
- Chaalal, O. A Modified Solvay Process, and Uses Thereof for Processing 2 CO Containing Gas Streams and for Desalination. Patent WO/2007/139392, 30 May 2007. [Google Scholar]
- Steinhauser, G. Cleaner production in the Solvay Process: General strategies and recent developments. J. Clean. Prod. 2008, 16, 833–841. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; Al Musharfy, M.; Al-Marzouqi, A.H. A new process for the capture of CO2 and reduction of water salinity. Desalination 2017, 411, 69–75. [Google Scholar] [CrossRef]
- Pinto, P.C.D.C.; Da Silva, T.R.; Linhares, F.M.; De Andrade, F.V.; Carvalho, M.M.D.O.; De Lima, G.M. A integrated route for CO2 capture in the steel industry and its conversion into CaCO3 using fundamentals of Solvay process. Clean Technol. Environ. Policy 2016, 18, 1123–1139. [Google Scholar] [CrossRef]
- El-Naas, M.; Mohammad, A.; Suleiman, M.; Al Musharfy, M.; Al-Marzouqi, A. Statistical Analysis and Optimization of a Process for CO2 capture. Int. J. Pf Chem. Mol. Nucl. Mater. Metall. Eng. 2016, 10, 350–357. [Google Scholar]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; al Musharfy, M.; Al-Marzouqi, A.H. Evaluation of a novel gas-liquid con-tactor/reactor system for natural gas applications. J. Nat. Gas Sci. Eng. 2017, 39, 133–142. [Google Scholar] [CrossRef]
- Mohammad, A.F.; Mourad, A.A.-H.I.; Mustafa, J.; Al-Marzouqi, A.H.; El-Naas, M.; Al-Marzouqi, M.; Alnaimat, F.; Suleiman, M.I.; Al Musharfy, M.; Firmansyah, T. Computational fluid dynamics simulation of an Inert Particles Spouted Bed Reactor (IPSBR) system. Int. J. Chem. React. Eng. 2020, 18, 5–6. [Google Scholar] [CrossRef]
- Mohammad, A.; Mourad, A.; Al-Marzouqi, A.; El-Naas, M.; Van der Bruggen, B.; Al-Marzouqi, M.; Alnaimat, F.; Suleiman, M.; Al Musharfy, M. CFD and statistical approach to optimize the average air velocity and air volume fraction in an inert-particles spouted-bed reactor (IPSBR) system. Heliyon 2021, 7, e06369. [Google Scholar] [CrossRef] [PubMed]
- Jirsa, F. The Electrolysis of Aqueous Solutions of Ammonium Iodide. J. Am. Chem. Soc. 1950, 72, 2831–2834. [Google Scholar] [CrossRef]
- Roine, A. Hsc-Software Ver. 3.0 for Thermodynamic Calculations. In Proceedings of the International Symposium on Computer Software in Chemical and Extractive Metallurgy; Thompson, W.T., Ajersch, F., Eriksson, G., Eds.; Pergamon, 1989. [Google Scholar]








| Method | Ammonia Recovery/Removal Percentage | Strength | Weakness |
|---|---|---|---|
| Ammonia air-stripping process, wherein caustic soda (lime) is used to obtain a high pH level [3]. | 45–75% |
|
|
| Membrane contactors, wherein the gas-permeable hollow fiber membrane removes ~99% of ammonia. The dissolved ammonia diffuses from the gas-permeable membrane under vacuum pressure [4,5]. | 99–100% |
|
|
| Chemical precipitation, wherein ammonium ions are precipitated by forming magnesium ammonium phosphate solids [21,22]. | 95–98% |
|
|
| Ion exchange, wherein treated water is passed through a resin bed of zeolite and ammonium ions are exchanged with the resin’s free ions [23,24]. | - |
|
|
| Atmospheric and vacuum distillation, wherein ammonia from different concentrations of landfill leachates evaporates under both atmospheric and vacuum pressure conditions [25]. | 95–98% |
