Conservation Status of Milkcaps (Basidiomycota, Russulales, Russulaceae), with Notes on Poorly Known Species
Abstract
:1. Introduction
2. Compilation of Data
2.1. Red Lists
2.2. Taxonomy and Nomenclature
2.3. Distribution Data
3. Results and Discussion
3.1. Efforts in Macrofungi Conservation
3.2. Milkcaps Diversity: A Global Perspective
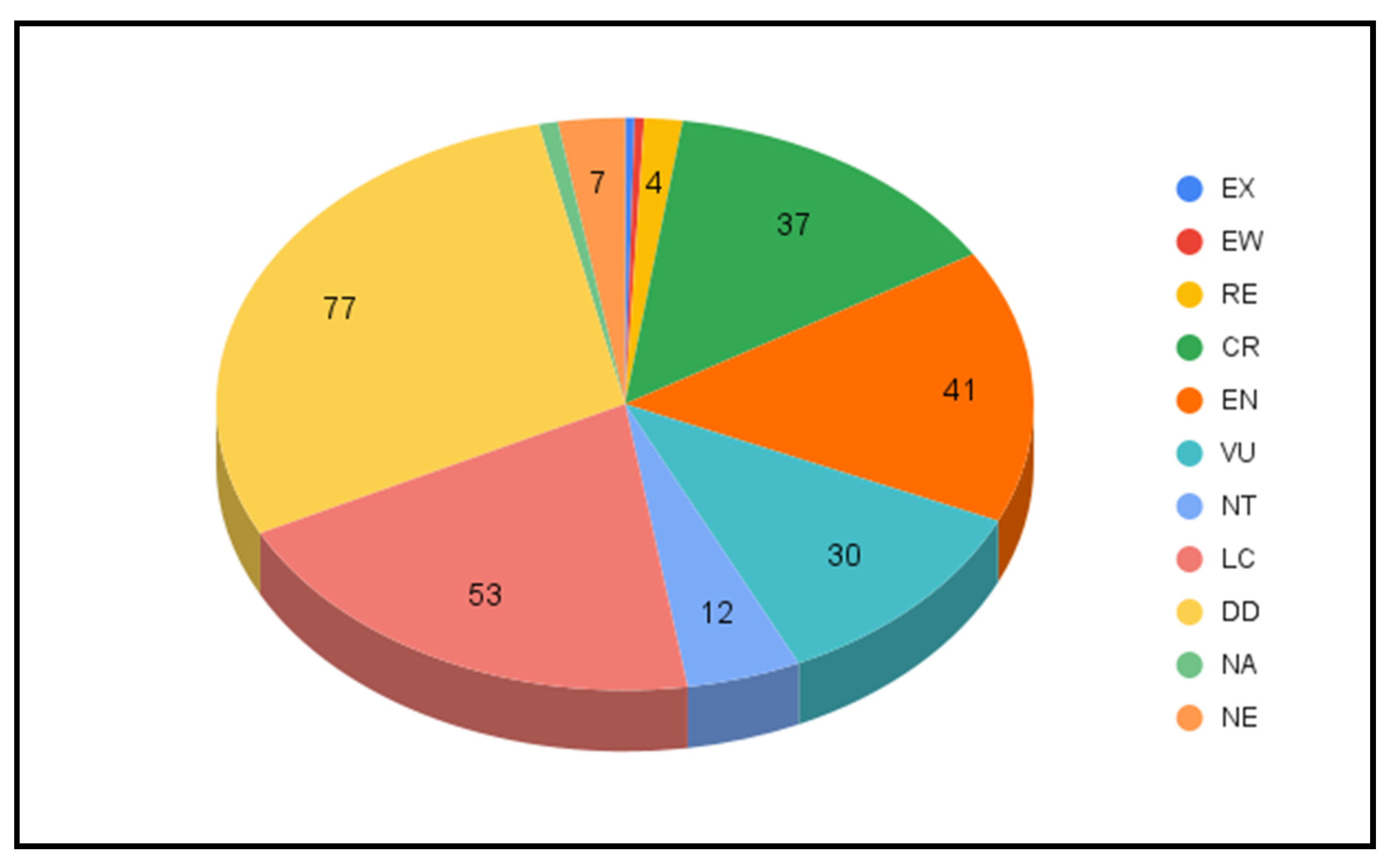
3.3. Milkcaps in Red Lists
3.4. Protecting Milkcaps
3.5. Milkcaps Distribution Data: The Good, the Bad, the Weird
3.6. Rare and Endangered, or Simply Poorly Known and Questionable?
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buyck, B.; Hofstetter, V.; Verbeken, A.; Walleyn, R. 1919 Proposal to conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Taxon 2010, 59, 295–296. [Google Scholar] [CrossRef]
- Stubbe, D.; Wang, X.-H.; Verbeken, A. New combinations in Lactifluus. 2. L. subgen. Gerardii. Mycotaxon 2012, 119, 483–485. [Google Scholar] [CrossRef] [Green Version]
- Verbeken, A.; Nuytink, J.; Buyck, B. New combinations in Lactifluus. 1. L. subgenera Edules, Lactariopsis and Russulopsis. Mycotaxon 2011, 118, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Verbeken, A.; Van de Putte, K.; De Crop, E. New combinations in Lactifluus. 3. L. subgenera Lactifluus and Piperati. Mycotaxon 2012, 120, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Delgat, L.; Courtecuisse, R.; De Crop, E.; Hampe, F.; Hofmann, T.A.; Manz, C.; Piepenbring, M.; Roy, M.; Verbeken, A. Lactifluus (Russulaceae) diversity in Central America and the Caribbean: Melting pot between realms. Persoonia 2020, 44, 278–300. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, L.J. Lactarius. In Ectomycorrhizal Fungi. Key Genera in Profile; Cairney, J.W.G., Chambers, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 269–285. [Google Scholar]
- Rinaldi, A.C.; Comandini, O.; Kuyper, T.W. Ectomycorrhizal fungal diversity: Separating the wheat from the chaff. Fungal Divers. 2008, 33, 1–45. [Google Scholar]
- Comandini, O.; Rinaldi, A.C.; Kuyper, T.W. Measuring and estimating ectomycorrhizal fungal diversity: A continuous challenge. In Mycorrhiza: Occurrence in Natural and Restored Environments; Pagano, M., Ed.; Nova Science Publishers: New York, NY, USA, 2012; pp. 165–200. [Google Scholar]
- Homola, R.L.; Czapowskyj, M.M. Ectomycorrhizae of Maine 2. A listing of Lactarius with the associated hosts (with additional information on edibility). Life Sci. Agric. Exp. Stn. Bull. 1981, 779, 1–19. [Google Scholar]
- Dell, B.; Havel, J.J.; Malajczuk, N. The Jarrah Forest: A Complex Mediterranean Ecosystem; Kluwer Aacademic Publishers: Dordrecht, The Netherlands, 1989. [Google Scholar]
- Comandini, O.; Pacioni, G.; Rinaldi, A.C. Fungi in ectomycorrhizal associations of silver fir (Abies alba Miller) in Central Italy. Mycorrhiza 1998, 7, 323–328. [Google Scholar] [CrossRef]
- Eberhardt, U.; Oberwinkler, F.; Verbeken, A.; Pacioni, G.; Rinaldi, A.C.; Comandini, O. Lactarius ectomycorrhizae on Abies alba: Morphological description, molecular characterization, and taxonomic remarks. Mycologia 2000, 92, 860–873. [Google Scholar] [CrossRef]
- Nuytinck, J.; Verbeken, A.; Leonardi, M.; Pacioni, G.; Rinaldi, A.C.; Comandini, O. Characterization of Lactarius tesquorum ectomycorrhizae on Cistus sp., and molecular phylogeny of related European Lactarius taxa. Mycologia 2004, 96, 272–282. [Google Scholar] [CrossRef]
- Montoya, L.; Bandala, V.M. Revision of Lactarius from Mexico. Persoonia 2005, 18, 471–483. [Google Scholar]
- Le, H.T.; Stubbe, D.; Verbeken, A.; Nuytinck, J.; Lumyong, S.; Desjardin, D.E. Lactarius in Northern Thailand: 2. Lactarius subgenus Plinthogali. Fungal Divers. 2007, 27, 61–94. [Google Scholar]
- Mueller, G.M.; Halling, R.E. Macrofungi of Costa Rica. 2010. Available online: https://www.nybg.org/bsci/res/hall/ (accessed on 30 June 2021).
- Verbeken, A.; Walleyn, R. Monograph of Lactarius in Tropical Africa; National Botanic Garden: Meise, Belgium, 2010. [Google Scholar]
- Comandini, O.; Erős-Honti, Z.; Jakucs, E.; Flores Arzú, R.; Leonardi, M.; Rinaldi, A.C. Molecular and morpho-anatomical description of mycorrhizas of Lactarius rimosellus on Quercus sp., with ethnomycological notes on Lactarius in Guatemala. Mycorrhiza 2012, 22, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Flores Arzú, R.; Comandini, O.; Rinaldi, A.C. A preliminary checklist of macrofungi of Guatemala, with notes on edibility and traditional knowledge. Mycosphere 2012, 3, 1–21. [Google Scholar] [CrossRef]
- Joshi, S.; Bhatt, R.P.; Stephenson, S.L. The current status of the family Russulaceae in the Uttarakhand Himalaya, India. Mycosphere 2012, 3, 486–501. [Google Scholar] [CrossRef]
- Leonard, P.L.; Cooper, J.A. Lactarius Novae-Zelandiae. The IUCN Red List of Threatened Species. 2017, e.T80188416A80188421. Available online: https://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T80188416A80188421.en (accessed on 30 July 2021).
