Lipids from Hermetia illucens, an Innovative and Sustainable Source
Abstract
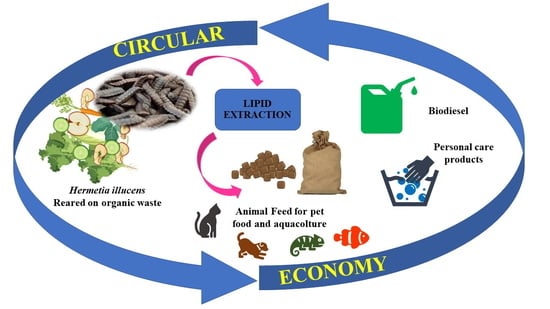
:1. Introduction
2. Lipids from the Black Soldier Fly
2.1. Larval Biomass Lipid Content Based on Different Diet
2.2. Fatty Acids Composition in Black Soldier Fly Lipids
3. Lipid Extraction
- -
- sample reduction, drying or hydrolysis;
- -
- sample homogenization with organic solvent;
- -
- separation of the organic and aqueous phase (organic phase contains lipids);
- -
- removal of non-lipid contaminants (if necessary);
- -
- drying of the extract to remove the organic solvent.
3.1. Traditional Lipid Extraction Methods
3.2. Industrial Extraction Method
4. Black Soldier Fly Lipid Applications
4.1. Biodiesel Production
4.2. Animal Feed
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Scudder, G.G.E. The Importance of Insects in Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2017. [Google Scholar]
- Pires, C.S.S.; Maués, M.M. Insect pollinators, major threats and mitigation measures. Neotrop. Entomol. 2020, 49, 469–471. [Google Scholar] [CrossRef]
- Mauricio da Rocha, J.R.; De Almeida, J.R.; Lins, G.A.; Durval, A. Insects as indicators of environmental changing and pollution: A review of appropriate species and their monitoring. Holos Environ. 2010, 10, 250. [Google Scholar] [CrossRef]
- Cusumano, A.; Volkoff, A.N. Influence of parasitoid-associated viral symbionts on plant-insect interactions and biological control. Curr. Opin. Insect Sci. 2021, 44, 64–71. [Google Scholar] [CrossRef]
- Adamski, Z.; Bufo, S.A.; Chowański, S.; Falabella, P.; Lubawy, J.; Marciniak, P.; Pacholska-Bogalska, J.; Salvia, R.; Scrano, L.; Słocińska, M.; et al. Beetles as model organisms in physiological, biomedical and environmental studies–A review. Front. Physiology 2019, 10, 319. [Google Scholar] [CrossRef] [Green Version]
- Snell-Rood, E. Bring biologists into biomimetics. Nature 2016, 529, 277–278. [Google Scholar] [CrossRef] [Green Version]
- Diener, S.; Zurbrügg, C.; Tockner, K. Conversion of organic material by black soldier fly larvae: Establishing optimal feeding rates. Waste Manag. Res. J. Sustain. Circ. Econ. 2009, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kalová, M.; Borkovcová, M. Voracious larvae Hermetia illucens and treatment of selected types of biodegradable waste. Acta. Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Tomberlin, J.K.; Zheng, L.; Yu, Z.; Zhang, J. Developmental and Waste Reduction Plasticity of Three Black Soldier Fly Strains (Diptera: Stratiomyidae) Raised on Different Livestock Manures. J. Med. Entomol. 2013, 50, 1224–1230. [Google Scholar] [CrossRef]
- Sheppard, C. House Fly and Lesser Fly Control Utilizing the Black Soldier Fly in Manure Management Systems for Caged Laying Hens. Environ. Entomol. 1983, 12, 1439–1442. [Google Scholar] [CrossRef]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of Vegetable and Fruit Substrates as Potential Rearing Media for Hermetia illucens (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2017, 46, 1415–1423. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Tomberlin, J.K.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Webster, C.D.; Rawles, S.D.; Koch, J.F.; Thompson, K.R.; Kobayashi, Y.; Gannam, A.L.; Twibell, R.G.; Hyde, N.M. Bio-Ag reutilization of distiller’s dried grains with solubles as a substrate for black soldier fly larvae, Hermetia illucens, along with poultry by-product meal and soybean meal, as total replacement of fish meal in diets for. Aquac. Nutr. 2016, 22, 976–988. [Google Scholar] [CrossRef]
- Ruhnke, I.; Normant, C.; Campbell, D.L.M.; Iqbal, Z.; Lee, C.; Hinch, G.N.; Roberts, J. Impact of on-range choice feeding with black soldier fly larvae (Hermetia illucens) on flock performance, egg quality, and range use of free-range laying hens. Anim. Nutr. 2018, 4, 452–460. [Google Scholar] [CrossRef]
- Engel, M.S. Insect evolution. Curr. Biol. 2015, 25, R868–R872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Poshadri, A. Insects as an Alternate Source for Food to Conventional Food Animals. Int. J. Pure Appl. Biosci. 2018, 6, 697–705. [Google Scholar] [CrossRef]
- FAO. Global Food Losses and Food Waste–Extent, Causes and Prevention. SAVE FOOD: An Initiative on Food Loss and Waste Reduction; FAO: Rome, Italy, 2011. [Google Scholar]
- Fowles, T.M.; Nansen, C. Insect-Based Bioconversion: Value from Food Waste. In Food Waste Management; Springer International Publishing: Cham, Switzerland, 2020; pp. 321–346. [Google Scholar]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- FAO. Agriculture Organization of the United Nations. Food Wastage Footprint: Impacts on Natural Resources; Summary Report; Natural Resources Management and Environment Department: Rome, Italy, 2013. Available online: http://www.fao.org/3/i3347e/i3347e.pdf (accessed on 1 January 2021).