|
|
| Membrane distillation, wherein the vapor volatility and pressure through the membrane is controlled to concentrate ammonia on the permeate side [26]. | 20–70% |
|
|
| Ammonia recovery by a bioelectrochemical system, wherein the organic content in the treated water is oxidized by the exoelectrogens growing on an anode electrode, the released electrons are freely flowing from anode to cathode [27,28,29,30]. | 70–80% |
|
|
| Ammonium chloride leaching process, wherein ammonium chloride solutions are reacted with metal oxides such as zinc to form zinc ammine chloride [31,32]. | 84–95% |
|
|
| Osmotic membrane bioreactor, wherein ammonium ions are recovered via struvite precipitation. Adding sodium hydroxide is required for increasing the pH level to ensure the struvite precipitation [33]. | - |
|
|
| Recovery the Ammonia from Ammonium Chloride Solution | |||||
|---|---|---|---|---|---|
| # of EXP. | Applying EC | Run Time (h) | Temperature (°C) | Current Density mA/cm2 | Calcium Hydroxide CaOH g/l |
| 1 | Yes | 9 | 38–43 | 9.8 | 20 |
| 2 | Yes | 9 | 38–43 | 9.8 | 40 |
| 3 | Yes | 9 | 38–43 | 9.8 | 74 |
| 4 | No | 9 | 23–35 | 9.8 | 20 |
| 5 | Yes | 9 | 38–43 | 9.8 | 20 |
| 6 | No | 9 | 155–170 | 9.8 | 74 |
| 7 | Yes | 9 | 38–47 | 9.8 | 74 |
| Recovering the ammonia from ammonium hydroxide/bicarbonate | |||||
| 8 | Yes | 4 | 37–40 | 1.9 | 0 |
| 9 | Yes | 4 | 37–40 | 4.9 | 0 |
| 10 | Yes | 4 | 37–40 | 9.8 | 0 |
| 11 | Yes | 4 | 37–40 | 14.7 | 0 |
| 12 | Yes | 4 | 37–40 | 19.6 | 0 |
| 13 | Yes | 4 | 37–40 | 1.9 | 20 |
| 14 | Yes | 4 | 37–40 | 4.9 | 20 |
| 15 | Yes | 4 | 37–40 | 9.8 | 20 |
| 16 | Yes | 4 | 37–40 | 14.7 | 20 |
| 17 | Yes | 4 | 37–40 | 19.6 | 20 |
| 18 | Yes | 1 | 37–40 | 9.8 | 20 |
| 19 | Yes | 1 | 40 | 9.8 | 20 |
| 20 | Yes | 1 | 60 | 9.8 | 20 |
| 21 | Yes | 1 | 80 | 9.8 | 20 |
| 22 | No | 4 | 37–40 | 9.8 | 20 |
| 23 | No | 4 | 40 | 9.8 | 20 |
| 24 | No | 4 | 60 | 9.8 | 20 |
| 25 | No | 4 | 80 | 9.8 | 20 |
| Maximum Ammonium Chloride Removal (%) | Ammonia Recovery (%) | Energy Consumption | Time Required for 95% Ammonium Chloride Removal | |
|---|---|---|---|---|
| EC | 99.99 | 77.14 | 2.301 kWh/kg NH3 | 6 h |
| Heating up to 160 °C | 99.99 | 98.06 | 7.77 Wh/kg NH3 * | 3 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, A.F.; Al-Marzouqi, A.H.; El-Naas, M.H.; Van der Bruggen, B.; Al-Marzouqi, M.H. A New Process for the Recovery of Ammonia from Ammoniated High-Salinity Brine. Sustainability 2021, 13, 10014. https://doi.org/10.3390/su131810014
Mohammad AF, Al-Marzouqi AH, El-Naas MH, Van der Bruggen B, Al-Marzouqi MH. A New Process for the Recovery of Ammonia from Ammoniated High-Salinity Brine. Sustainability. 2021; 13(18):10014. https://doi.org/10.3390/su131810014
Chicago/Turabian StyleMohammad, Ameera F., Ali H. Al-Marzouqi, Muftah H. El-Naas, Bart Van der Bruggen, and Mohamed H. Al-Marzouqi. 2021. "A New Process for the Recovery of Ammonia from Ammoniated High-Salinity Brine" Sustainability 13, no. 18: 10014. https://doi.org/10.3390/su131810014
APA StyleMohammad, A. F., Al-Marzouqi, A. H., El-Naas, M. H., Van der Bruggen, B., & Al-Marzouqi, M. H. (2021). A New Process for the Recovery of Ammonia from Ammoniated High-Salinity Brine. Sustainability, 13(18), 10014. https://doi.org/10.3390/su131810014