- Heilmann-Clausen, J.; Verbeken, A.; Vesterholt, J. The Genus Lactarius. Fungi of Northern Europe; Svampetryk and Danish Mycological Society: Greve, Denmark, 1998; Volume 2. [Google Scholar]
- Basso, M.T. Lactarius Pers. Fungi Europaei 7; Mykoflora: Alassio, Italy, 1999. [Google Scholar]
- Van de Putte, K.; Nuytinck, J.; De Crop, E.; Verbeken, A. Lactifluus volemus in Europe: Three species in one revealed by a multilocus genealogical approach, Bayesian species delimitation and morphology. Fungal Biol. 2016, 120, 1–25. [Google Scholar] [CrossRef] [PubMed]
- De Crop, E.; Nuytinck, J.; Van de Putte, K.; Wisitrassameewong, K.; Hackel, J.; Stubbe, D.; Hyde, K.D.; Roy, M.; Halling, R.E.; Moreau, P.-A.; et al. A multi-gene phylogeny of Lactifluus (Basidiomycota, Russulales) translated into a new infrageneric classification of the genus. Persoonia 2017, 38, 58–80. [Google Scholar] [CrossRef] [Green Version]
- Boa, E. Wild Edible Fungi: A Global Overview of Their Use and Importance to People; FAO: Roma, Italy, 2004. [Google Scholar]
- Pérez-Moreno, J.; Guerin-Laguette, A.; Rinaldi, A.C.; Yu, F.; Verbeken, A.; Hernàndez-Santiago, F.; Martìnez-Reyes, M. Edible ectomycorrhizal fungi: How can they contribute to forest sustainability, food security, biocultural conservation and mitigation of hunger and climate change? Plants People Planet 2021, 3, 471–490. [Google Scholar] [CrossRef]
- Guerin-Laguette, A.; Cummings, N.; Butler, R.C.; Willows, A.; Hesom-Williams, N.; Li, S.; Wang, Y. Lactarius deliciosus and Pinus radiata in New Zealand: Towards the development of innovative gourmet mushroom orchards. Mycorrhiza 2014, 24, 511–523. [Google Scholar] [CrossRef]
- Senn-Irlet, B.; Heilmann-Clausen, J.; Genney, D.; Dahlberg, A. Guidance for Conservation of Macrofungi in Europe. European Council for the Conservation of Fungi. 2007. Available online: http://www.eccf.eu/Guidance_Fungi.pdf (accessed on 30 July 2021).
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willis, K.J. (Ed.) State of the World’s Fungi 2018 Report; Royal Botanic Gardens: Kew, UK, 2018; Available online: https://stateoftheworldsfungi.org/ (accessed on 7 May 2021).
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012. [Google Scholar]
- Nuytinck, J.; Wang, X.H.; Verbeken, A. Descriptions and taxonomy of the Asian representatives of Lactarius sect. Deliciosi. Fungal Divers. 2006, 22, 171–203. [Google Scholar]
- Favre, A.; Guichard, C. Lactarius helodes nov. sp. Bull. Mycol. Bot. Dauphiné-Savoie 2002, 164, 17–23. [Google Scholar]
- Nuytinck, J.; Verbeken, A.; Miller, S.L. Worldwide phylogeny of Lactarius section Deliciosi inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 2007, 99, 820–832. [Google Scholar] [CrossRef]
- Stubbe, D.; Verbeken, A. Lactarius subg. Plinthogalus: The European taxa and American varieties of L. lignyotus re-evaluated. Mycologia 2012, 104, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H. Type studies of Lactarius species published from China. Mycologia 2007, 99, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Bessette, A.E.; Harris, D.B.; Bessette, A.R. Milk Mushrooms of North America. A Field Identification Guide to the Genus Lactarius; Syracuse University Press: Syracuse, NY, USA, 2009. [Google Scholar]
- Bas, B. Les Lactaires en Franche-Comté: Généralités, Classification, Monographies, Cartographies, Toxicités & Recherches Scientifiques. Ph.D. Thesis, Université de Franche-Comté, Besançon, France, 2016. Available online: http://mycofme.free.fr/publications/these_pharmacie_les_lactaires.pdf (accessed on 21 April 2021).
- Della Maggiora, M.; Nuytinck, J. Lactifluus oedematopus e Lactifluus subvolemus, due specie poco conosciute raccolte in Toscana. Riv. Micol. 2018, 61, 157–172. [Google Scholar]
- De Crop, E.; Nuytinck, J.; Van de Putte, K.; Lecomte, M.; Eberhardt, U.; Verbeken, A. Lactifluus piperatus (Russulales, Basidiomycota) and allied species in Western Europe and a preliminary overview of the group worldwide. Mycol. Prog. 2014, 13, 493–511. [Google Scholar] [CrossRef]
- Silva-Filho, A.G.S.; Sulzbacher, M.A.; Ferreira, R.J.; Baseia, I.G.; Wartchow, F. Lactarius taedae (Russulales): An unexpected new gasteroid fungus from Brazil. Phytotaxa 2018, 379, 234–246. [Google Scholar] [CrossRef]
- Methven, A.S. North American and European Species of Lactarius. Scr. Bot. Belgica 2013, 51, 91–105. [Google Scholar]
- Stubbe, D.; Nuytinck, J.; Verbeken, A. Critical assessment of the Lactarius gerardii species complex (Russulales). Fungal Biol. 2010, 114, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Nuytinck, J.; Verbeken, A. Morphology and taxonomy of the European species in Lactarius sect. Deliciosi (Russulales). Mycotaxon 2005, 92, 125–168. [Google Scholar]
- Luo, X.; Karunarathna, S.C.; Luo, Y.H.; Xu, K.; Xu, J.C.; Chamyuang, S.; Mortimer, P.E. Drivers of macrofungal composition and distribution in Yulong Snow Mountain, southwest China. Mycosphere 2016, 7, 727–740. [Google Scholar] [CrossRef]
- De Crop, E.; Delgat, L.; Nuytinck, J.; Halling, R.E.; Verbeken, A. A short story of nearly everything in Lactifluus (Russulaceae). Fungal Syst. Evol. 2021, 7, 133–164. [Google Scholar] [CrossRef]
- Leonardi, M.; Furtado, A.N.M.; Comandini, O.; Geml, J.; Rinaldi, A.C. Halimium as an ectomycorrhizal symbiont. New records and an appreciation of known fungal diversity. Mycol. Prog. 2020, 19, 1495–1509. [Google Scholar] [CrossRef]
- Van de Putte, K.; Nuytinck, J.; Verbeken, A. Lactarius volemus (Russulales): From a traditional morphological species to a worldwide species complex. Scr. Bot. Belgica 2013, 51, 152–161. [Google Scholar]
- Moore, D.; Nauta, M.M.; Evans, S.E.; Rotheroe, M. Fungal Conservation, Issues and Solutions; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Mueller, G.M.M. Progress in conserving fungi. BG J. 2017, 14, 30–33. [Google Scholar]
- Nuytinck, J.; De Crop, E.; Delgat, L.; Bafort, Q.; Rivas Ferreiro, M.; Verbeken, A.; Wang, X.-H. Recent insights in the phylogeny, species diversity and culinary uses of milkcap genera Lactarius and Lactifluus. In Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences; Pérez-Moreno, J., Guerin-Laguette, A., Flores Arzú, R., Yu, F., Eds.; Springer: Cham, Switzerland, 2020; pp. 273–286. [Google Scholar]
- Nuytinck, J.; Ammirati, J.F. A new species of Lactarius sect. Deliciosi (Russulales, Basidiomycota) from western North America. Botany 2014, 92, 767–774. [Google Scholar]
- Das, K.; Verbeken, A.; Nuytinck, J. Morphology and phylogeny of four new Lactarius species from Himalayan India. Mycotaxon 2015, 130, 105–130. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-H.; Nuytinck, J.; Verbeken, A. Lactarius vividus sp. nov. (Russulaceae, Russulales), a widely distributed edible mushroom in central and southern China. Phytotaxa 2015, 231, 63–72. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Nuytinck, J.; Thanh Le, H.; De Crop, E.; Hampe, F.; Hyde, K.D.; Verbeken, A. Lactarius subgenus Russularia (Russulaceae) in South-East Asia, 3: New diversity in Thailand and Vietnam. Phytotaxa 2015, 207, 215–241. [Google Scholar] [CrossRef]
- Wang, X.-H. Three new species of Lactarius sect. Deliciosi from subalpine-alpine regions of central and southwestern China. Cryptogam. Mycol. 2016, 37, 493–508. [Google Scholar] [CrossRef]
- Lee, H.; Wissitrassameewong, K.; Park, M.S.; Verbeken, A.; Eimes, J.; Lim, Y.W. Taxonomic revision of the genus Lactarius (Russulales, Basidiomycota) in Korea. Fungal Divers. 2019, 95, 275–335. [Google Scholar] [CrossRef] [Green Version]