- Xiao, X.; Jin, P.; Zheng, L.; Cai, M.; Yu, Z.; Yu, J.; Zhang, J. Effects of black soldier fly (Hermetia illucens) larvae meal protein as a fishmeal replacement on the growth and immune index of yellow catfish (Pelteobagrus fulvidraco). Aquac. Res. 2018, 49, 1569–1577. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0893 (accessed on 1 January 2021).
- Gasco, L.; Biancarosa, I.; Liland, N.S. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Marone, P.A. Food safety and regulatory concerns. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J., Rojas, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 203–221. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Quebec City, QC, Canada, 2013; Volume 97, ISBN 9789251075951. [Google Scholar]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect Rearing: Potential, Challenges, and Circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Madau, F.A.; Arru, B.; Furesi, R.; Pulina, P. Insect Farming for Feed and Food Production from a Circular Business Model Perspective. Sustainability 2020, 12, 5418. [Google Scholar] [CrossRef]
- Tao, J.; Li, Y.O. Edible insects as a means to address global malnutrition and food insecurity issues. Food Qual. Saf. 2018, 2, 17–26. [Google Scholar] [CrossRef]
- Mather, J.A. Ethics and care: For animals, not just mammals. Animals 2019, 9, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Wanaratana, S.; Amonsin, A.; Chaisingh, A.; Panyim, S.; Sasipreeyajan, J.; Pakpinyo, S. Experimental assessment of houseflies as vectors in avian influenza subtype H5N1 transmission in chickens. Avian Dis. 2013, 57, 266–272. [Google Scholar] [CrossRef]
- Pezzi, M.; Leis, M.; Chicca, M.; Falabella, P.; Salvia, R.; Scala, A.; Whitmore, D. Morphology of the antenna of Hermetia illucens (Diptera: Stratiomyidae): An ultrastructural investigation. J. Med Entomol. 2017, 54, 925–933. [Google Scholar] [CrossRef]
- Newton, L.; Sheppard, C.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, as a Value-Added Tool for the Management of Swine Manure; Animal Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005; p. 17. [Google Scholar]
- Purschke, B.; Scheibelberger, R.; Axmann, S.; Adler, A.; Jäger, H. Impact of substrate contamination with mycotoxins, heavy metals and pesticides on the growth performance and composition of black soldier fly larvae (Hermetia illucens) for use in the feed and food value chain. Food Addit. Contam. Part A 2017, 34, 1410–1420. [Google Scholar] [CrossRef]
- Romdhana, M.H.; Lecomte, D.; Ladevie, B.; Sablayrolles, C. Monitoring of pathogenic microorganisms contamination during heat drying process of sewage sludge. Process. Saf. Environ. Prot. 2009, 87, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EU) 2017/1017 of 15 June 2017 Amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017R1017&from=EN (accessed on 1 January 2021).
- Skrivervik, E. Insects’ contribution to the bioeconomy and the reduction of food waste. Heliyon 2020, 6, e03934. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, F.M.; Burnham, L. The geological record of insects. Annu. Rev. Earth Planet. Sci. 1985, 13, 297. [Google Scholar] [CrossRef]
- Lowman, M.D.; Rinker, H.B. Forest Canopies; Lowman, M.D., Rinker, H.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch. 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Ecological Implications of Minilivestock—Potential of Insects, Rodents, Frogs and Sails; Paoletti, M.G., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 263–291. [Google Scholar]
- Caligiani, A.; Marseglia, A.; Leni, G.; Baldassarre, S.; Maistrello, L.; Dossena, A.; Sforza, S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res. Int. 2018, 105, 812–820. [Google Scholar] [CrossRef]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [PubMed]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Scieuzo, C.; Hahn, T.; Zibek, S.; Salvia, R.; Falabella, P. Insect Chitin-Based Nanomaterials for Innovative Cosmetics and Cosmeceuticals. Cosmetics 2021, 8, 40. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11. [Google Scholar] [CrossRef]
- Manniello, M.D.; Moretta, A.; Salvia, R.; Scieuzo, C.; Lucchetti, D.; Vogel, H.; Sgambato, A.; Falabella, P. Insect antimicrobial peptides: Potential weapons to counteract the antibiotic resistance. Cell. Mol. Life Sci. 2021, 1, 3. [Google Scholar] [CrossRef]
- Mai, H.C.; Dao, N.D.; Lam, T.D.; Nguyen, B.V.; Nguyen, D.C.; Bach, L.G. Purification Process, Physicochemical Properties, and Fatty Acid Composition of Black Soldier Fly (Hermetia illucens Linnaeus) Larvae Oil. J. Am. Oil Chem. Soc. 2019, 96, 1303–1311. [Google Scholar] [CrossRef]
- Fasakin, E.A.; Balogun, A.M.; Ajayi, O.O. Evaluation of full-fat and defatted maggot meals in the feeding of clariid catfish Clarias gariepinus fingerlings. Aquac. Res. 2003, 34, 733–738. [Google Scholar] [CrossRef]