- Delgat, L.; Dierickx, G.; De Wilde, S.; Angelini, C.; De Crop, E.; De Lange, R.; Halling, R.; Manz, C.; Nuytinck, J.; Verbeken, A. Looks can be deceiving: The deceptive milkcaps (Lactifluus, Russulaceae) exhibit low morphological variance but harbour high genetic diversity. IMA Fungus 2019, 10, 14. [Google Scholar] [CrossRef]
- Duque Barbosa, J.A.É.; Delgat, L.; Elias, S.G.; Verbeken, A.; Neves, M.A.; de Carvalho, A.A., Jr. A new section, Lactifluus section Neotropicus (Russulaceae), and two new Lactifluus species from the Atlantic Forest, Brazil. System. Biodivers. 2020, 18, 347–361. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; David, J.C.; Stalpers, J.A. (Eds.) Ainsworth and Bisby’s Dictionary of the Fungi, 10th ed.; CABI Publishing: Wallingford, UK, 2008. [Google Scholar]
- Vidal, J.M. Arcangeliella borziana and A. stephensii, two gasteroid fungi often mistaken. A taxonomic revision of Lactarius-related sequestrate fungi. Rev. Catalana Micol. 2004, 26, 59–82. [Google Scholar]
- Vidal, J.M.; Alvarado, P.; Loizides, M.; Konstantinidis, G.; Chachuła, P.; Mleczko, P.; Moreno, G.; Vizzini, A.; Krakhmalnyi, M.; Paz, A.; et al. A phylogenetic and taxonomic revision of sequestrate Russulaceae in Mediterranean and temperate Europe. Persoonia 2019, 42, 127–185. [Google Scholar] [CrossRef] [PubMed]
- Silva-Filho, A.G.S.; Sulzbacher, M.A.; Grebenc, T.; Wartchow, F. Not every edible orange milkcap is Lactarius deliciosus: First record of Lactarius quieticolor (sect. Deliciosi) from Brazil. J. Appl. Bot. Food Qual. 2020, 93, 289–299. [Google Scholar]
- Comandini, O.; Contu, M.; Rinaldi, A.C. An overview of Cistus ectomycorrhizal fungi. Mycorrhiza 2006, 16, 381–395. [Google Scholar] [CrossRef]
- Comandini, O.; Rinaldi, A.C. Lactarius cistophilus Bon & Trimbach + Cistus sp. Descr. Ectomycorrhizae 2008, 11/12, 83–88. [Google Scholar]
- Verbeken, A.; Nuytinck, J.; Noordeloos, M.E. Russulales, part I. In Flora Agaricina Neerlandica; Nooerdeloos, M.E., Kuyper, T.W., Somhorst, I., Vellinga, E.C., Eds.; Candusso Editrice: Origgio, Italy, 2018; Volume 7, pp. 226–356. [Google Scholar]
- Buscardo, E.; Rodríguez-Echeverría, S.; Barrico, L.; García, M.Á.; Freitas, H.; Martín, M.P.; De Angelis, P.; Muller, L.A.H. Is the potential for the formation of common mycorrhizal networks influenced by fire frequency? Soil Biol. Biochem. 2012, 46, 136–144. [Google Scholar] [CrossRef]
- Vellinga, E.C.; Bérubé, J.; Castellano, M.; Mueller, G.M. Adding North American mushrooms to the IUCN Global Red List. Inoculum 2015, 66, 3–4. [Google Scholar]
- Dahlberg, A.; Mueller, G.M. Applying IUCN red-listing criteria for assessing and reporting on the conservation status of fungal species. Fungal Ecol. 2011, 4, 147–162. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0; IUCN: Gland, Switzerland; Cambridge, UK, 2012. [Google Scholar]
- McNabb, R.F.R. The Russulaceae of New Zealand 1. Lactarius DC ex S. F. Gray. N. Z. J. Bot. 1971, 9, 46–66. [Google Scholar]
- Hosaka, K.; Kobayashi, T.; Castellano, M.A.; Orihara, T. The status of voucher specimens of mushroom species thought to be extinct from Japan. Bull. Natl. Mus. Nat. Sci. (Ser. B) 2018, 44, 53–66. [Google Scholar]
- Ito, S.; Imai, S. Fungi of the Bonin Islands. IV. Trans. Sapporo Nat. Hist. Soc. 1940, 16, 45–56. [Google Scholar]
- Kibby, G. British Milkcaps Lactarius & Lactifluus. Privately Published. 2014.
- Ing, B. A Provisional Red Data List of British Fungi. Mycologist 1992, 6, 124–128. [Google Scholar] [CrossRef]
- Evans, S.; Henrici, A.; Ing, B. Preliminary Assessment: Red Data List of threatened British Fungi. British Mycological Society. 2006. Available online: https://www.britmycolsoc.org.uk/application/files/2013/3537/5755/RDL_of_Threatened_British_Fungi.pdf (accessed on 25 March 2021).
- Yorou, N.S.; De Kesel, A. Champignons supérieurs/Larger fungi. In Protection de la Nature en Afrique de l’Ouest: Une Liste Rouge pour le Bénin/Nature Conservation in West Africa: Red List for Benin; Neuenschwander, P., Sinsin, B., Goergen, G., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2011; pp. 47–60. [Google Scholar]
- Dahlberg, A.; Genney, D.R.; Heilmann-Clausen, J. Developing a comprehensive strategy for fungal conservation in Europe: Current status and future needs. Fungal Ecol. 2010, 3, 50–64. [Google Scholar] [CrossRef]
- Barge, E.G.; Cripps, C.L. New reports, phylogenetic analysis, and a key to Lactarius Pers. in the Greater Yellowstone Ecosystem informed by molecular data. MycoKeys 2016, 15, 1–58. [Google Scholar] [CrossRef] [Green Version]
- Barge, E.G.; Cripps, C.L.; Osmundson, T.W. Systematics of the ectomycorrhizal genus Lactarius in the Rocky Mountain alpine zone. Mycologia 2016, 108, 414–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stubbe, D.; Le, H.T.; Wang, X.-H.; Nuytinck, J.; Van de Putte, K.; Verbeken, A. The Australasian species of Lactarius subgenus Gerardii (Russulales). Fungal Divers. 2012, 52, 141–167. [Google Scholar] [CrossRef]
- Das, K. Lactarius fennoscandicus (Russulaceae), a new record for India. Nelumbo 2013, 55, 214–218. [Google Scholar]
- Calledda, F.; Boerio, G.; Cartabia, M.; Carbone, M. Studio del genere Lactarius. 1° contributo. Lactarius flavoaspideus e prima segnalazione per l’Italia. Studio e tipificazione di Lactarius aspideus. Prime evidenze genetiche di Lactarius flavidus. Micol. Toscana 2020, 2, 9–46. [Google Scholar]
- Zhishu, B.; Guoyang, Z.; Taihui, L. The Macrofungus flora of China’s Guangdong Province; Chinese University Press: Hong Kong, China, 1993. [Google Scholar]
- Vellinga, E.C.; Wolfe, B.E.; Pringle, A. Global patterns of ectomycorrhizal introductions. New Phytol. 2009, 181, 960–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryzenhout, M. The need to engage with citizen scientists to study the rich fungal biodiversity in South Africa. IMA Fungus 2015, 6, A58–A64. [Google Scholar] [CrossRef] [Green Version]
- Irga, P.J.; Barker, K.; Torpy, F.R. Conservation mycology in Australia and the potential role of citizen science. Conserv. Biol. 2018, 32, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Heilmann-Clausen, J.; Bruun, H.H.; Ejrnæs, R.; Frøslev, T.G.; Læssøe, T.; Petersen, J.H. How citizen science boosted primary knowledge on fungal biodiversity in Denmark. Biol. Conserv. 2019, 237, 366–372. [Google Scholar] [CrossRef]
- Feldman, M.J.; Imbeau, L.; Marchand, P.; Mazerolle, M.J.; Darveau, M.; Fenton, N.J. Trends and gaps in the use of citizen science derived data as input for species distribution models: A quantitative review. PLoS ONE 2021, 16, e0234587. [Google Scholar] [CrossRef]
- Schwab, N. Nomenclatural novelties. Index Fungorum 2019, 409, 1. [Google Scholar]
- Sarnari, M. Una nuova specie di Lactarius dell’area mediterranea Lactarius castanopus sp.nov. Riv. Micol. 1992, 35, 229–232. [Google Scholar]
- Basso, M.T. Revisione “Lactaires du Maroc”. In Compléments à la Flore des Champignons Supérieurs du Maroc de G. Malençon et R. Bertault; Maire, J.C., Moreau, P.-A., Robich, G., Eds.; CEMM: Nice, France, 2009; pp. 653–688. [Google Scholar]
- Pérez-De-Gregorio, M.A.; Campos, J.C.; Illescas, T. Lactarius purpureobadius Malençon ex Basso, en España. Rev. Catalana Micol. 2012, 34, 81–86. [Google Scholar]
- Basso, M.T. Lactarius cyanopus une nouvelle espéce de la sect. Dapetes Fries. Bull. Trimest. Soc. Mycol. Fr. 1998, 114, 67. [Google Scholar]
- Nuytinck, J.; Verbeken, A. Species delimitation and phylogenetic relationships in Lactarius section Deliciosi in Europe. Mycol. Res. 2007, 11, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.T. Contributo allo studio dei Lactarius mediterranei: L. cyanopus e L. pseudoscrobiculatus. Micol. Veg. Mediterr. 2001, 16, 97–104. [Google Scholar]
- Polemis, E.; Nuytinck, J.; Fryssouli, V.; Pera, U.; Zervakis, G.I. Phylogeny, ecology and distribution of the rare Mediterranean species Lactarius pseudoscrobiculatus (Basidiomycota, Russulales). Plant Syst. Evol. 2019, 305, 755–764. [Google Scholar] [CrossRef]
- Buchanan, P.K.; May, T.W. Conservation of New Zealand and Australian fungi. N. Z. J. Bot. 2003, 41, 407–421. [Google Scholar] [CrossRef]
- Zotti, M.; Persiani, A.M.; Ambrosio, E.; Vizzini, A.; Venturella, G.; Donnini, D.; Angelini, P.; Di Piazza, S.; Pavarino, M.; Lunghini, D.; et al. Macrofungi as ecosystem resources: Conservation versus exploitation. Plant Biosyst. 2013, 147, 219–225. [Google Scholar] [CrossRef] [Green Version]
- May, T.W.; Cooper, J.A.; Dahlberg, A.; Furci, G.; Minter, D.W.; Mueller, G.M.; Pouliot, A.; Yang, Z. Recognition of the discipline of conservation mycology. Conserv. Biol. 2019, 33, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Wang, K.; Yu, X.; Li, Y.; Wu, H.; Wu, H.; Wang, Y.; Wei, X.; Li, B.; Jiang, L.; et al. Assessment of the threatened status of macro-basidiomycetes in China. Biodivers. Sci. 2020, 28, 41–53. [Google Scholar]




| Species § | Distribution | List(s) * | Category # |
|---|---|---|---|
| Lactarius acatlanensis Bandala, Montoya, and Ramos | Mexico | GFRLI | EN |
| Lactarius acerrimus Britzelm. | widely distributed in Europe, Russia, China (?), Colombia (?) | ARL BuRL CRL DRL ECCF ERL FRL FrRL GRL LRL NBIC NRL PRL SRL SwRL | NT VU LC VU LC VU CR LC TH EN EN TNB R VU LC |
| Lactarius acris1 (Bolton) Gray | widely distributed in Europe, Russia, Japan (?), China (?), India (?) | ARL CRL CrRL CRRL DRL ECCF FrRL GRL MRL NBIC NMRL NRL PRL SlRL SwRL | LC LC NT EN LC LC NT/LC TH EKSP NT NT VN R NT NT |
| Lactarius acrissimus Verbeken and Van Rooij | Benin | BeRL | VU |
| Lactarius aestivus Nuytinck and Ammirati | USA | GFRLI | LC |
| Lactarius afroscrobiculatus Verbeken and Van Rooij | Benin, Togo | BeRL | VU |
| Lactarius agglutinatus Burl. | Canada, USA, China (?) | CRL GFRLI | DD DD |
| Lactarius albivellus 2 Romagn. | France, Belgium | FrRL | DD |
| Lactarius albocarneus 3 Britzelm. | widely distributed in Europe, Russia, USA (?), China (?) | ARL CRL CRRL DRL ECCF FrRL GRL NMRL PRL SwRL | VU DD CR VU LC NT/LC/DD NT NT R NA |
| Lactarius alpinus Peck | Germany, Austria, Italy, France, Switzerland, Russia, Greenland, Alaska, USA, Canada, Ecuador (?), Argentina (?) | ARL GRL | NT ER |
| Lactarius angustifolius Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius aquizonatus Kytöv. | central and more frequently northern Europe, Russia, USA | ARL DRL ECCF ERL FRL GRL NBIC SwRL | EN CR LC LC LC ER NT LC |
| Lactarius areolatus Hesler and A.H. Sm. | USA, Mexico, China (?) | CRL | DD |
| Lactarius aspideus (Fr.: Fr.) Fr. | widely distributed in central and northern Europe, Russia, USA, Canada, China (?), Japan (?) | ARL CRL CRRL DRL ECCF ERL FRL FrRL GRL NBIC NRL PRL RRL SRL SwRL | EN DD EN LC LC LC LC CR/EN/NT HT LC KW V NT EN LC |
| Lactarius atlanticus Bon | southern Europe, up to northern Italy, southern France, Slovenia, Malta | ECCF FrRL | LC NT/LC |
| Lactarius atro-olivaceus Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius atrosquamulosus X. He | China | CRL | DD |
| Lactarius atroviridis Peck | USA, Canada, Costa Rica, Colombia, China (?) | CRL | DD |
| Lactarius aurantiacoochraceous 4 Lar.N. Vassiljeva | Russia, China (?) | CRL | DD |
| Lactarius aurantiacus 5 (Pers.) Gray | widely distributed in Europe, Russia, Greenland, USA (?), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SwRL | LC LC LC LC LC LC LC/DD NotT LC KW LC |
| Lactarius aurantifolius Verbeken | eastern Africa, Benin, Zambia, Burundi, Zimbabwe, Madagascar | BeRL | CR |
| Lactarius aurantiofulvus 6 J. Blum ex Bon | central Europe | FrRL | LC |
| Lactarius aurantiosordidus Nuytinck and S.L. Mill. | Canada, USA, China (?) | GFRLI | LC |
| Lactarius auriolla Kytöv. | Norway, Finland, Sweden, Estonia, Russia | ERL FRL NBIC SwRL | CR LC DD LC |
| Lactarius austrostratus Wisitr. and Verbeken | China, Thailand | GFRLI | Proposed |
| Lactarius azonites 7 (Bull.) Fr. | widely distributed in Europe, Russia, USA (?), China (?), Japan (?), India (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC NMRL NRL SRL SwRL | LC DD DD LC LC LC NT/LC NotT VU NT GE VU LC |
| Lactarius badiosanguineus Kühner and Romagn. | central and northern Europe, Russia, USA, Canada, China | ARL CRL CRRL DRL ECCF ERL FRL FrRL GRL HRL NBIC SwRL | LC DD EN DD LC LC LC VU/LC TUE 1 LC LC |
| Lactarius bisporus Verbeken and F. Hampe | Thailand, China (?) | CRL | DD |
| Lactarius blennius 8 (Fr.) Fr. | widely distributed in Europe, Russia, China (?) | ARL CRL DRL ECCF FRL FrRL GRL NBIC SwRL | LC LC LC LC LC LC/DD NotT LC LC |
| Lactarius blumii 9 Bon | Spain, Germany, Estonia, China (?) | CRL | DD |
| Lactarius borzianus 10 (Cavara) Verbeken and Nuytinck | central and southern Europe | ARL SRL | NT NT |
| Lactarius bresadolanus 11 Singer | Austria, Italy, Switzerland, Sweden, China (?) | CRL | DD |
| Lactarius britannicus 12 D.A. Reid | central and southern Europe, Great Britain | ARL FrRL GRL | LC LC/DD DD |
| Lactarius brunneohepaticus M.M. Moser | Germany, Austria, France, Poland, Greenland | ARL GRL | LC DD |
| Lactarius brunneoviolaceus M.P. Christ. | central and northern Europe, Russia, Iceland, Svalbard, | ARL FRL NBIC SwRL | VU DD NE LC |
| Lactarius californiensis Hesler and A.H. Sm. | USA | GFRLI | LC |
| Lactarius camphoratus (Bull.) Fr. | central and northern Europe, Russia, North America, Mexico, Costa Rica, Colombia, Korea (?), Japan (?), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL SwRL | LC LC LC LC LC LC LC NotT 3 LC TNB LC |
| Lactarius carbonicola A.H. Sm. | USA, Canada, China (?) | CRL | DD |
| Lactarius castaneus W.F. Chiu | China | CRL | LC |
| Lactarius castanopsidis Hongo | Japan, Malaysia, Korea (?), China (?) | CRL | DD |
| Lactarius chamaeleontinus R. Heim | central and eastern Africa, Benin, Togo, Democratic Republic of Congo, Zambia | BeRL | CR |
| Lactarius changbaiensis Y. Wang and Z.X. Xie | China | CRL | VU |
| Lactarius chelidonium Peck | USA, Canada, Haiti, China (?) | CRL | LC |
| Lactarius chiapanensis Montoya, Bandala, and Guzmán | Mexico | GFRLI | VU |
| Lactarius chichuensis W.F. Chiu | China, Thailand | CRL | LC |
| Lactarius chrysorrheus Fr. | widely distributed in Europe, Russia, North America, Mexico, Colombia, Japan (?), China (?) | ARL CRL DRL ECCF ERL FrRL GRL NBIC PRL SwRL URL | LC LC LC LC EN LC NotT LC R LC VU |
| Lactarius cinereus Peck | USA, Canada, China (?) | CRL | DD |
| Lactarius cinnamomeus W.F. Chiu | China, Korea, Thailand, Vietnam | CRL | DD |
| Lactarius circellatus Fr. | central and northern Europe, Russia, North America, Japan (?), China (?) | ARL CRL DRL ECCF ERL FrRL GRL NBIC SwRL | LC LC LC LC NA LC/DD NotT NA LC |
| Lactarius cistophilus Bon and Trimbach | southern Europe, Mediterranean area | CrRL ECCF | VU LC |
| Lactarius citriolens Pouzar | central and northern Europe, Russia, Iceland, China (?) | ARL CRL CRRL DRL ECCF ERL FRL FrRL GRL NBIC RRL SRL SwRL | CR DD EN DD Relevant NT LC EN/VU HT NT NT VU LC |