- Delicato, C.; Schouteten, J.J.; Dewettinck, K.; Gellynck, X.; Tzompa-Sosa, D.A. Consumers’ perception of bakery products with insect fat as partial butter replacement. Food Qual. Prefer. 2020, 79, 103755. [Google Scholar] [CrossRef]
- Smetana, S.; Leonhardt, L.; Kauppi, S.-M.; Pajic, A.; Heinz, V. Insect margarine: Processing, sustainability and design. J. Clean. Prod. 2020, 264, 121670. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Tan, C.K.; Tey, L.H. Comparative study on the effect of organic waste on lauric acid produced by Hermetia illucens larvae via bioconversion. J. Eng. Sci. Technol. 2015, 8, 52–63. [Google Scholar]
- Barragán-Fonseca, K.; Pineda-Mejia, J.; Dicke, M.; van Loon, J.J.A. Performance of the Black Soldier Fly (Diptera: Stratiomyidae) on Vegetable Residue-Based Diets Formulated Based on Protein and Carbohydrate Contents. J. Econ. Entomol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Anim. Feed Sci. Technol. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Leong, S.Y.; Kutty, S.R.M.; Malakahmad, A.; Tan, C.K. Feasibility study of biodiesel production using lipids of Hermetia illucens larva fed with organic waste. Waste Manag. 2016, 47, 84–90. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Pieterse, E.; Pretorius, Q. Nutritional evaluation of dried larvae and pupae meal of the housefly (Musca domestica) using chemical- and broiler-based biological assays. Anim. Prod. Sci. 2014, 54, 347. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Yao, H. Comprehensive Resource Utilization of Waste Using the Black Soldier Fly (Hermetia illucens L.) (Diptera: Stratiomyidae). Animals 2019, 9, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, M.; Binggeli, M.; Kurt, F.; de Wouters, T.; Reichlin, M.; Zurbrügg, C.; Mathys, A.; Kreuzer, M. Novel Experimental Methods for the Investigation of Hermetia illucens (Diptera: Stratiomyidae) Larvae. J. Insect Sci. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of Resources on Hermetia illucens (Diptera: Stratiomyidae) Larval Development. J. Med. Entomol. 2013, 50, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Li, M.; Zheng, L.; Liu, Y.; Zhang, Y.; Yu, Z.; Ma, Z.; Li, Q. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels 2015, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, A.C.; Montali, A.; Bruno, D.; Tettamanti, G. Metabolic adjustment of the larval fat body in Hermetia illucens to dietary conditions. J. Asia. Pac. Entomol. 2017, 20, 1307–1313. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia illucens L.) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Somroo, A.A.; ur Rehman, K.; Zheng, L.; Cai, M.; Xiao, X.; Hu, S.; Mathys, A.; Gold, M.; Yu, Z.; Zhang, J. Influence of Lactobacillus buchneri on soybean curd residue co-conversion by black soldier fly larvae (Hermetia illucens) for food and feedstock production. Waste Manag. 2019, 86, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Arango Gutiérrez, G.P.; Vergara Ruiz, R.A.; Mejía Vélez, H. Compositional, microbiological and protein digestibility analysis of the larva meal of Hermetia illuscens L. (Diptera: Stratiomyiidae) at Angelópolis-Antioquia, Colombia. Rev. Fac. Nac. Agron. Medellín 2004, 57, 2491–2500. [Google Scholar]
- Yang, S.Y.; Li, W.J.; Liu, C.X.; Hu, W.F. Effects of fermented swine manure on the conversion ratio of Hermetia illucens and nutritional components detection of Hermetia illucens larva and sandworm. J. Anhui Agricul. Sci. 2016, 44, 69–70. [Google Scholar]
- Julita, U.; Suryani, Y.; Kinasih, I.; Yuliawati, A.; Cahyanto, T.; Maryeti, Y.; Permana, A.D.; Fitri, L.L. Growth performance and nutritional composition of black soldier fly, Hermetia illucens (L), (Diptera: Stratiomyidae) reared on horse and sheep manure. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 187, p. 012071. [Google Scholar]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, S.; Yamashita, O. Metabolic shift from lipogenesis to glycogenesis in the last instar larval fat body of the silkworm, Bombyx mori. Insect Biochem. 1986, 16, 327–331. [Google Scholar] [CrossRef]
- Ushakova, N.A.; Brodskii, E.S.; Kovalenko, A.A.; Bastrakov, A.I.; Kozlova, A.A.; Pavlov, D.S. Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl. Biochem. Biophys. 2016, 468, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Surendra, K.C.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364, 345–352. [Google Scholar] [CrossRef]
- Mashkovskii, M.D. Lekarstvennye Sredstva; Novaya Volna: Moscow, Russia, 2012. [Google Scholar]
- Diclaro, J.W., II; Kaufman, P.E. Black Soldier Fly Hermetia Illucens Linnaeus (Insecta: Diptera: Stratiomyidae). Available online: https://edis.ifas.ufl.edu/pdf/IN/IN83000.pdf (accessed on 1 January 2021).