| Lactarius clethrophilus 13 Romagnesi | France | FrRL | VU |
| Lactarius coccolobae O.K. Mill. and Lodge | British Virgin Islands, Puerto Rico | GFRLI | EN |
| Lactarius controversus Pers. | widely distributed in Europe, Great Britain, Russia, North America, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL MRL NBIC PRL SRL SwRL | NT LC LC LC EN LC LC NotT EKSP VU E VU LC |
| Lactarius cookei 14 Z. Schaef. | Austria, Germany, Slovakia | GRL SlRL | DD DD |
| Lactarius cordovaensis Hesler and A.H. Sm. | USA | GFRLI IUCN | DD DD |
| Lactarius corrugis 15 Peck | North America, Japan (?), China (?) | CRL | LC |
| Lactarius crassus (Singer and A.H. Sm.) Pierotti | USA | GFRLI | NE |
| Lactarius cremor 16 Fr. | central and northern Europe, Russia (?) | CRRL FrRL | DD NT/LC/DD |
| Lactarius croceus Burl. | USA, Canada, Costa Rica, China (?) | CRL | DD |
| Lactarius cyathula 17 (Fr.) Fr. | Great Britain, France, Germany, The Netherlands, China (?) | CRL | DD |
| Lactarius cyathuliformis Bon | central and northern Europe, Great Britain, Ireland | ARL DRL ECCF ERL FRL FrRL GRL NBIC NMRL SwRL | VU LC LC LC LC VU/DD NotT LC DD LC |
| Lactarius decipiens Quél | widespread in Europe, Great Britain, Russia, China (?) | ARL CRL DRL ECCF FrRL GRL NBIC NRL SwRL | LC LC VU LC LC/DD NT NA BE NT |
| Lactarius delicatus Burl. | USA, China (?) | CRL | DD |
| Lactarius deliciosus 18 (L.) Gray | Europe, Turkey, Morocco, Russia, North America, China, a cosmopolitan species, introduced in many areas together with its host plants (Pinus spp.). In some cases (e.g., Guatemala, North America), the name has been probably misapplied to indicate distinct, local taxa | ARL CRL DRL ECCF ERL FRL FrRL GRL GuRL HRL NBIC NMRL NRL SwRL | LC LC LC LC LC LC NT/LC/NA NotT 3 4 LC LC TNB LC |
| Lactarius deterrimus Gröger | widespread in Europe, Russia, Turkey, North America (?), China (?), Japan (?). In some cases, the name has been probably misapplied to indicate distinct, local taxa | ARL CRL DRL ECCF ERL FRL FrRL GFRLI GRL NBIC NMRL SwRL | LC LC LC LC LC LC LC/NA Proposed NotT LC NT LC |
| Lactarius dryadophilus Kühner | central and northern Europe, Russia, Iceland, Svalbard, Greenland, USA, Canada | ARL ECCF FRL GRL NBIC SRL SwRL | VU LC NT ER LC EN LC |
| Lactarius duplicatus A.H. Sm. | Sweden, Finland, Norway, Russia, Greenland, USA, Canada | SwRL | LC |
| Lactarius echinatus Thiers | USA, Mexico, China | CRL | DD |
| Lactarius edulis Verbeken and Buyck | Benin, Togo, Burundi, Democratic Republic of Congo, Malawi, Tanzania, Zambia, Zimbabwe, Madagascar | BeRL | VU |
| Lactarius evosmus Kūhner and Romagn. | Italy, central and northern Europe, Great Britain | ARL CRRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SwRL | VU CR EN LC LC NT LC/DD NT NT TNB LC |
| Lactarius fallax A.H. Sm. and Hesler | Canada, USA | GFRLI | DD |
| Lactarius fascinans (Fr.) Fr. | Switzerland, Austria, Germany, Slovenia, Finland, Alaska | ARL FrRL SRL | LC CR/EN CR |
| Lactarius favrei 19 H. Jahn | France | FrRL | LC |
| Lactarius fennoscandicus Verbeken and Vesterh. | northern Europe, India (?) | ERL FRL SwRL | NT LC LC |
| Lactarius firmus 20 Pacioni and Lalli | Italy, France, China (?) | CRL | DD |
| Lactarius flavidulus S. Imai | Japan, Korea, China (?) | CRL | DD |
| Lactarius flavidus Boud. | central and northern Europe, Great Britain, Italy, Greenland | ARL DRL ECCF FrRL GRL HRL PRL SRL SwRL | NT CR LC NT/LC/DD HT 3 V VU NT |
| Lactarius flavoaspideus Kytöv. | Finland, Italy | FRL | LC |
| Lactarius flavopalustris Kytöv. | northern Europe, Great Britain, Austria | ARL ERL FRL SwRL | EN NT LC NE |
| Lactarius flexuosus 21 (Pers.) Gray | central and northern Europe, Great Britain, Russia, Iceland, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC SRL SwRL | NT DD DD LC LC LC VU/NT/LC/DD TUE 1 LC VU LC |
| Lactarius fluens Boud. | central and northern Europe, Great Britain, Russia, Spain, Italy | ARL CRRL DRL ECCF FrRL GRL NBIC NRL SwRL | LC DD LC LC LC/DD NotT LC TNB LC |
| Lactarius foetens Verbeken and Van Rooij | Benin, Togo | BeRL | CR |
| Lactarius fraxineus Romagn. | France, Belgium, Italy, Turkey | FrRL | DD |
| Lactarius fuliginellus A.H. Sm. and Hesler | USA, Canada, Mexico | GFRLI | DD |
| Lactarius fuliginosus (Fr.:Fr.) Fr. | central and northern Europe, Great Britain, Russia, Spain, Italy, North America, China (?), Japan (?), India (?) | ARL CRL CRRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SwRL | LC LC DD DD LC NT LC LC/DD NotT LC BE LC |
| Lactarius fulvissimus 22 Romagn. | central and northern Europe, Great Britain, Spain, Italy, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC SwRL | LC DD LC LC NT NT LC/DD NotT NE LC |
| Lactarius fuscomarginatus Montoya, Bandala, and I. Haug | Mexico | GFRLI | EN |
| Lactarius fusco-olivaceous Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius fuscus 23 Rolland | central Europe, Greenland | FrRL | EN/VU/DD |
| Lactarius gerardii Peck | North America, Costa Rica, Colombia, Japan (?), Malaysia (?), China (?) | CRL | LC |
| Lactarius glyciosmus Fr. | central and northern Europe, Great Britain, Russia, Iceland, Svalbard, Greenland, North America, China (?) | ARL CRL DRL ERL FRL FrRL GRL NBIC SwRL | LC LC LC LC LC LC NotT LC LC |
| Lactarius gracilis Hongo | Japan, Korea, Thailand, China | CRL | LC |
| Lactarius grandisporus Lar.N. Vassiljeva | Russia, China (?) | CRL | DD |
| Lactarius griseus Peck | North America, Greenland, China (?) | CRL | DD |
| Lactarius hatsudake Nobuj. Tanaka | Japan, eastern Russia, Korea, Laos, Thailand, China | CRL | LC |
| Lactarius haugiae Bandala, Montoya, and Ramos | Mexico | GFRLI IUCN | VU VU |
| Lactarius helodes 24 A. Favre and Guichard | France | ECCF | DD |
| Lactarius helvus Plowr. | central and northern Europe, Great Britain, Russia, North America, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL SRL SwRL | LC LC LC LC LC LC NT/LC NotT 1 LC TNB VU LC |
| Lactarius hepaticus Plowr. | widespread in Europe, Great Britain, Russia, Greenland, North America (?), China (?) | ARL CRL CRRL DRL ECCF FrRL GRL NBIC NRL SRL SwRL | VU DD DD LC LC EN/LC/DD/NA NotT LC TNB VU LC |
| Lactarius hirtipes J.Z. Ying | China | CRL | DD |
| Lactarius hortensis 25 Velen. | Denmark, Germany, The Netherlands, Estonia, Finland, Sweden | GRL | NotT |
| Lactarius hygrophoroides 26 Berk and M.A. Curtis | North America, French Guiana (?), China (?) | CRL | LC |
| Lactarius hysginoides Korhonen and T. Ulvinen | central and northern Europe, Great Britain, Russia, Svalbard, Iceland, Greenland | ARL FRL GRL NBIC SwRL | EN LC DD LC LC |
| Lactarius hysginus (Fr.:Fr.) Fr. | central and northern Europe, Great Britain, Russia, Iceland, North America, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL PRL SRL SwRL | NT LC DD LC CR LC NT/LC/DD/NA HT 1 LC BE V VU LC |
| Lactarius ichoratus 27 (Batsch) Fr. | Sweden, Denmark, The Netherlands, Germany, France, Austria, Switzerland | FrRL NRL | LC KW |
| Lactarius ilicis Sarnari | southern Europe, Spain, France, Italy, Croatia | FrRL | VU/LC |
| Lactarius illyricus Piltaver | Slovenia, Switzerland, Germany, Spain, Italy | ARL FrRL GRL | NT DD DD |
| Lactarius imbricatus M.X. Zhou and H.A. Wen | China | CRL | DD |
| Lactarius imperceptus Beardslee and Burl. | eastern USA, China (?) | CRL | DD |
| Lactarius indigo 28 (Schwein.) Fr. | eastern USA, Mexico, Belize, Guatemala, Costa Rica, central America, Colombia, Japan (?), China (?), India (?) | CRL GuRL | LC 3 |