- Almeida, C.; Rijo, P.; Rosado, C. Bioactive Compounds from Hermetia illucens Larvae as Natural Ingredients for Cosmetic Application. Biomolecules. 2020, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Briegel, H. Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J. Insect Physiol. 1990, 36, 165–172. [Google Scholar] [CrossRef]
- Venkatesh, K.; Morrison, P.E. Studies of weight changes and amount of food ingested by the stable fly, Stomoxys calcitrans (Diptera: Muscidae). Can. Entomol. 1980, 112, 141–149. [Google Scholar] [CrossRef]
- Bennett, V.; Lee, R. Modeling seasonal changes in intracellular freeze-tolerance of fat body cells of the gall fly Eurosta solidaginis (Diptera, Tephritidae). J. Exp. Biol. 1997, 200, 185–192. [Google Scholar] [CrossRef]
- Holmes, L.A.; VanLaerhoven, S.L.; Tomberlin, J.K. Lower temperature threshold of black soldier fly (Diptera: Stratiomyidae) development. J. Insects Food Feed 2016, 2, 255–262. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Kao, M.C.; Fang, J.-Y.; Zouboulis, C.C.; Zhang, L.; Gallo, R.L.; Huang, C.-M. Antimicrobial Property of Lauric Acid Against Propionibacterium Acnes: Its Therapeutic Potential for Inflammatory Acne vulgaris. J. Invest Dermatol. 2009, 129, 2480–2488. [Google Scholar] [CrossRef] [Green Version]
- Park, K.-M.; Lee, S.J.; Yu, H.; Park, J.-Y.; Jung, H.-S.; Kim, K.; Lee, C.J.; Chang, P.-S. Hydrophilic and lipophilic characteristics of non-fatty acid moieties: Significant factors affecting antibacterial activity of lauric acid esters. Food Sci. Biotechnol. 2018, 27, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, K.; Popp, J.; Becker, A.; Hankel, J.; Visscher, C.; Klein, G.; Meemken, D. Lauric acid as feed additive—An approach to reducing Campylobacterspp. in broiler meat. PLoS ONE 2017, 12, e0175693. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Hu, Y.; Xu, D.; Cai, K. Surface functionalization of titanium substrates with chitosan–lauric acid conjugate to enhance osteoblasts functions and inhibit bacteria adhesion. Colloids Surf. B Biointerfaces 2014, 119, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Mohana Devi, S.; Kim, I. Effect of medium chain fatty acids (MCFA) and probiotic (Enterococcus faecium) supplementation on the growth performance, digestibility and blood profiles in weanling pigs. Vet. Med. 2014, 59, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Skřivanová, E.; Marounek, M.; Benda, V.; Březina, P. Susceptibility of Escherichia coli, Salmonella sp and Clostridium perfringens to organic acids and monolaurin. Veterinární Med. 2006, 51, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Regulation (EC) 1831/2003/EC on Additives for Use in Animal Nutrition, Replacing Directive 70/524/EEC on Additives in Feeding-Stuffs. Available online: https://ec.europa.eu/jrc/sites/default/files/EC-1831-2003.pdf (accessed on 1 January 2021).
- Kim, S.A.; Rhee, M.S. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans -cinnamaldehyde, thymol, and vanillin) against Escherichia coli O1. Food Control. 2016, 60, 447–454. [Google Scholar] [CrossRef]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Erucic acid in feed and food. EFSA J. 2016, 14, e04593. [Google Scholar]
- Bovera, F.; Loponte, R.; Marono, S.; Piccolo, G.; Parisi, G.; Iaconisi, V.; Gasco, L.; Nizza, A. Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 2016, 94, 639–647. [Google Scholar] [CrossRef] [Green Version]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Anim. Feed Sci. Technol. 2016, 220, 34–45. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Kates, M. Techniques of lipidology: Isolation, analysis and identification of lipids. In Laboratory Techniques in Biochemistry and Molecular Biology; Newport Somerville: Somerville, MA, USA, 1986. [Google Scholar]
- Lee, A.G. Lipid–protein interactions in biological membranes: A structural perspective. Biochim. Biophys. Acta Biomembr. 2003, 1612, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; Freeman, W.H.: New York, NY, USA, 2018. [Google Scholar]
- Gil, A.; Zhang, W.; Wolters, J.C.; Permentier, H.; Boer, T.; Horvatovich, P.; Heiner-Fokkema, M.R.; Reijngoud, D.J.; Bischoff, R. One- vs two-phase extraction: Re-evaluation of sample preparation procedures for untargeted lipidomics in plasma samples. Anal. Bioanal. Chem. 2018, 410, 5859–5870. [Google Scholar] [CrossRef] [Green Version]
- Pomeranz, Y. Food Analysis: Theory and Practice; Pomeranz, Y., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Wongkittipong, R.; Prat, L.; Damronglerd, S.; Gourdon, C. Solid-liquid extraction of andrographolide from plants—Experimental study, kinetic reaction and model. Sep. Purif. Technol. 2004, 40, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, M.; Ali, M.W.; Ripin, A.; Ahmad, A. Effect of Extraction Process Parameters on the Yield of Bioactive Compounds from the Roots of Eurycoma longifolia. J. Teknol. 2013, 60. [Google Scholar] [CrossRef] [Green Version]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef] [Green Version]
- Pernet, F.; Tremblay, R. Effect of ultrasonication and grinding on the determination of lipid class content of microalgae harvested on filters. Lipids 2003, 38, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Q.; Chen, J.-C.; Liu, D.-H.; Ye, X.-Q. Simultaneous extraction of phenolic compounds of citrus peel extracts: Effect of ultrasound. Ultrason. Sonochem. 2009, 16, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.E.; Ngvainti, J.N.; Lee, S. Lipid Analysis in: Methods of Analysis for Nutrition Labeling; Sulivan, D.M., Carpenter, D.E., Eds.; AOAC Press: Rockville, MD, USA, 1993; pp. 85–104. [Google Scholar]
- Christie, W.W. Lipid Analysis; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid Extraction Methods from Microalgae: A Comprehensive Review. Front. Energy Res. 2015, 2. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Salem, M.; Bernach, M.; Bajdzienko, K.; Giavalisco, P. A Simple Fractionated Extraction Method for the Comprehensive Analysis of Metabolites, Lipids, and Proteins from a Single Sample. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [Green Version]
- Dennison, C.; Lovrien, R. Three Phase Partitioning: Concentration and Purification of Proteins. Protein Expr. Purif. 1997, 11, 149–161. [Google Scholar] [CrossRef]
- Dennison, C. Three-phase partitioning. In Methods in Protein Biochemistry; Tschesche, H., Ed.; de Gruyter: Berlin/Heidelberg, Germany, 2011; pp. 1–12. [Google Scholar]
- Gómez-González, S.; Ruiz-Jiménez, J.; Priego-Capote, F.; Luque de Castro, M.D. Qualitative and Quantitative Sugar Profiling in Olive Fruits, Leaves, and Stems by Gas Chromatography−Tandem Mass Spectrometry (GC-MS/MS) after Ultrasound-Assisted Leaching. J. Agric. Food Chem. 2010, 58, 12292–12299. [Google Scholar] [CrossRef]
- Isengard, H.D.; Morlock, G.; Breithaupt, D. Food Analysis in Food Science and Technology; Campbell-Platt, G., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 31–75. [Google Scholar]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Nelson Education: Toronto, ON, USA, 2013. [Google Scholar]
- Brunner, G. Supercritical fluids: Technology and application to food processing. J. Food Eng. 2005, 67, 21–33. [Google Scholar] [CrossRef]
- Eawag-Aquatic Research. Fractioning of Black Soldier Fly Larvae into Protein Meal and Fat. Available online: https://www.eawag.ch/fileadmin/Domain1/Abteilungen/sandec/schwerpunkte/swm/Practical_knowhow_on_BSF/bsf_factsheet_fractioning.pdf (accessed on 1 January 2021).