| Lactarius iners 29 Kühner | France, Denmark | FrRL | DD |
| Lactarius insulsus 30 (Fr.) Fr. | central Europe, Sweden, USA (?), Japan (?) | GRL NRL PRL | DD TNB E |
| Lactarius intermedius (Krombh.) Berk. and Broome | Germany, Austria, Switzerland, France, Spain, Italy | ARL ECCF FrRL GRL | LC LC LC NotT |
| Lactarius kauffmanii Hesler and A.H. Sm. | Canada, USA | GFRLI | LC |
| Lactarius kesiyae Verbeken and K.D. Hyde | China, India, Korea, Thailand, Vietnam | GFRLI | Proposed |
| Lactarius lacunarum Romagn. ex Hora | widespread in Europe, Russia, China (?) | ARL CRL CrRL CRRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL PRL SRL SwRL | VU DD VU NT LC LC LC LC LC/DD TUE 2 LC TNB E VU LC |
| Lactarius lanceolatus O.K. Mill. and Laursen | Norway, Sweden, Finland, Great Britain, Svalbard, Greenland, Russia, North America | FRL NBIC SwRL | DD LC NA |
| Lactarius lapponicus 31 Harmaja | Norway, Sweden, Finland, Russia, Greenland | FRL NBIC | LC LC |
| Lactarius leonis Kytöv. | Norway, Sweden, Finland, Estonia, Germany, Austria, Switzerland, Italy, Russia | ARL DRL ERL FRL GRL NBIC SwRL | NT DD NT LC DD DD LC |
| Lactarius lepidotus 32 Hesler and A.H. Sm. | Germany, Austria, France, Norway, Russia | ARL FrRL GRL NBIC | NT VU DD NE |
| Lactarius lignicola W.F. Chiu | China | CRL | LC |
| Lactarius lignyotus 33 Fr. | widely distributed in Europe, Russia, Ukraine, North America (?), Colombia (?), Japan (?), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL LRL NBIC SwRL URL | LC LC EN LC NT LC VU/LC TH VU LC LC R |
| Lactarius lilacinus (Lasch:Fr.) Fr. | widely distributed in Europe, Russia, China (?) | ARL CRL CrRL CRRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NMRL NRL PRL SloRL SlRL SwRL | VU DD VU EN LC LC LC LC EN/VU/NT/LC TH 1 LC VU BE R V NT LC |
| Lactarius luculentus Burl. | western North America, China (?) | CRL | DD |
| Lactarius luridus (Pers.) Gray | widely distributed in Europe, Iceland | ARL CRRL DRL ECCF ERL FrRL GRL NBIC SwRL | LC DD DD DD NT NT/LC/DD TUE NT NE |
| Lactarius luteocanus Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius luteus 34 A. Blytt | Norway, Austria, France | NBIC | NE |
| Lactarius maculatus Peck | USA, Canada, China (?) | CRL | DD |
| Lactarius mairei 35 Malençon | mostly distributed in southern Europe, but present also in central and northern European countries, Great Britain, Russia, Svalbard | ARL BRL CRRL DRL ECCF ERL FrRL GRL MRL NBIC NRL RRL SRL SwRL | EN NT EN CR LC CR NT/LC/DD ER EKSP NE EB NT EN VU |
| Lactarius mammosus Fr. | central and northern Europe, Great Britain, Russia, Iceland, North America (?) | ARL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL SRL SwRL | LC VU LC LC LC VU/NT/LC NotT 1 LC KW VU LC |
| Lactarius maruiaensis McNabb | New Zealand | GFRLI NZTCS | EW NC |
| Lactarius mediterranensis Listosella and Bellù | distributed in the Mediterranean area of southern Europe | FrRL | VU |
| Lactarius miniatescens Verbeken and Van Rooij | Benin, Togo, Ghana, Burkina Faso | BeRL | CR |
| Lactarius minimus 36 W.G. Sm. | Great Britain, China (?) | CRL | EN |
| Lactarius mucidus Burl. | USA, Canada, China (?) | CRL | DD |
| Lactarius muscicola Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius musteus Fr. | a mostly north European species, including Great Britain, present also in central Europe and Russia, China (?) | ARL BRL CRL CRRL DRL ECCF ERL FRL FrRL GRL MRL NBIC SloRL SRL SwRL | EN NT LC EN EN Relevant NT LC EN/DD HT EKSP LC V EN NT |
| Lactarius mutabilis Peck | USA, Canada, China (?) | CRL | DD |
| Lactarius nanus J. Favre | linked to dwarf willows (e.g., Salix herbacea L., S. arctica Pall.) in artic and alpine areas of central and northern Europe; Great Britain, Iceland, Greenland, Svalbard, Russia, North America, China (?) | ARL CRL ECCF GRL NBIC SlRL SwRL | VU DD LC ER LC DD LC |
| Lactarius necator 37 (Bull.) Pers. | widely distributed in Europe, Russia, Iceland, Greenland, North America, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC SwRL | LC LC LC LC LC LC LC NotT 3 LC LC |
| Lactarius nigroviolascens 38 G.F. Atk. | USA, Canada, Japan (?), China (?) | CRL | DD |
| Lactarius nothofagi R. Heim (nom. inval.) | New Zealand | NZTCS | DD |
| Lactarius novae-zelandiae McNabb | New Zealand | GFRLI IUCN | EN EN |
| Lactarius obliquus 39 Fr. | China (?) | CRL | LC |
| Lactarius obscuratus 40 (Lasch) Fr. | widely distributed in Europe, Russia, Greenland, North America (?), Colombia (?), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC SwRL | NT DD LC LC LC LC EN/LC NotT 2 LC LC |
| Lactarius occidentalis A.H. Sm. | Canada, USA, China (?) | CRL GFRLI | DD LC |
| Lactarius oedehyphosus 41 Izderda and Noordeloos | France | FrRL | DD |
| Lactarius oedematopus 42 (Scop.) Kuntze | Germany, Austria, Belgium, Slovakia, France, Bulgaria, Italy | GRL | DD |
| Lactariusogasawarashimensis S. Ito and S. Imai | Japan | JRL | EX |
| Lactarius olivinus Kytöv. | Norway, Finland, Sweden, Estonia | ECCF ERL FRL NBIC SwRL | Relevant LC LC DD NT |
| Lactarius omeiensis W.F. Chiu | China | CRL | DD |
| Lactarius omphaliformis Romagn. | central and northern Europe, Great Britain, Russia | ARL CrRL CRRL DRL ECCF FRL FrRL GRL HRL NBIC NMRL NRL SRL SwRL | VU VU DD LC LC LC EN/NT NT 1 LC CR KW VU LC |
| Lactarius pallescens Hesler and A.H. Sm. | Canada, USA | GFRLI | LC |
| Lactarius pallidiolivaceus Hesler and A.H. Sm. | USA | GFRLI | DD |
| Lactarius pallidus Pers. | widely distributed in Europe, Russia, Greenland, China (?) | ARL CRL DRL ECCF FrRL GRL NBIC NRL SwRL | LC LC LC LC LC NotT LC BE LC |
| Lactarius paludinellus Peck | USA, China (?) | CRL | DD |
| Lactarius paradoxus Beardslee and Burl. | USA, Canada, Dominican Republic, Cuba | CuRL | CR |
| Lactarius parvus Peck | USA, Canada, China (?) | CRL | DD |
| Lactarius paulus P.M. Kirk | USA | GFRLI | Proposed |
| Lactarius peckii Burl. | USA, Costa Rica, China (?) | CRL | DD |
| Lactarius pergamenus 43 (Sw.) Fr. | central Europe, Great Britain, Ireland, Sweden, China (?) | CRL FrRL | DD LC/DD |
| Lactarius picinus Fr. | central and northern Europe, Great Britain, Russia, Iceland, China (?) | ARL CRL ECCF FrRL GRL HRL NBIC PRL RRL SwRL | LC LC LC LC NotT 2 LC R NT LC |
| Lactarius pilatii Z. Schaef. | Czech Republic, Germany, Austria, Denmark, Finland, Sweden, Estonia, Norway, Greenland | ARL CRRL ERL FRL GRL NBIC SwRL | CR DD NT LC DD LC LC |
| Lactarius pinastri 44 Romagn. | France | FrRL | LC |
| Lactarius pinckneyensis Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius porninsis 45 Rolland | Italy, central and northern Europe, Great Britain, Russia, Japan, China (?), strictly associated with Larix | ARL CRL CrRL DRL ECCF FRL FrRL GRL SwRL | LC LC EN EN LC DD NA NotT NA |
| Lactarius pseudomucidus Hesler and A.H. Sm. | Canada, USA | GFRLI | LC |
| Lactarius pseudoscrobiculatus Basso, Neville, and Poumarat | southern Europe, Mediterranean area, France, Spain, Italy | GFRLI | Proposed |
| Lactarius pseudouvidus Kühner | mostly northern Europe, Great Britain, France, Austria, Iceland, Russia, Svalbard, Faroe Islands, Greenland, Canada, USA | ARL ECCF FRL NBIC SwRL | VU LC DD LC LC |
| Lactarius pterosporus Romagn. | widely distributed in Europe, Russia, Japan (?), China (?) | ARL CRL CRRL DRL ECCF FrRL GRL NBIC NRL SwRL | LC DD EN LC LC LC NotT VU EB LC |
| Lactarius pubescens (Schrad). Fr. | widely distributed in Europe, Russia, Iceland, Greenland, Canada, USA, China (?), Australia, New Zealand (introduced with its mycorrhizal host Betula) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC SwRL | LC LC LC LC LC LC LC NotT 3 LC LC |
| Lactarius pyrogalus (Bull.) Fr. | widely distributed in Europe, Russia, USA, Canada, China (?), linked to Corylus | ARL CRL DRL ERL FRL FrRL NBIC SwRL | LC DD LC LC LC LC LC LC |
| Lactarius quieticolor 46 Romagn. | widely distributed in Europe, Brazil (introduced), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SRL SwRL | NT LC LC LC LC LC EN/LC/DD/NA NotT LC TNB EN LC |
| Lactarius quietus (Fr.) Fr. | widely distributed in Europe, Russia, USA, Canada, Japan (?), China (?) | ARL CRL DRL ERL FRL FrRL GRL NBIC SwRL | LC LC LC LC LC LC NotT LC LC |
| Lactarius repraesentaneus Britzelm. | central and northern Europe, Great Britain, Russia, Greenland, Iceland, North America, Japan, China (?) | ARL BRL CRL CRRL DRL ECCF ERL FRL FrRL GRL HRL LRL NBIC PRL SlRL SRL SwRL | VU NT LC EN EN LC NT LC EN/VU HT 1 VU LC E NT VU LC |
| Lactarius resimus (Fr.) Fr. | widely distributed across Europe, more common in the northern part, Great Britain, Russia, North America, China (?) | ARL BRL CRL CRRL DRL ECCF ERL FRL FrRL GRL HRL LRL NBIC NRL PRL SRL SwRL | EN VU DD CR RE LC NT LC DD TE 1 VU NT EB E EN LC |
| Lactarius rimosellus Peck | Canada, USA, Mexico, Guatemala, Costa Rica, Colombia, China (?) | CRL | DD |
| Lactarius robertianus 47 Bon | France, Spain, Germany, Italy, Russia, Svalbard, with Salix in alpine zones | GRL | DD |
| Lactarius romagnesii 48 Bon. | central and northern Europe, North America, China (?) | ARL CRL CRRL DRL ECCF FrRL GRL NBIC SRL SwRL | NT DD DD LC LC LC/DD TUE LC VU LC |
| Lactarius roseozonatus (H. Post) Britzelm. | France, Denmark, Finland, Sweden, Estonia, Russia | ECCF FRL NRL | DD LC VN |
| Lactarius rostratus Heilmann-Clausen | Switzerland, Sweden, Estonia, Austria, Belgium, France, Germany, Denmark, Norway, Great Britain | ARL DRL ECCF ERL NBIC SRL SwRL | NT DD Relevant NA NA VU NE |
| Lactarius rubidus (Hesler and A.H. Sm.) Methven | USA, Canada, Colombia | GFRLI IUCN | LC LC |
| Lactarius rubrilacteus Hesler and A.H. Sm. | Canada, USA | GFRLI | LC |
| Lactarius rubriviridis Desjardin, H.M. Saylor, and Thiers | USA | GFRLI | NE |
| Lactarius rubrocintus Fr. | central and northern Europe, Great Britain | ARL CRRL DRL ECCF FrRL GRL HRL NBIC SwRL | LC CR LC LC EN/VU/LC TUE 1 NE LC |
| Lactarius rufomarginatus Verbeken and Van Rooij | Benin | BeRL | CR |
| Lactarius rufulus 49 Peck | USA, Mexico | GFRLI | LC |
| Lactarius rufus (Scop.) Fr. | widespread in Europe, Russia, Iceland, Greenland, Svalbard, North America. Introduced in several distant areas (e.g., Brazil, New Zealand) with its host plants, Pinus and Picea | ARL CRL DRL ECCF ERL FRL FrRL GFRLI GRL HRL NBIC NRL SwRL | LC LC LC LC LC LC LC/DD Proposed NotT 3 LC TNB LC |
| Lactarius ruginosus Romagn. | central and northern Europe, Great Britain, Russia | ARL CRRL DRL ECCF ERL FrRL GRL NBIC NRL SRL SwRL | NT EN LC LC NA LC/DD NotT NE EB NT LC |
| Lactarius sakamotoi S. Imai | Japan, China | CRL | DD |
| Lactarius salicis-herbaceae Kühner | Norway, Sweden, Finland, alpine areas of France, Austria, Switzerland and Italy, Russia, Iceland, Greenland, Canada, Alaska, always linked to its host plants, dwarf and shrubby Salix | ARL ECCF FRL NBIC SRL SwRL | VU LC NT LC VU LC |
| Lactarius salicis-reticulatae Kühner | Norway, Sweden, Finland, alpine areas of France, Austria, Switzerland, Italy and Spain (Pyrenees), Great Britain, Poland, Russia, Svalbard, Greenland, USA, always linked to its host plants, dwarf and shrubby Salix or Dryas | ARL BRL ECCF GRL NBIC SlRL SRL SwRL | VU VU LC DD LC NT EN LC |
| Lactarius salmoneus Peck | USA, Mexico, Belize, China (?) | CRL | DD |
| Lactarius salmonicolor 50 R. Heim and Leclair | widely distributed in southern and central Europe, Great Britain, Russia, linked to Abies, reported from North America (?), Mexico (?), Guatemala (?), China (?) | ARL BHRL CRL CRRL ECCF FrRL GRL GuRL HRL RRL | LC EN LC VU LC LC/NA NotT 2 1 NT |
| Lactarius sanguifluus 51 (Paulet) Fr. | widely distributed in Europe, Great Britain, Russia, Malta, Algeria, Morocco, North America (?), Pakistan, Japan, China, Nepal | ARL CRL CRRL ECCF ERL FrRL GFRLI GRL MaRL NMRL NRL PRL SRL SwRL URL | LC LC CR LC NT EN/LC/NA NE HT R LC GE V NT LC VU |
| Lactarius sanguineus 52 Teng | France (?) | FrRL | NA |
| Lactarius saylorii (Thiers) P.M. Kirk | USA | GFRLI | DD |
| Lactarius scoticus Berk. and Broome | central and northern Europe, Great Britain, Ireland, Iceland, Russia, China (?) | ARL BRL CRL CRRL DRL ECCF ERL FRL GRL NBIC NRL SRL SwRL | VU VU DD DD LC LC LC LC DD LC GE VU LC |
| Lactarius scrobiculatus 53 (Scop.) Fr. | widely distributed in Europe, Russia, North America (?), China (?), Japan (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC SwRL | LC LC CR LC LC LC LC TH LC LC |
| Lactarius semisanguifluus R. Heim and Leclair | widely distributed in Europe, Russia, China (?) | CRL CRRL ECCF ERL FRL FrRL GRL NMRL NRL SRL SwRL | DD DD LC NT DD VU/LC/NA TH LC BE NT LC |
| Lactarius serifluus (DC.) Fr. | widely distributed in Europe, India (?), China (?) | CRL DRL ECCF ERL FRL FrRL GRL NBIC SRL SwRL | LC LC LC CR NT LC NT LC VU LC |
| Lactarius silviae 54 | USA, Canada | GFRLI | EN |
| Lactarius similissimus 55 A.H. Sm. and Hesler | USA, China (?) | CRL | DD |
| Lactarius sordidus see note 37 Peck | USA, Canada, China (?) | CRL | DD |
| Lactarius sphagneti (Fr.) Neuhoff | mostly northern Europe, but distributed also in the central part of the continent, Russia. In GBIF, also records from Canada and USA | ARL BuRL CRRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL PRL SwRL | VU DD NT DD LC NT LC VU/NT TH 1 LC VN E LC |
| Lactarius spinosulus Quél. and Le Bret. | central and northern Europe, Russia, Iceland, China (?) | ARL BuRL CRL CRRL DRL ECCF ERL FRL FrRL GRL NBIC PRL SRL SwRL | VU EN DD EN VU Relevant LC LC VU/NT TH LC V EN LC |
| Lactarius squamulosus 56 Z.S. Bi and T.H. Li | China | CRL | DD |
| Lactarius stephensii 57 (Berk.) Verbeken and Walleyn | widely distributed in central and southern Europe, Great Britain | ARL SRL | VU VU |
| Lactarius strigosipes Montoya and Bandala | Mexico | GFRLI | EN |
| Lactarius subcircellatus Kühner | northern Europe, Russia, Iceland, Greenland, Alaska | FRL GRL NBIC SwRL | LC DD LC LC |
| Lactarius subdulcis 58 (Pers.) Gray | widely distributed in Europe, Russia, Greenland, North America (?), China (?) | ARL CRL DRL ECCF FrRL GRL NBIC SwRL | LC LC LC LC LC NotT LC LC |
| Lactarius subflammeus Hesler and A.H. Sm. | USA, Canada, China (?) | CRL | DD |
| Lactarius subolivaceus Hesler and A.H. Sm. | USA, China (?) | CRL | DD |
| Lactarius subplinthogalus Coker | USA, Japan, Thailand, Nepal, China | CRL | LC |
| Lactarius subruginosus 59 J. Blum ex Bon | Austria, France, Spain | ARL FrRL | LC VU |
| Lactarius subsericeus 60 Deenis, Orton, and Hora | France | FrRL | NA |
| Lactarius subserifluus Longyear | USA, Malaysia (?), China (?) | CRL | DD |
| Lactarius subtomentosus 61 Z. Schaefer | Slovakia | SlRL | DD |
| Lactarius subumbonatus Lindgr. | southern and central Europe, Great Britain, Denmark, Sweden | ARL ECCF FrRL SRL | LC LC LC EN |
| Lactarius subvillosus Hesler and A.H. Sm. | USA | GFRLI | DD |
| Lactarius subzonarius Hongo | Japan, China, Korea, Thailand | CRL | LC |
| Lactarius sumstinei Peck | USA, Malaysia (?) China (?) | CRL | DD |