- Fitzherbert, E.; Struebig, M.; Morel, A.; Danielsen, F.; Bruhl, C.; Donald, P.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. The importance of bioethanol and biodiesel from biomass. Energy Sources Part B Econ. Plan. Policy 2008, 3, 177–185. [Google Scholar] [CrossRef]
- Islam, M.M.; Hasanuzzaman, M.; Pandey, A.K.; Rahim, N.A. Modern energy conversion technologies. In Energy for Sustainable Development; Hasanuzzaman, M., Rahim, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 19–39. [Google Scholar]
- Bozbas, K. Biodiesel as an alternative motor fuel: Production and policies in the European Union. Renew. Sustain. Energy Rev. 2008, 12, 542–552. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.C.; Liang, S.H.; Li, S.Y.; Su, C.H.; Chien, C.C.; Chen, Y.J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (Hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Balat, M. Potential alternatives to edible oils for biodiesel production–A review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Biorefin. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Exploring the potential of grease from yellow mealworm beetle (Tenebrio molitor) as a novel biodiesel feedstock. Appl. Energy 2013, 101, 618–621. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, X.; Xi, Y. Fat body remodeling and homeostasis control in Drosophila. Life Sci. 2016, 167, 22–31. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste. 2011. Available online: http://www.fao.org/fileadmin/user_upload/suistainability/pdf/Global_Food_Losses_and_Food_Waste.pdf (accessed on 1 January 2021).
- Wong, C.Y.; Aris, M.N.M.; Daud, H.; Lam, M.K.; Yong, C.S.; Hasan, H.A.; Chong, S.; Show, P.L.; Hajoeningtijas, O.D.; Ho, Y.C.; et al. In-situ yeast fermentation to enhance bioconversion of coconut endosperm waste into larval biomass of Hermetia illucens: Statistical augmentation of larval lipid content. Sustainability 2020, 12, 1558. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Y.; Zhang, X.; Tan, T. Biodiesel production by direct transesterification of microalgal biomass with co-solvent. Bioresour. Technol. 2015, 196, 712–715. [Google Scholar] [CrossRef]
- Rahimi, M.; Mohammadi, F.; Basiri, M.; Parsamoghadam, M.A.; Masahi, M.M. Transesterification of soybean oil in four-way micromixers for biodiesel production using a cosolvent. J. Taiwan Inst. Chem. Eng. 2016, 64, 203–210. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Sulaiman, S.; Abdul Aziz, A.R.; Aroua, M.K. Reactive extraction of solid coconut waste to produce biodiesel. J. Taiwan Inst. Chem. Eng. 2013, 44, 233–238. [Google Scholar] [CrossRef]
- Ishak, S.; Kamari, A. A review of optimum conditions of transesterification process for biodiesel production from various feedstocks. Int. J. Environ. Sci. Technol. 2019, 16, 2481–2502. [Google Scholar] [CrossRef]
- Su, C.H.; Nguyen, H.C.; Bui, T.L.; Huang, D.L. Enzyme-assisted extraction of insect fat for biodiesel production. J. Clean. Prod. 2019, 223, 436–444. [Google Scholar] [CrossRef]
- Feng, W.; Xiong, H.; Wang, W.; Duan, X.; Yang, T.; Wu, C.; Yang, F.; Xiong, J.; Wang, T.; Wang, C. Energy consumption analysis of lipid extraction from black soldier fly biomass. Energy 2019, 185, 1076–1085. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour. Technol. 2015, 184, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae mediated valorization of industrial, agriculture and food wastes: Biorefinery concept through bioconversion, processes, procedures, and products. Processes 2020, 8, 857. [Google Scholar] [CrossRef]
- Lai, E.P.C. Biodiesel: Environmental Friendly Alternative to Petrodiesel. J. Pet. Environ. Biotechnol. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The Future of Aquatic Protein: Implications for Protein Sources in Aquaculture Diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef] [Green Version]
- Khusro, M.; Andrew, N.R.; Nicholas, A. Insects as poultry feed: A scoping study for poultry production systems in Australia. Worlds. Poult. Sci. J. 2012, 68, 435–446. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Waagbø, R.; Biancarosa, I.; Pelusio, N.; Li, Y.; Krogdahl, Å.; Lock, E.J. Potential of insect-based diets for Atlantic salmon (Salmo salar). Aquaculture 2018, 491, 72–81. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.-J.; Segura, M.; Morote, E.; Torres, A.; Ramos, R.; Guil, J.-L. Insects as food: Enrichment of larvae of Hermetia illucens with omega 3 fatty acids by means of dietary modifications. J. Food Compos. Anal. 2017, 62, 8–13. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbø, R.; Krogdahl, Å.; Lock, E.J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Belghit, I.; Waagbø, R.; Lock, E.J.; Liland, N.S. Insect-based diets high in lauric acid reduce liver lipids in freshwater Atlantic salmon. Aquac. Nutr. 2019, 25, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Dietary DHA and health: Cognitive function ageing. Nutr. Res. Rev. 2016, 29, 281–294. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 1–16. [Google Scholar] [CrossRef]