| Lactarius syringinus 62 Z. Schaef. | very scattered distribution, with records from Norway, Sweden, Estonia, Russia, Great Britain, Austria, Czech Republic, Slovakia | ARL | LC |
| Lactarius tabidus Fr. | widely distributed, especially in central and northern Europe, Iceland, Svalbard, Russia, Greenland, eastern North America, Korea, China (?) | ARL CRL DRL ERL FRL FrRL GRL NBIC SwRL | LC DD LC LC LC LC NotT LC LC |
| Lactarius terenopus 63 Romagnesi | reported from France, Spain, Slovenia, Great Britain | FrRL | EN |
| Lactarius tesquorum Malençon | a Mediterranean species, Portugal, Spain, France, Italy, Croatia, Malta, Morocco | CrRL ECCF | NT LC |
| Lactarius theiogalus 64 (Bull.) Gray | central and northern Europe, Svalbard, Greenland, North America, China (?) | CRL FrRL HRL | DD VU/LC 2 |
| Lactarius tithymalinus 65 (Scop.) Fr. | reported from France, Spain, Germany, Austria, Denmark, Norway, China (?) | CRL FrRL | DD EN/DD |
| Lactarius torminosulus Knudsen and T. Borgen | a northern European species, also reported from France, Austria, Germany, Russia, Iceland, Greenland, Svalbard, Canada | FRL GRL NBIC SwRL | LC DD LC LC |
| Lactarius torminosus (Schaeff.) Gray | widely distributed in central and northern Europe, Russia, Iceland, Greenland, North America, Japan, China, Morocco, Australia, New Zealand, introduced in many areas with its host Betula | ARL CRL DRL ECCF ERL FRL FrRL GFRLI GRL HRL NBIC NRL SwRL | LC LC LC LC LC LC LC Proposed NotT 4 LC KW LC |
| Lactarius tristis 66 J. Blum | France | FrRL | DD |
| Lactarius trivialis (Fr.) Fr. | widely distributed in central and northern Europe, Russia, Svalbard, Greenland, North America, Japan, China | ARL CRL DRL ECCF ERL FRL FrRL GFRLI GRL NBIC NRL PRL SwRL | LC DD VU LC LC LC LC/DD Proposed TUE LC KW R LC |
| Lactarius tuomikoskii Kytöv. | Finland, Sweden, Norway, Germany, Austria, records also from northern Italy | ARL FRL GRL NBIC SwRL | LC LC ER LC LC |
| Lactarius umbrinus 67 (Paulet) Fr. | just a few, mostly very old reports from Germany, Sweden, Great Britain and Estonia | GRL | DD |
| Lactarius utilis (Weinm.) Fr. | central and more frequently northern Europe, Great Britain, Estonia, Russia, Greenland | ARL ECCF FRL FrRL | LC LC LC DD |
| Lactarius uvidus 68 (Fr.:Fr.) Fr. | widely distributed in Europe, Russia, Iceland, Svalbard, Greenland, North America, Japan, China (?) | ARL CRL CRRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NRL RRL SwRL | LC LC EN EN LC LC LC EN/LC/DD TH 3 LC EB NT LC |
| Lactarius vietus 69 (Fr.) Fr. | widely distributed in Europe, Russia, Iceland, Greenland, North America, Korea, China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SwRL | LC LC LC LC LC LC EN/NT/LC/DD NotT LC KW LC |
| Lactarius vinaceorufescens A.H. Sm. | USA, Canada, China (?) | CRL | DD |
| Lactarius vinosus 70 (Quél.) Bataille | southern Europe, Mediterranean area, Turkey | ECCF FrRL | LC EN/DD/NA |
| Lactarius violascens (J. Otto) Fr. | widely distributed in Europe, Russia, Japan (?), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL HRL NBIC NMRL NRL PRL SwRL | LC LC EN Relevant LC DD VU/LC/DD TE 3 LC NT VN E NT |
| Lactarius waltersii Hesler and A.H. Sm | USA, China (?) | CRL | DD |
| Lactarius wangii 71 J.Z. Ying and H.A. Wen | China | CRL | DD |
| Lactarius wenquanensis 72 Y. Wang and Z.X. Xie | China, Russia (?) | CRL | DD |
| Lactarius xanthogalactus 73 Peck | USA, Mexico, reported also from Canada, Colombia, China (?) | GFRLI | LC |
| Lactarius zonarioides Kühner and Romagn. | central and northern Europe, Russia, China (?) | ARL CRL CRRL ECCF ERL FRL FrRL GRL NBIC PRL SwRL | LC DD EN LC NT LC LC/NA TUE LC E LC |
| Lactarius zonarius 74 (Bull.) Fr. | widely distributed in Europe, Russia, North America, Japan, India, China (?), typically (but not exclusively) associated to Quercus | ARL CRL CRRL DRL ECCF ERL FrRL GRL NBIC SwRL | LC LC VU EN LC NT LC TH NE DD |
| Lactifluus atrovelutinus (J.Z. Ying) X.H. Wang | China, Malaysia | CRL | DD |
| Lactifluus bertillonii 75 (Neuhoff ex Z. Schaef.) Verbeken | widely distributed in Europe, Russia | ARL DRL ECCF FRL FrRL GRL NBIC SRL SwRL | LC LC LC LC EN/DD DD LC EN LC |
| Lactifluus glaucescens 76 (Crossl.) Verbeken | widely distributed in Europe, Russia, North America (?), Japan, China (?) | ARL CRL DRL ECCF FRL FrRL GRL NBIC NRL SRL SwRL | LC DD DD LC LC LC/DD NotT LC GE VU LC |
| Lactifluus hallingii Delgat and De Wilde | Costa Rica, Panama, Colombia | GFRLI IUCN | VU VU |
| Lactifluus luteolus 77 (Peck) Verbeken | Italy, Spain, France, Slovenia, Switzerland, Morocco, China (?) | CRL ECCF FrRL SRL | LC Relevant DD CR |
| Lactifluus ochrogalactus (Hashiya) X.H. Wang | Japan, Korea, China India, Malaysia, Borneo. Records also from Australia (see GBIF) | CRL | DD |
| Lactifluus pilosus (Verbeken, H.T. Le and Lumyong) Verbeken | Thailand, Korea, France (?) | FrRL | DD |
| Lactifluus piperatus 78 (L.) Roussel | widely distributed in Europe, Russia, North America (?), China (?) | CRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SwRL | LC LC LC NT LC LC NotT LC EB LC |
| Lactifluus puberulus (H.A. Wen and J.Z. Ying) Nuytinck | China | CRL | DD |
| Lactifluus rugatus 79 (Kühner and Romagn.) Verbeken | a typical Mediterranean species, Portugal, Spain, Italy, France, Greece, Morocco, Germany (?), China (?) | CRL ECCF FrRL GRL | DD LC LC/DD DD |
| Lactifluus subgerardii (Hesler and A.H. Sm.) D. Stubbe | USA, Canada, China (?) | CRL | LC |
| Lactifluus subpiperatus (Hongo) Verbeken | Japan, Korea (?), China (?) | CRL | LC |
| Lactifluus subvellereus (Peck) Nuytinck | USA, Canada, Mexico, Japan (?), Korea (?), China (?) | CRL | LC |
| Lactifluus subvolemus Van de Putte & Verbeken | recorded from Italy, Austria, Slovenia, France, Belgium, Denmark, Sweden, Norway, Estonia, Bulgaria | DRL | DD |
| Lactifluus tenuicystidiatus (X.H. Wang and Verbeken) X.H. Wang | China, Laos | CRL | DD |
| Lactifluus vellereus 80 (Fr.) Kuntze | widely distributed in Europe, Russia, Iceland, Nepal (?), China (?) | ARL CRL DRL ECCF ERL FRL FrRL GRL NBIC NRL SwRL | LC LC LC LC NT LC LC NotT LC KW LC |
| Lactifluus vitellinus (Van de Putte and Verbeken) Van de Putte | Thailand, China (?) | CRL | DD |
| Lactifluus volemus 81 (Fr.) Kuntze | widely distributed in Europe, Russia, North America (?), China (?) | AlRL CRL DRL ECCF ERL FRL FrRL GRL HRL LRL NBIC NMRL NRL SlRL SwRL | NT LC LC LC NT LC LC NT 3 NT LC LC EB VU LC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, M.; Comandini, O.; Sanjust, E.; Rinaldi, A.C. Conservation Status of Milkcaps (Basidiomycota, Russulales, Russulaceae), with Notes on Poorly Known Species. Sustainability 2021, 13, 10365. https://doi.org/10.3390/su131810365
Leonardi M, Comandini O, Sanjust E, Rinaldi AC. Conservation Status of Milkcaps (Basidiomycota, Russulales, Russulaceae), with Notes on Poorly Known Species. Sustainability. 2021; 13(18):10365. https://doi.org/10.3390/su131810365
Chicago/Turabian StyleLeonardi, Marco, Ornella Comandini, Enrico Sanjust, and Andrea C. Rinaldi. 2021. "Conservation Status of Milkcaps (Basidiomycota, Russulales, Russulaceae), with Notes on Poorly Known Species" Sustainability 13, no. 18: 10365. https://doi.org/10.3390/su131810365
APA StyleLeonardi, M., Comandini, O., Sanjust, E., & Rinaldi, A. C. (2021). Conservation Status of Milkcaps (Basidiomycota, Russulales, Russulaceae), with Notes on Poorly Known Species. Sustainability, 13(18), 10365. https://doi.org/10.3390/su131810365