- Nekrasov, R.; Zelenchenkova, A.; Chabaev, M.; Ivanov, G.; Antonov, A.; Pastukhova, N. PSIII-37 Dried Black Soldier Fly larvae as a dietary supplement to the diet of growing pigs. J. Anim. Sci. 2018, 96, 314. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Li, J.; Ma, X. Use of Hermetia illucens larvae as a dietary protein source: Effects on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2019, 158, 107837. [Google Scholar] [CrossRef] [PubMed]
- van Heugten, E.; Martinez, G.; McComb, A.; Koutsos, E. 285 Black soldier fly (Hermetia illucens) larvae oil improves growth performance of nursery pigs. J. Anim. Sci. 2019, 97, 118. [Google Scholar] [CrossRef]
- Cullere, M.; Schiavone, A.; Dabbou, S.; Gasco, L.; Zotte, A.D. Meat quality and sensory traits of finisher broiler chickens fed with black soldier fly (Hermetia illucens L.) larvae fat as alternative fat source. Animals 2019, 9, 140. [Google Scholar] [CrossRef] [Green Version]
- Brede, A.; Wecke, C.; Liebert, F. Does the optimal dietary methionine to cysteine ratio in diets for growing chickens respond to high inclusion rates of insect meal from Hermetia illucens? Animals 2018, 8, 187. [Google Scholar] [CrossRef] [Green Version]
- Cutrignelli, M.I.; Messina, M.; Tulli, F.; Randazzi, B.; Olivotto, I.; Gasco, L.; Loponte, R.; Bovera, F. Evaluation of an insect meal of the Black Soldier Fly (Hermetia illucens) as soybean substitute: Intestinal morphometry, enzymatic and microbial activity in laying hens. Res. Vet. Sci. 2018, 117, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.-M.; Park, Y.-K.; Yang, Y.-C.; Jung, B.-G.; Lee, B.-J. Black soldier fly (Hermetia illucens) larvae enhances immune activities and increases survivability of broiler chicks against experimental infection of Salmonella Gallinarum. J. Vet. Med. Sci. 2018, 80, 736–740. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, K.; Hashimoto, Y.; Hori, A.; Kawasaki, T.; Hirayasu, H.; Iwase, S.I.; Hashizume, A.; Ido, A.; Miura, C.; Miura, T.; et al. Evaluation of black soldier fly (Hermetia illucens) larvae and pre-pupae raised on household organic waste, as potential ingredients for poultry feed. Animals 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kierończyk, B.; Sypniewski, J.; Rawski, M.; Czekała, W.; Swiatkiewicz, S.; Józefiak, D. From Waste to Sustainable Feed Material: The Effect of Hermetia illucens Oil on the Growth Performance, Nutrient Digestibility, and Gastrointestinal Tract Morphometry of Broiler Chickens. Ann. Anim. Sci. 2020, 20, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Mbhele, F.G.T.; Mnisi, C.M.; Mlambo, V. A nutritional evaluation of insect meal as a sustainable protein source for jumbo quails: Physiological and meat quality responses. Sustainability 2019, 11, 6592. [Google Scholar] [CrossRef] [Green Version]
- Mwaniki, Z.; Neijat, M.; Kiarie, E. Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn-soybean meal diet fed to Shaver White Leghorns from wk 19 to 27 of age. Poult. Sci. 2018, 97, 2829–2835. [Google Scholar] [CrossRef]
- Mwaniki, Z. Complete Replacement of Soybean Meal with Defatted Black Soldier Fly Larva Meal (BSFLM) in Laying Hen Feeding Programs: Impact on Egg Production and Quality. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2019. [Google Scholar]
- Nery, J.; Gasco, L.; Dabbou, S.; Schiavone, A. Protein composition and digestibility of black soldier fly larvae in broiler chickens revisited according to the recent nitrogen-protein conversion ratio. J. Insects Food Feed 2018, 4, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for Income Generation Through Animal Feed: Effect of Dietary Replacement of Soybean and Fish Meal With Black Soldier Fly Meal on Broiler Growth and Economic Performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, E.; Erasmus, S.W.; Uushona, T.; Hoffman, L.C. Black soldier fly (Hermetia illucens) pre-pupae meal as a dietary protein source for broiler production ensures a tasty chicken with standard meat quality for every pot. J. Sci. Food Agric. 2019, 99, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.T.; Bergagna, S.; Dezzutto, D.; Meneguz, M.; et al. Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Dabbou, S.; Petracci, M.; Zampiga, M.; Sirri, F.; Biasato, I.; Gai, F.; Gasco, L. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on carcass traits, breast meat quality and safety. Animal 2019, 13, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Bovera, F.; Nizza, S.; Baronti, N.; Gasco, L.; Conte, G.; Serra, A.; Bonelli, A.; Parisi, G. Quality of eggs from Lohmann Brown Classic laying hens fed black soldier fly meal as substitute for soya bean. Animal 2018, 12, 2191–2197. [Google Scholar] [CrossRef]
- Surendra, K.C.; Tomberlin, J.K.; van Huis, A.; Cammack, J.A.; Heckmann, L.H.L.; Khanal, S.K. Rethinking organic wastes bioconversion: Evaluating the potential of the black soldier fly (Hermetia illucens L.) (Diptera: Stratiomyidae) (BSF). Waste Manag. 2020, 117, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Moula, N.; Scippo, M.L.; Douny, C.; Degand, G.; Dawans, E.; Cabaraux, J.F.; Hornick, J.-L.; Medigo, R.C. Leroy, P. Performances of local poultry breed fed black soldier fly larvae reared on horse manure. Anim. Nutr. 2018, 4, 73–78. [Google Scholar] [CrossRef]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef]
- Maurer, V.; Holinger, M.; Amsler, Z.; Früh, B.; Wohlfahrt, J.; Stamer, A.; Leiber, F. Replacement of soybean cake by Hermetia illucens meal in diets for layers. J. Insects Food Feed 2016, 2, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.B.; Kim, D.H.; Jeong, S.B.; Lee, J.W.; Kim, T.H.; Lee, H.G.; Lee, K.W. Black soldier fly larvae oil as an alternative fat source in broiler nutrition. Poult. Sci. 2020, 99, 3133–3143. [Google Scholar] [CrossRef]
- Kozłowski, K.; Ognik, K.; Stępniowska, A.; Juśkiewicz, J.; Zduńczyk, Z.; Kierończyk, B.; Benzertiha, A.; Benzertiha, A.; Józefiak, D. Growth performance, immune status and intestinal fermentative processes of young turkeys fed diet with additive of full fat meals from Tenebrio molitor and Hermetia illucens. Anim. Feed Sci. Technol. 2021, 278, 114994. [Google Scholar] [CrossRef]
- Jankowski, J.; Kozłowski, K.; Zduńczyk, Z.; Stępniowska, A.; Ognik, K.; Kierończyk, B.; Józefiak, D.; Juśkiewicz, J. The effect of dietary full-fat Hermetia illucens larvae meal on gut physiology and growth performance in young turkeys. Anim. Feed Sci. Technol. 2021, 275, 114879. [Google Scholar] [CrossRef]
- Giannetto, A.; Oliva, S.; Ceccon Lanes, C.F.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef]
- Chun, C.Y.; Yoong, L.S.; Kim, L.P.; Hock, T.L.; Ling, L.J. Comparison of Hermetia illucens larvae and pre-pupae as potential aqua feed derived from the biotransformation of organic waste. AIP Conference Proceedings. 2019, 2157, 020008. [Google Scholar]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Salomone, R.; Saija, G.; Mondello, G.; Giannetto, A.; Fasulo, S.; Savastano, D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. J. Clean. Prod. 2017, 140, 890–905. [Google Scholar] [CrossRef]
- Wang, S.Y.; Wu, L.; Li, B.; Zhang, D. Reproductive Potential and Nutritional Composition of Hermetia illucens (Diptera: Stratiomyidae) Prepupae Reared on Different Organic Wastes. J. Econ. Entomol. 2020, 113, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Deng, W.; Zhu, F. Reference gene selection for quantitative gene expression analysis in black soldier fly (Hermetia illucens). PLoS ONE 2019, 14, e0221420. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Bondari, K.; Sheppard, D.C. Soldier fly, Hermetia illucens L., larvae as feed for channel catfish, Ictalurus punctatus (Rafinesque), and blue tilapia, Oreochromis aureus (Steindachner). Aquac. Res. 1987, 18, 209–220. [Google Scholar] [CrossRef]
- Hu, J.; Wang, G.; Huang, Y.; Sun, Y.; He, F.; Zhao, H.; Li, N. Effects of substitution of fish meal with black soldier fly (Hermetia illucens) larvae meal, in yellow catfish (Pelteobagrus fulvidraco) diets. Israeli J. Aquacul. 2017, 69, 1–9. [Google Scholar]
- Fawole, F.J.; Adeoye, A.A.; Tiamiyu, L.O.; Ajala, K.I.; Obadara, S.O.; Ganiyu, I.O. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haemato-physiological response, and oxidative stress biomarker. Aquaculture 2020, 518, 734849. [Google Scholar] [CrossRef]
- Muin, H.; Taufek, N.M.; Kamarudin; Razak, S. A. Growth performance, feed Utilization and body composition of nile tilapia, Oreochromis niloticus (Linnaeus, 1758) fed with different levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) maggot meal diet. Iran. J. Fish. Sci. 2017, 16, 567–577. [Google Scholar]
- Devic, E.; Leschen, W.; Murray, F.; Little, D.C. Growth performance, feed utilization and body composition of advanced nursing Nile tilapia (Oreochromis niloticus) fed diets containing Black Soldier Fly (Hermetia illucens) larvae meal. Aquac. Nutr. 2018, 24, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Toriz-Roldan, A.; Ruiz-Vega, J.; García-Ulloa, M.; Hernández-Llamas, A.; Fonseca-Madrigal, J.; Rodríguez-González, H. Assessment of Dietary Supplementation Levels of Black Soldier Fly, Hemertia illucens, Pre-Pupae Meal for Juvenile Nile Tilapia, Oreochromis niloticus. Southwest. Entomol. 2019, 44, 251. [Google Scholar] [CrossRef]
- Borgogno, M.; Dinnella, C.; Iaconisi, V.; Fusi, R.; Scarpaleggia, C.; Schiavone, A.; Monteleone, E.; Gasco, L.; Parisi, G. Inclusion of Hermetia illucens larvae meal on rainbow trout (Oncorhynchus mykiss) feed: Effect on sensory profile according to static and dynamic evaluations. J. Sci. Food Agric. 2017, 97, 3402–3411. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Randazzo, B.; Messina, M.; Zarantoniello, M.; Giorgini, E.; Zimbelli, A.; Bruni, L.; Parisi, G.; Olivotto, I.; Tulli, F. Effects of graded dietary inclusion level of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss). Animals 2019, 9, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimoldi, S.; Gini, E.; Iannini, F.; Gasco, L.; Terova, G. The effects of dietary insect meal from Hermetia illucens prepupae on autochthonous gut microbiota of rainbow trout (Oncorhynchus mykiss). Animals 2019, 9, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruni, L.; Belghit, I.; Lock, E.J.; Secci, G.; Taiti, C.; Parisi, G. Total replacement of dietary fish meal with black soldier fly (Hermetia illucens) larvae does not impair physical, chemical or volatile composition of farmed Atlantic salmon (Salmo salar L.). J. Sci. Food Agric. 2020, 100, 1038–1047. [Google Scholar] [CrossRef]
- Mancini, S.; Medina, I.; Iaconisi, V.; Gai, F.; Basto, A.; Parisi, G. Impact of black soldier fly larvae meal on the chemical and nutritional characteristics of rainbow trout fillets. Animal 2018, 12, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Raggi, T.; Barkhouse, J.; Lewis, E.; Weltzien, E. The oil fraction and partially defatted meal of black soldier fly larvae (Hermetia illucens) affect differently growth performance, feed efficiency, nutrient deposition, blood glucose and lipid digestibility of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 492, 24–34. [Google Scholar] [CrossRef]
- Huyben, D.; Vidaković, A.; Werner Hallgren, S.; Langeland, M. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens). Aquaculture 2019, 500, 485–491. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Speckmann, H.; Schlüter, O.; Kloas, W.; Prochnow, A. Potentials of a biogenic residue-based production of Hermetia illucens as fish meal replacement in aquafeed for Oncorhynchus mykiss in Germany. J. Insects Food Feed 2018, 4, 5–18. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory Analysis of Rainbow Trout, Oncorhynchus mykiss, Fed Enriched Black Soldier Fly Prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Lock, E.R.; Arsiwalla, T.; Waagbø, R. Insect larvae meal as an alternative source of nutrients in the diet of Atlantic salmon (Salmo salar) postsmolt. Aquac. Nutr. 2016, 22, 1202–1213. [Google Scholar] [CrossRef]
- Biancarosa, I.; Sele, V.; Belghit, I.; Ørnsrud, R.; Lock, E.J.; Amlund, H. Replacing fish meal with insect meal in the diet of Atlantic salmon (Salmo salar) does not impact the amount of contaminants in the feed and it lowers accumulation of arsenic in the fillet. Food Addit. Contam.Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 1191–1205. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, O.K.; Holen, E.; Piemontese, L.; Liland, N.S.; Lock, E.J.; Espe, M.; Belghit, I. Effect of dietary replacement of fish meal with insect meal on in vitro bacterial and viral induced gene response in Atlantic salmon (Salmo salar) head kidney leukocytes. Fish Shellfish. Immunol. 2019, 91, 223–232. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Truzzi, C.; Giorgini, E.; Marcellucci, C.; Vargas-Abúndez, J.A.; Zimbelli, A.; Annibaldi, A.; Parisi, G.; Tulli, F.; et al. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Caimi, C.; Renna, M.; Lussiana, C.; Bonaldo, A.; Gariglio, M.; Meneguz, M.; Dabbou, S.; Schiavone, A.; Gai, F.; Elia, A.C.; et al. First insights on Black Soldier Fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture 2020, 515, 734539. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Tian, J.; Zhou, J.; Yu, H. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2016, 465, 43–52. [Google Scholar] [CrossRef]
- Zhou, J.S.; Liu, S.S.; Ji, H.; Yu, H.B. Effect of replacing dietary fish meal with black soldier fly larvae meal on growth and fatty acid composition of Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2018, 24, 424–433. [Google Scholar] [CrossRef]
- Guerreiro, I.; Castro, C.; Antunes, B.; Coutinho, F.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; et al. Catching black soldier fly for meagre: Growth, whole-body fatty acid profile and metabolic responses. Aquaculture 2020, 516, 734613. [Google Scholar] [CrossRef]
- Vongvichith, B.; Morioka, S.; Sugita, T.; Phousavanh, N.; Phetsanghanh, N.; Chanthasone, P.; Pommachan, P.; Nakamura, S. Evaluation of the efficacy of aquaculture feeds for the climbing perch Anabas testudineus: Replacement of fishmeal by black soldier fly Hermetia illucens prepupae. Fish. Sci. 2020, 86, 145–151. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Scieuzo, C.; Salvia, R.; Petrone, A.M.; Tafi, E.; Moretta, A.; Schmitt, E.; Falabella, P. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability 2021, 13, 10198. https://doi.org/10.3390/su131810198
Franco A, Scieuzo C, Salvia R, Petrone AM, Tafi E, Moretta A, Schmitt E, Falabella P. Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability. 2021; 13(18):10198. https://doi.org/10.3390/su131810198
Chicago/Turabian StyleFranco, Antonio, Carmen Scieuzo, Rosanna Salvia, Anna Maria Petrone, Elena Tafi, Antonio Moretta, Eric Schmitt, and Patrizia Falabella. 2021. "Lipids from Hermetia illucens, an Innovative and Sustainable Source" Sustainability 13, no. 18: 10198. https://doi.org/10.3390/su131810198
APA StyleFranco, A., Scieuzo, C., Salvia, R., Petrone, A. M., Tafi, E., Moretta, A., Schmitt, E., & Falabella, P. (2021). Lipids from Hermetia illucens, an Innovative and Sustainable Source. Sustainability, 13(18), 10198. https://doi.org/10.3390/su131810198